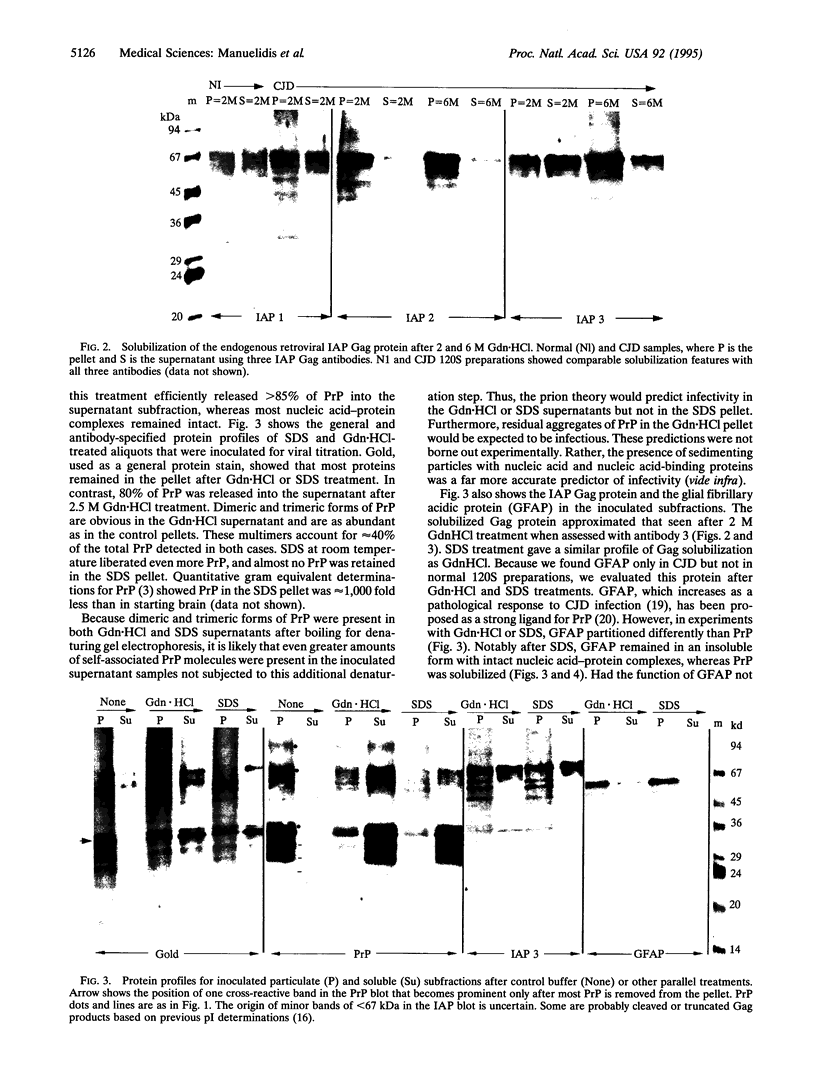
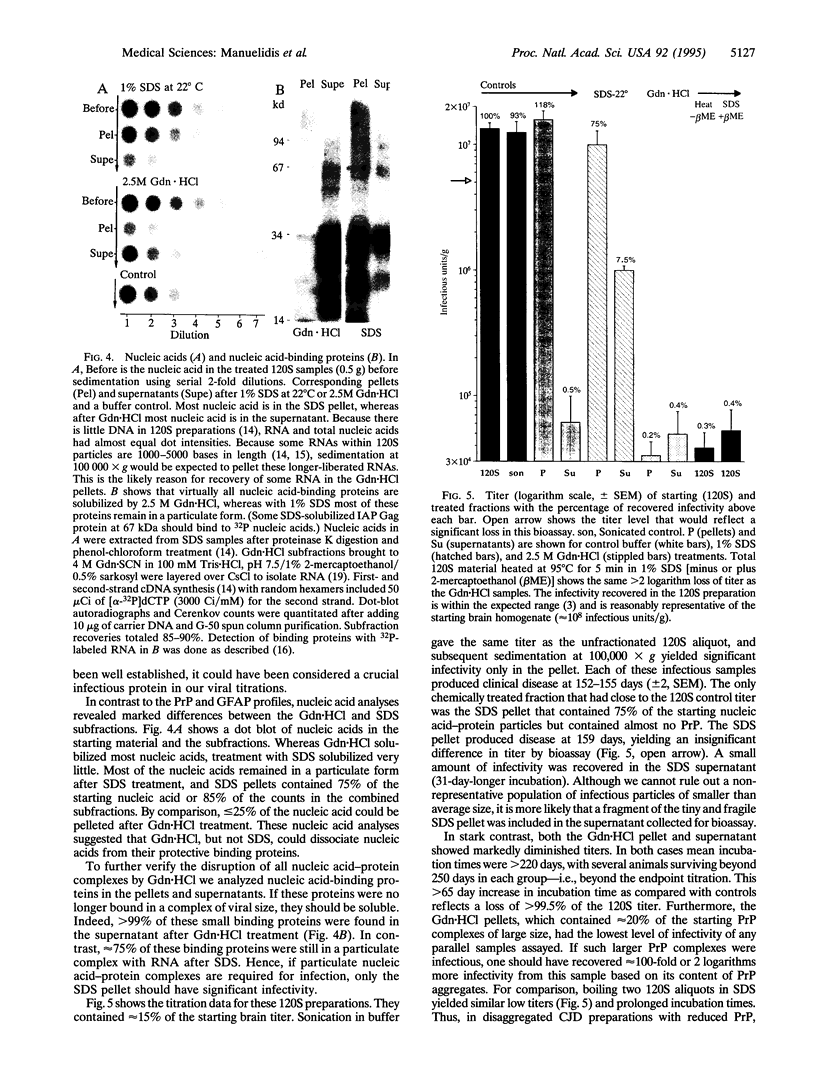
Abstract
Several models have been proposed for the infectious agents that cause human Creutzfeldt-Jakob disease (CJD) and sheep scrapie. Purified proteins and extracted nucleic acids are not infectious. To further identify the critical molecular components of the CJD agent, 120S infectious material with reduced prion protein (PrP) was treated with guanidine hydrochloride or SDS. Particulate and soluble components were then separated by centrifugation and molecularly characterized. Conditions that optimally solubilized residual PrP and/or nucleic acid-protein complexes were used to produce subfractions that were assayed for infectivity. All controls retained > 90% of the 120S titer (approximately 15% of that in total brain) but lost > 99.5% of their infectivity after heat-SDS treatment (unlike scrapie fractions enriched for PrP). Exposure to 1% SDS at 22 degrees C produced particulate nucleic acid-protein complexes that were almost devoid of host PrP. These sedimenting complexes were as infectious as the controls. In contrast, when such complexes were solubilized with 2.5 M guanidine hydrochloride, the infectious titer was reduced by > 99.5%. Sedimenting PrP aggregates with little nucleic acid and no detectable nucleic acid-binding proteins had negligible infectivity, as did soluble but multimeric forms of PrP. These data strongly implicate a classical viral structure, possibly with no intrinsic PrP, as the CJD infectious agent. CJD-specific protective nucleic acid-binding protein(s) have already been identified in 120S preparations, and preliminary subtraction studies have revealed several CJD-specific nucleic acids. Such viral candidates deserve more attention, as they may be of use in preventing iatrogenic CJD and in solving a fundamental mystery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akowitz A., Sklaviadis T., Manuelidis E. E., Manuelidis L. Nuclease-resistant polyadenylated RNAs of significant size are detected by PCR in highly purified Creutzfeldt-Jakob disease preparations. Microb Pathog. 1990 Jul;9(1):33–45. doi: 10.1016/0882-4010(90)90038-r. [DOI] [PubMed] [Google Scholar]
- Akowitz A., Sklaviadis T., Manuelidis L. Endogenous viral complexes with long RNA cosediment with the agent of Creutzfeldt-Jakob disease. Nucleic Acids Res. 1994 Mar 25;22(6):1101–1107. doi: 10.1093/nar/22.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K., Masters C. L. Neurobiology. Catching the culprit prion. Nature. 1994 Aug 11;370(6489):419–420. doi: 10.1038/370419a0. [DOI] [PubMed] [Google Scholar]
- Brown P., Liberski P. P., Wolff A., Gajdusek D. C. Conservation of infectivity in purified fibrillary extracts of scrapie-infected hamster brain after sequential enzymatic digestion or polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7240–7244. doi: 10.1073/pnas.87.18.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. E., McConnell I., Fraser H., Dickinson A. G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol. 1991 Mar;72(Pt 3):595–603. doi: 10.1099/0022-1317-72-3-595. [DOI] [PubMed] [Google Scholar]
- Collee J. G. BSE: stocktaking 1993. Lancet. 1993 Sep 25;342(8874):790–793. doi: 10.1016/0140-6736(93)91546-x. [DOI] [PubMed] [Google Scholar]
- Forloni G., Angeretti N., Chiesa R., Monzani E., Salmona M., Bugiani O., Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993 Apr 8;362(6420):543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Spontaneous generation of infectious nucleating amyloids in the transmissible and nontransmissible cerebral amyloidoses. Mol Neurobiol. 1994 Feb;8(1):1–13. doi: 10.1007/BF02778003. [DOI] [PubMed] [Google Scholar]
- Hsiao K. K., Groth D., Scott M., Yang S. L., Serban H., Rapp D., Foster D., Torchia M., Dearmond S. J., Prusiner S. B. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocisko D. A., Come J. H., Priola S. A., Chesebro B., Raymond G. J., Lansbury P. T., Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994 Aug 11;370(6489):471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Dementias, neurodegeneration, and viral mechanisms of disease from the perspective of human transmissible encephalopathies. Ann N Y Acad Sci. 1994 Jun 6;724:259–281. doi: 10.1111/j.1749-6632.1994.tb38916.x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Murdoch G., Manuelidis E. E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Sklaviadis T., Manuelidis E. E. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987 Feb;6(2):341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Tesin D. M., Sklaviadis T., Manuelidis E. E. Astrocyte gene expression in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5937–5941. doi: 10.1073/pnas.84.16.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. The dimensions of Creutzfeldt-Jakob disease. Transfusion. 1994 Oct;34(10):915–928. doi: 10.1046/j.1537-2995.1994.341095026981.x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Teplow D. B., Stahl N., Serban D., Hood L. E., Prusiner S. B. Identification of cellular proteins binding to the scrapie prion protein. Biochemistry. 1990 Jun 19;29(24):5848–5855. doi: 10.1021/bi00476a029. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D., Serban A., Stahl N., Gabizon R. Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2793–2797. doi: 10.1073/pnas.90.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T. K., Manuelidis L., Manuelidis E. E. Physical properties of the Creutzfeldt-Jakob disease agent. J Virol. 1989 Mar;63(3):1212–1222. doi: 10.1128/jvi.63.3.1212-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T., Akowitz A., Manuelidis E. E., Manuelidis L. Nucleic acid binding proteins in highly purified Creutzfeldt-Jakob disease preparations. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5713–5717. doi: 10.1073/pnas.90.12.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]







