Abstract
Background
The complexity of multiple-item criteria in acute respiratory distress syndrome (ARDS) often causes inconvenience for physicians in the management of patients with severe acute pancreatitis (SAP). We evaluated whether serum SP-A levels in the presence of diffuse alveolar damage (DAD) can be qualitatively assessed for diagnosis of SAP-induced ARDS.
Material/Methods
Eighty rats were randomly divided into 2 groups (n=40 each) – the sham-operated (SO) group and the SAP group – and then randomly subdivided into 4 subgroups in a time-course manner. Furthermore, rats in the SAP group were subdivided into the SAP induced-ARDS group (ARDS group) and the SAP without ARDS group (non-ARDS group) according to the diagnostic standard of ARDS. The diagnostic cut-off values of SP-A for SAP-induced ARDS were determined by the receiver operating characteristic curve (ROC).
Results
Serum SP-A levels in Baseline, SO group, SAP group, ARDS group, and non-ARDS group were 43.15±14.29, 51.91±16.99, 193.4±35.37, 198.0+29.73, and 185.7±43.21 ug/ml, respectively. The best cut-off value for the serum SP-A level for the diagnosis of SAP-induced ARDS was 150 ug/ml and the area under the ROC curve of SP-A was 0.88. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of SP-A in the diagnosis of SAP-induced ARDS were 100.0%, 81.8%, 71.4%, 100.0%, and 87.5%, respectively.
Conclusions
Serum SP-A levels may allow the detection of SAP-induced ARDS and may help to support the clinical diagnosis of ARDS. The optimal serum SP-A cut-off value to discriminate SAP-induced ARDS and other groups (SO group and non-ARDS group) is around 150 ug/ml.
MeSH Keywords: Acute Respiratory Distress Syndrome, Pancreatitis, Pulmonary Surfactant-Associated Proteins
Background
Acute pancreatitis is an inflammatory disease characterized by the presence of acute abdominal pain and/or increased levels of serum pancreatic enzymes [1]. The primary causes of acute pancreatitis are gallstones and excessive alcohol consumption. More importantly, Takeda et al. [2] have indicated that the mortality rate was of approximately 20% when acute pancreatitis developed to severe acute pancreatitis (SAP). Similarly, several lines of evidence have demonstrated that systemic inflammatory response syndrome (SIRS) and multiple-organ dysfunction syndrome (MODS) are the 2 most common causes of death in the early stage (within approximately 2 weeks) of patients with SAP [3]. Previous research has suggested that acute respiratory distress syndrome (ARDS), which is a clinical manifestation of lung injury, is one of the earliest manifestations of functional deterioration in human organs and is one of the most common and serious complications of SAP [4].
The most severe clinical manifestations of ARDS exhibit significant hypoxemia with bilateral pulmonary infiltrates consistent with edema [5,6]. Currently, the criteria for the diagnosis of ARDS are mainly based on the Berlin definition [7], which includes the original diagnosis of acute lung injury (ALI) and ARDS: acute onset of respiratory distress, hypoxemia (ALI: PaO2/FiO2 £300 mmHg, ARDS: PaO2/FiO2 £200 mmHg), bilateral consolidation of chest radiograph, and absence of clinical findings of cardiogenic pulmonary edema [8]. In practice, the complexity of multiple-item criteria of ARDS often causes inconvenience for physicians in the management of patients with SAP. If ARDS also causes changes in several related bio-markers, similar to the characteristic elevation of troponin in myocardial infarction, this bio-marker might be useful to help diagnose of SAP-induced ARDS.
Previous reports indicate that pulmonary surfactant consists principally of various lipids and proteins (surfactant proteins) [9]. Furthermore, the major constituent of surfactant proteins is SP-A, and the remainder includes SP-B, SP-C, and SP-D. Recently, a study suggested that SP-A is a large multimeric protein found in the airways and alveoli [10]. Several lines of evidence have shown that SP-A not only mediates the inflammatory response, but also controls vascular permeability, which are considered to be important factors in the development of ARDS [10,11]. A large number of studies [12,13] have shown that SP-A levels in bronchoalveolar lavage fluid (BALF) are significantly lower in patients with Bleomycin-induced ARDS compared with healthy volunteers. In contrast, the serum levels of SP-A were raised in patients with Bleomycin-induced ARDS. Thus, the serum levels of SP-A may be suggested as a sensitive and specific serum marker on the diagnosis of SAP-induced ARDS. However, until now, this has received little research attention.
It has been suggested that the presence of qualitatively assessed (yes or no) diffuse alveolar damage (DAD) should be considered the criterion standard for the diagnosis of ARDS [14,15]. To improve the early detection and diagnosis of SAP-induced ARDS, determination of serum levels of SP-A in SAP-induced ARDS rats was used. In the present study, we therefore evaluated serum SP-A levels according to the criterion standard for the diagnosis of ARDS to evaluate the qualitative assessment of presence of diffuse alveolar damage (DAD) in the diagnosis of SAP-induced ARDS in an animal model.
Material and Methods
Animals and groups
One hundred male Wistar rats weighing 250–300 g were purchased from Cavens Laboratory Animal Company, Changzhou, Jiangsu, China. Six rats were housed per cage with food and water available ad libitum and maintained on a 12-h light/dark cycle (lights on at 07:00 AM). Food, but not water, was withdrawn 12 h before the experiment.
Eighty rats were randomly divided into 2 groups (n=40 each): the sham-operated (SO group) and the Severe Acute Pancreatitis (SAP group) and then randomly subdivided into 1-h, 2-h, 3-h, and 4-h subgroups, with 10 rats in each subgroup. The rats in the 1-h, 2-h, 3-h, and 4-h subgroups of the SAP group, a total of 40 rats, were subdivided into a SAP-induced ARDS group (ARDS group) and a SAP without ARDS group (non-ARDS group) according to the diagnosis [14,15] of ARDS for lung tissue, including the presence of DAD, qualitatively assessed (yes or no) after the operation. The other 20 rats were regarded as the “baseline” and euthanized without any operation in initial experiments.
Intra-peritoneal injection of 1% sodium pentobarbital (50 mg/kg; Sigma, St. Louis, MO, USA) was used for abdominal cavity anesthesia to all rats of the 1-h, 2-h, 3-h, 4-h subgroups. Animal care was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Animal Use and Protection Committee of Soochow University.
SAP and ARDS induction
The abdominal surgery of the SO group (n=40) consisted of opening the abdomen, flipping the pancreas, returning them to their original position, then closing the abdominal cavity with 2 layers of sutures. SAP model were performed according to previous studies [16–20]. Sodium taurocholate solution (Sigma, St. Louis, MO, USA) was administered into the common biliopancreatic duct of the SAP group (n=40) by retrograde injection. We identified the duodenal papilla inside the duodenum duct wall, and then used a No. 5 needle to drill a hole in the avascular area of the mesentery. A segmental epidural catheter was inserted into the duodenum cavity through the hole, and inserted retrogradely into the biliary-pancreatic duct through the papilla. This was followed by retrograde transfusion of 4.5% sodium taurocholate solution (4.5%; 0.1 ml/100 g) by a microinjection pump at 0.1 ml/min. The hole in the lateral duodenal wall was then sutured. All rats of the 2 groups were injected with normal saline (20 ml/kg) through the vena caudalis to compensate for loss of fluid during surgery.
Determination of serum SP-A levels
The rats in all groups were euthanized at 1, 2, 3 and 4 hours, according to the subgroup, after the operation. Blood samples (5 ml) were collected from the heart, and were mixed with EDTA anticoagulative liquor, then were centrifuged at 3000 r/min for 10 min and stored at −70°C. Sera of their blood samples were collected and maintained at 4°C for the detection of SP-A. Simultaneously, we obtained pancreatic and lung tissue specimens for pathological analysis.
Serum levels of SP-A were determined by the ELISA method by using the “Rat SP-A ELISA kit” purchased from MyBioSource (San Diego, California, USA). The antigens were measured by ELISA inhibition assays using antibodies raised against alveolar proteinosis-derived SP-A. Briefly, in order to free the SP-A from any associated plasma or surfactant components, aliquots were first treated with EDTA, SDS, and Triton X-100. Serial dilutions of the samples in PBST containing 0.25% BSA (w/v) were incubated in an ELISA plate with aliquots of the respective antibody. Free antibody was captured using a second ELISA plate coated with purified SP-A (1 μg/ml) and the amount measured using alkaline phosphatase conjugated IgG against rabbit immunoglobulins and 15 mm disodium p-nitrophenyl phosphate in 1.0 M diethanolamine and 0.5 mm MgCl 2 as a substrate. After 1 h, the absorbance of the substrate was measured at 405 nm using a Dynatech MR5000 reader (Dynatech Laboratories, Chantilly, VA, USA). The AssayZap program (Biosoft, Ferguson, MO, USA) was used to generate a standard curve and to compute the concentration of SP-A in each sample. All samples were assayed in duplicate at 4 serial dilutions. Standards, assayed in quadruplicate, were included in each ELISA plate at 8 serial dilutions (range 1.95–250 ng/ml for SP-A, r>0.99). The antibodies used do not react with any other known antigens and the assays have coefficients of variance of ~6%.
Pathologic criteria for pancreas and lung tissues
Rat pancreatic and lung tissues were collected, fixed in 10% formalin, embedded in paraffin, and sectioned into 4-μ sections, after which they were stained by hematoxylin and eosin (H&E) staining for histopathological observation. Histopathology of the pancreas and lungs was scored and classified by 2 professional pathologists using a double-blind method. Each group randomly selected 3 slices; for each slice, 10 high-power fields of vision were again randomly selected, and finally the extent of pancreatic tissue damage by edema, infection, hemorrhage, and necrosis were evaluated.
The pathological score for pancreatic tissue was calculated according to Rongiones’ standards [21] as a reference, with a minimum score of 0 and the highest score of 4. The pathological grade for lung tissue was according to Lei [22] as a reference, with a minimum grade of 0 and the highest grade of III.
Criteria for the diagnosis of ARDS for lung tissue included the presence of diffuse alveolar damage (DAD), qualitatively assessed (yes/positive result or no/negative result) as: intra-alveolar edema, alveolar type I cell necrosis, alveolar type II cell (cuboidal cells) proliferation progressively covering the denuded alveolar-capillary membrane, interstitial proliferation of fibroblasts and myofibroblasts, or organizing interstitial fibrosis [14,15].
Statistical analysis
Data are expressed as the means ±SD. Statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Positive rates were tested with χ2 and rank sum test. Quantitative data were compared with t-test. Correlations between 2 variants were tested with bivariate correlation analysis. P level<0.05 was considered significant.
The diagnostic cut-off values of SP-A for ARDS were determined by the receiver operating characteristic curve (ROC). The comparison among multiple groups was analyzed with 1-dimensional analysis of variance after data conversion. The comparison between 2 groups – ARDS group and SO group + non-ARDS group – was tested with nonparametric rank sum test. True- positive (TP) was defined as SP-A values higher than the cut-off value in rats with SAP induced-ARDS groups (ARDS group). False-positive (FP) included SAP rats without ARDS who scored positive. True-negative (TN) included SAP rats without ARDS who scored negative. False-negative (FN) included rats with ARDS but who scored negative. Sensitivity and specificity were used to evaluate the diagnostic accuracy.
Formulas for calculation were: sensitivity=TP/(TP+FN)×100%, specificity=TN/(TN+FP)×100%, diagnostic accuracy=(TP+TN)/(TP+FP+TN+FN)×100%, positive predictive value=TP/(TP+FP) ×100%, and negative predictive value =TN/(TN+FN)×100%.
Results
Expression of pathology in pancreas and lung tissues
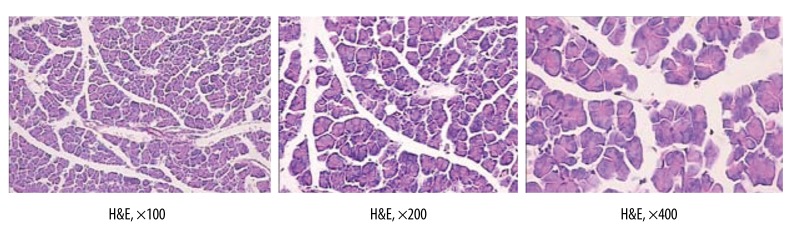
The glandular architecture of the pancreas in the SO group was entirely normal (H&E) (Figure 1).
Figure 1.
The glandular architecture of pancreas in SO group is entirely normal (H&E).
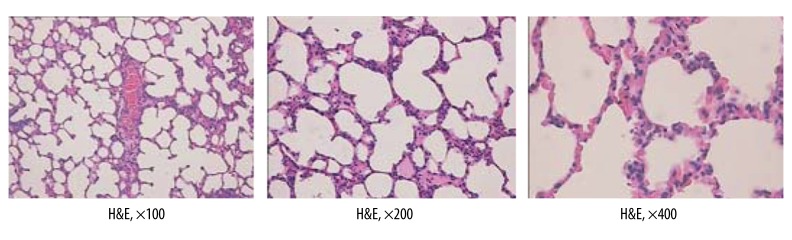
The architecture of the lung in the SO group was entirely normal (H&E) (Figure 2).
Figure 2.
The architecture of lung in SO group is entirely normal (H&E).
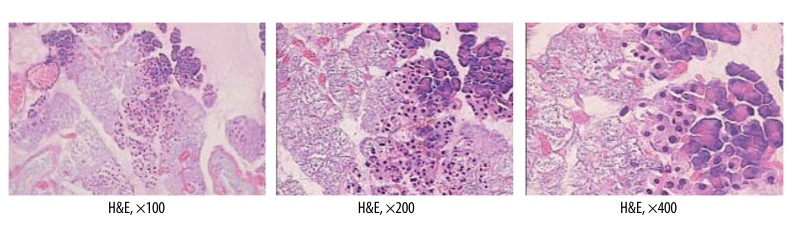
Histopathologic features of the pancreas in the SAP group included various degrees of edema, minimal necrosis, and inflammatory infiltration (H&E) (Figure 3).
Figure 3.
Histopathologic features of pancreas in SAP group.
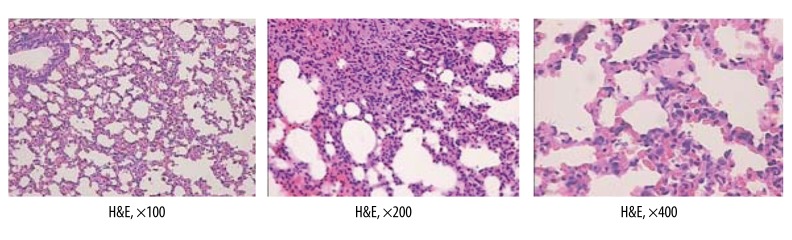
According to the criteria for the diagnosis of ARDS [14,15], the presence of DAD was qualitatively assessed (yes or no); in the 1-h, 2-h, 3-h, and 4-h subgroups we found 1, 5, 9, and 10 cases of ARDS, respectively. Therefore, the SAP group was then subdivided into the ARDS group (25 rats) and non-ARDS group (15 rats) according to the criteria for the diagnosis of ARDS. Histopathologic features of lung in the ARDS group included the presence of DAD, intra-alveolar edema, and organizing interstitial fibrosis (H&E) (Figure 4).
Figure 4.
Histopathologic features of lung in ARDS group.
Expression of Serum SP-A levels
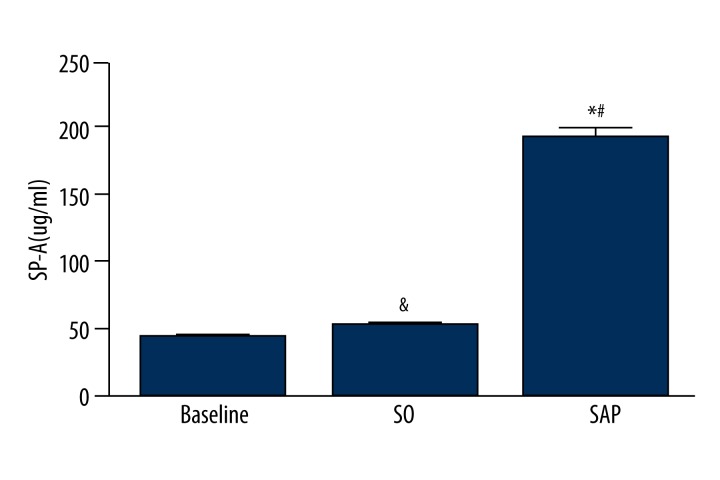
Serum SP-A levels (means ±SD) in the Baseline, SO, and SAP groups were 43.15±14.29, 51.91±16.99, and 193.4±35.37 ug/ml, respectively. There was no statistical difference between SO group and baseline in serum SP-A levels (&P>0.05). Serum SP-A levels of the SAP group were significantly increased compared to baseline and SO group (*P<0.05, #P<0.05) (Figure 5).
Figure 5.
Serum SP-A levels in SO group, SAP group, and baseline.
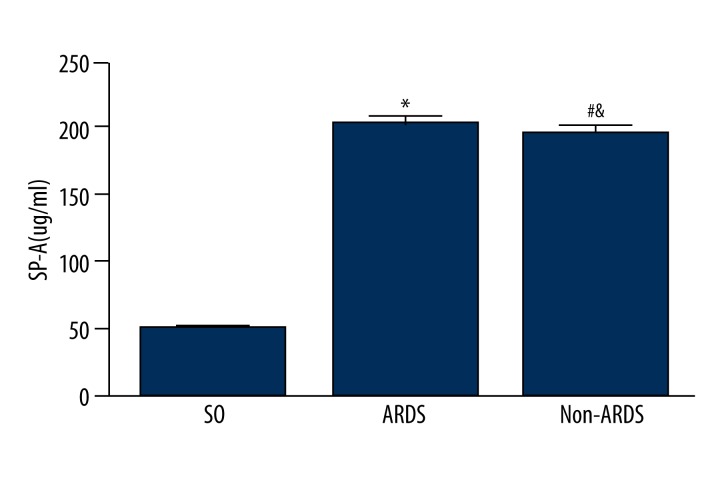
Serum SP-A levels (means ±SD) in the SO group, ARDS group, and non-ARDS group were 51.91±16.99, 198.0±29.73, and 185.7±43.21 ug/ml, respectively. Serum SP-A levels were significantly increased from the ARDS group to the SO group (*P<0.05) and from the non-ARDS group to the SO group (#P<0.05). There was no statistical difference between the ARDS group and non-ARDS group in serum SP-A levels (&P>0.05) (Figure 6).
Figure 6.
Serum SP-A levels in SO group, ARDS group, and non-ARDS group.
Diagnosis of SP-A according to ROC curves
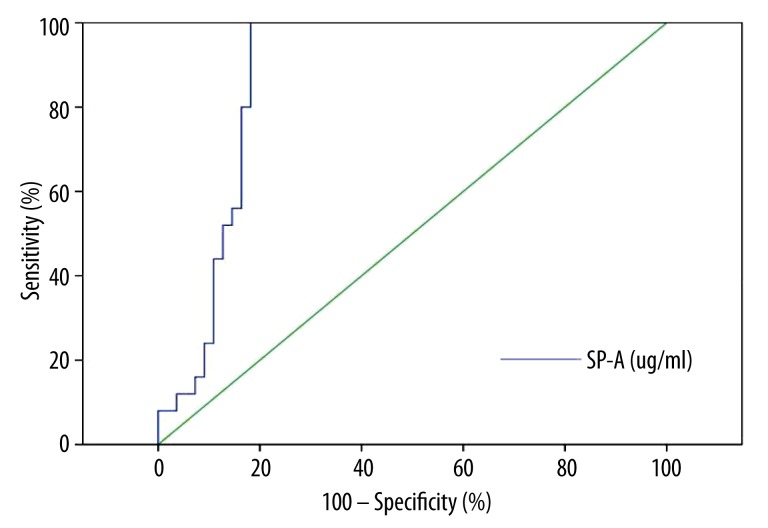
According to the analysis of ROC curves, the best cut-off value for the serum SP-A level for the diagnosis of SAP-induced ARDS was 150 ug/ml. In analysis of all SAP-induced ARDS rats, the area under the ROC curve of SP-A was 0.88 (Figure 7).
Figure 7.
ROC curves of SP-A for diagnosis of SAP-induced ARDS.
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of SP-A in the diagnosis of SAP-induced ARDS were 100.0%, 81.8%, 71.4%, 100.0%, and 87.5%, respectively (Table 1).
Table 1.
Evaluation of SP-A in the diagnosis of SAP-induced ARDS.
| Evaluation index | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy |
|---|---|---|---|---|---|
| SP-A, (n) | 100.0(%) (25/25) | 81.8(%) (45/55) | 71.4(%) (25/35) | 100.0(%) (45/45) | 87.5(%) (70/80) |
Discussion
In the present study, we evaluated serum SP-A levels for the diagnosis of SAP-induced ARDS in a rat model. Importantly, the SAP-induced ARDS rat model is mostly suitable for investigating the effectiveness and specificity of a particular therapeutic approach [23]. The model that we chose had to be similar to the etiology and convenient for the experimental operations. Some models focus on etiology, such as the duct ligation model and the taurocholate-induced pancreatitis model. But the latter is more simple, and less damaging to the animals, which mimic biliary pancreatitis in the clinical setting better than the duct ligation model [23]. Several lines of evidence have indicated that the taurocholate-induced pancreatitis model induced by ARDS was successfully 3–4 h after the operation [20,24,25]. Thus, we constructed this model by using sodium taurocholate solution injected into the common biliopancreatic duct.
According to the criteria for the diagnosis of ARDS [14,15], 1, 5, 9, and 10 cases of ARDS were determined as positive results in the 1-h, 2-h, 3-h, and 4-h subgroups, respectively. The results of the present study are consistent with previous findings that SAP leading to a systemic inflammatory response was characterized by widespread leukocyte activation and distant organ injury (e.g., ARDS) [26–28]. Our previous study showed that a definite correlation exists between pathological changes (pancreas and lung) and progression of SAP. In the present study, we found that the glandular architecture of the pancreas and the architecture of the lung in the SO group were entirely normal (H&E) (Figures 1 and 2). Furthermore, histopathologic features of the pancreas, various degrees of edema, minimal necrosis, and inflammatory infiltration (H&E) were detected in glandular architecture of the pancreas in the SAP group (Figure 3). The SAP group was subdivided into the ARDS group (25 rats) and non-ARDS group (15 rats) according to criteria including presence of DAD, intra-alveolar edema, and organizing interstitial fibrosis (H&E) (Figure 4). Similar results have also been reported in ARDS animal studies [25]. These results are similar to findings of the present study, suggested that the cases of positive results, which included the presence of DAD, was qualitatively assessed (yes or no), showing an increasing trend associated with longer time in the preceding 4 h in the rat model of SAP-induced ARDS.
This gap in serum levels of SP-A has previously been reported in many studies [12,13], with the results being similar to those in the present study – baseline, 43.15±14.29 ug/ml vs. SO group 51.91±16.99 ug/ml vs. SAP group 193.4±35.37 ug/ml (Figure 5); SO group, 51.91±16.99 ug/ml vs. ARDS group 198.0±29.73 ug/ml vs. non-ARDS group 185.7±43.21 ug/ml (Figure 6). These findings demonstrated that ARDS, the most common and serious complications of SAP, was induced in the SAP group and contributed to an elevation of SP-A in serum in this study. However, the underlying mechanism has not yet been fully elucidated and may be related to multiple factors. We propose that the possible mechanisms include epithelial injury and leak – increased secretion of SP-A per type II cell, increased leakage from the airspace to the interstitium, an increase in total number of type II cells per lung due to diffuse hyperplasia, and decreased clearance from the vascular compartment [29]. In addition, the degree of lung injury and SP-A levels leaked from the airspace to the serum was similar in the ARDS group and non-ARDS group. Moreover, the presence of DAD was not observed in the non-ARDS group because of multiple factors, such as the time of lung injury, individual differences, and the degree of lung injury, rather than the absence of lung injury [30]. Consistent with these findings, McIntosh et al. found that serum SP-A levels are upregulated by an acute inflammatory stress [30]; therefore, we used determination of serum levels of SP-A to diagnose SAP-induced ARDS in the rat model.
Our data analysis showed that SP-A>150 ug/ml statistically discriminates between SAP-induced ARDS and other groups (SO group and non-ARDS group) with 100.0% sensitivity and 81.8% specificity. At this cut-off value, the diagnostic accuracy was the highest, and the area under the ROC curve of SP-A was 0.88 (Figure 7). Furthermore, SP-A>150 ug/ml was diagnosed as SAP-induced ARDS with 71.4% positive predictive value, 100.0% negative predictive value, and 87.5% accuracy in this study. These data demonstrated that the accuracy of diagnosis of SP-A for SAP-induced ARDS is relatively high. Therefore, the SP-A, as lung-specific bioactive surface proteins, are valuable parameters for the prediction of SAP-induced ARDS. Interestingly, because it is impossible to collect the human pancreatic and lung tissues in the clinical course of treatment, there is little data on this value. We, therefore consider that our findings can be extrapolated to other animals and to humans.
Although further clinical studies are required to confirm this, rats with SP-A greater than 150 ug/ml and high pulmonary vascular permeability index may be typical for SAP-induced ARDS. In addition, ELLSA can detect small changes in SP-A content and serum SP-A level can be easily, quickly, and repeatedly measured at the bedside. Serum SP-A level correlates with the progression of lung injury and predicts progression to ARDS in patients with increased risk [31]. Thus, serum SP-A was chosen in this study as the first validated quantitative measure for SAP-induced ARDS and we believe that monitoring serum SP-A level in peripheral blood of SAP patients is helpful to evaluate whether SAP induces ARDS.
Conclusions
The serum levels of SP-A may be useful as a sensitive and specific serum marker for the diagnosis of SAP-induced ARDS, as confirmed in the present study. However, a great limitation of the present study is that we did not observe the long-term clinical significance, and future studies are required to investigate this.
Footnotes
Conflicts of interest
All the authors had no conflicts of interest to declare in relation to this article.
Source of support: This study was supported by Hospital-Level Care Special Pre-Research Fund of The First People’s Hospital of Changzhou (No: yy2013012)
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–52. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Yokoe M, Takada T, et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010;17:37–44. doi: 10.1007/s00534-009-0213-4. [DOI] [PubMed] [Google Scholar]
- 3.Wada K, Takada T, Hirata K, et al. Treatment strategy for acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:79–86. doi: 10.1007/s00534-009-0218-z. [DOI] [PubMed] [Google Scholar]
- 4.Raghu MG, Wig JD, Kochhar R, et al. Lung complications in acute pancreatitis. JOP. 2007;8:177–85. [PubMed] [Google Scholar]
- 5.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–23. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Pearson C, Chinchilli V, et al. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet. 2000;58:181–91. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- 10.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 11.Goto H, Ledford JG, Mukherjee S, et al. The role of surfactant protein A in bleomycin-induced acute lung injury. Am J Respir Crit Care Med. 2010;181:1336–44. doi: 10.1164/rccm.200907-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–50. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 13.Gunther A, Siebert C, Schmidt R, et al. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153:176–84. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage – the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976;85:209–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Thille AW, Esteban A, Fernandez-Segoviano P, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med. 2013;187:761–67. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 16.Aho HJ, Nevalainen TJ, Lindberg RL, et al. Experimental pancreatitis in the rat. The role of phospholipase A in sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:1027–31. doi: 10.3109/00365528009181808. [DOI] [PubMed] [Google Scholar]
- 17.Jha RK, Yong MQ, Chen SH. The protective effect of resveratrol on the intestinal mucosal barrier in rats with severe acute pancreatitis. Med Sci Monit. 2008;14(1):BR14–19. [PubMed] [Google Scholar]
- 18.Huang YX, Li WD, Jia L, et al. Infliximab enhances the therapeutic effectiveness of octreotide on acute necrotizing pancreatitis in rat model. Pancreas. 2012;41:849–54. doi: 10.1097/MPA.0b013e31823fbdc3. [DOI] [PubMed] [Google Scholar]
- 19.Li WD, Jia L, Ou Y, et al. Infliximab: protective effect to intestinal barrier function of rat with acute necrosis pancreatitis at early stage. Pancreas. 2013;42:366–67. doi: 10.1097/MPA.0b013e31825c5273. [DOI] [PubMed] [Google Scholar]
- 20.Zhou GX, Zhu XJ, Ding XL, et al. Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:201–7. [PubMed] [Google Scholar]
- 21.Rongione AJ, Kusske AM, Kwan K, et al. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–67. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 22.W L. The relationship between the endotoxemiaand damages to multiple organs in the experimental necrotic pancreatitis. Chinese J Exp Surg. 1995;12:131–32. [Google Scholar]
- 23.Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1–14. doi: 10.1097/01.mpa.0000246658.38375.04. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CB, Grewal HP, Gaber LW, et al. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg. 1996;171:274–80. doi: 10.1016/s0002-9610(97)89568-2. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Zhang H, Xu KY, et al. Role of the chemokine fractalkine in a rat model of acute necrotizing pancreatitis and the interventional effect of ulinastatin. Arch Iran Med. 2013;16:83–87. [PubMed] [Google Scholar]
- 26.Liu ZH, Peng JS, Li CJ, et al. A simple taurocholate-induced model of severe acute pancreatitis in rats. World J Gastroenterol. 2009;15:5732–39. doi: 10.3748/wjg.15.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwig W, Schimmel E, Hackert T, et al. A novel animal model of severe pancreatitis in mice and its differences to the rat. Surgery. 2008;144:394–403. doi: 10.1016/j.surg.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Dang SC, Zhang JX, Qu JG, et al. Ligustrazine alleviates gastric mucosal injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:213–18. [PubMed] [Google Scholar]
- 29.Endo S, Sato N, Nakae H, et al. Surfactant protein A and D (SP-A, AP-D) levels in patients with septic ARDS. Res Commun Mol Pathol Pharmacol. 2002;111:245–51. [PubMed] [Google Scholar]
- 30.McIntosh JC, Swyers AH, Fisher JH, et al. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15:509–19. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- 31.Kuroki Y, Tsutahara S, Shijubo N, et al. Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am Rev Respir Dis. 1993;147:723–29. doi: 10.1164/ajrccm/147.3.723. [DOI] [PubMed] [Google Scholar]