Abstract
The minimal ecological requirements for the fomation of regular vegetation patterns in semiarid systems have been recently questioned. Against the general belief that a combination of facilitative and competitive interactions is necessary, recent theoretical studies suggest that, under broad conditions, non-local competition among plants alone may induce patterns. In this paper, we review results along this line, presenting a series of models that yield spatial patterns when finite-range competition is the only driving force. A preliminary derivation of this type of model from a more detailed one that considers water–biomass dynamics is also presented.
Keywords: vegetation patterns, non-local interactions, competitive interactions
1. Introduction
Vegetation in semiarid regions around the world can form striking, highly organized patterns. Many approaches have been used to tackle the study of vegetation patterns from both a theoretical and an empirical perspective. Many studies have focused on measuring the different types of interactions among plants that are present in water-limited systems as well as their spatial ranges and strength [1,2]. On the theoretical side, which is the focus of this paper, mathematical models have been proposed accounting either for the evolution of the vegetation biomass alone [3–6] or coupled with the dynamics of the water in the system [7,8]. A common point of all these studies is the view that the pattern formation phenomenon is a symmetry-breaking process that induces instability in the uniform vegetation state [9–10].
Interest in plant patterns stems from the idea that these structures provide information about the physical and biological processes that generate them. However, the strength of the modern approach to vegetation patterns—that is, its universality—becomes a great disadvantage when searching for relationships between patterns and processes, as many different processes can give rise to the same spatial structures. As a result, it is useful on the theoretical side to find the minimal set of biophysical mechanisms under which typically observed patterns may appear in water-limited systems. Most existing mathematical models of vegetation pattern formation assume an interplay between short-range facilitation and long-range competition. While it is clear that such a combination of mechanisms is probably responsible for patterns in some conditions—for example, regular stripes on hillsides [9]—whether or not both mechanisms must always be present for pattern formation is an open question. While competition for water is likely to be the key factor for semiarid systems, some studies [11,12] have suggested that local facilitative interactions may be unnecessary, or of only minor importance, for pattern formation. Following these ideas, the authors have recently introduced a model of vegetation density for water-limited regions where only competition among plants is considered [3]. Here, the interaction enters by allowing the growth rate of a plant to diminish with the number of other individuals competing with it for resources (water). Despite the fact that facilitation is ignored, this non-local competition model produces a spectrum of spatial patterns similar to the one observed in models assuming that both facilitation and competition are necessary.
In this paper, we extend the results of Martínez-García et al. [3] to address several open questions. (i) Do patterns depend on how competition enters in the dynamical equations? (ii) What is the role of nonlinearities? (iii) Can simple models featuring non-local competition be derived from more fundamental ones that consider the dynamics of plants and water sources? To answer these questions, we present a set of non-local models with only competitive interactions that enter in the equations either linearly or nonlinearly. In the latter case, we complement our previous work by also allowing non-local competition to enter in the death term. Patterns emerge in all of these models, and in a sequence related to the one observed in standard facilitative–competitive models. We also present preliminary results on how the non-local density equations can be derived from a more mechanistic dynamics that considers biomass and water interactions.
The outline of the paper is as follows. In §2, we give an overview of previous non-local models and describe new ones: §2a shows a review of standard kernel-based descriptions with facilitative and competitive interactions; in §2b, we review the competition-only model introduced by Martínez-García et al. [3]; then in §2c, we study the model where the non-locality enters in the death term; in §2d, the model studied is of competition entering linearly in the equations. In §3, the derivation of density models from water–biomass dynamics is discussed, and in §4, we give our conclusion and summary.
2. Spatially non-local models for tree density
Vegetation patterns arise from a self-organization mechanism as a result of dynamic interactions among plants, and between plants and their environmental conditions. Existing studies [2,4,7,9,10,13,14] consider two typical length scales to account for facilitative (short-range) and competitive (long-range) interactions. As mentioned above, the need for these two types of mechanism has been recently questioned by Martínez-García et al. [3] from a mathematical point of view. In this section, we review the standard models that include both facilitation and competition, and then present the competition-only model described by Martínez-García et al. [3].
(a). Kernel-based models with facilitative and competitive mechanisms
The kernel-based models [15] express vegetation density mathematically as integro-differential equations with a spatially non-local interaction function. Roughly speaking, two types exist: (i) those where the non-locality enters linearly (nonlinearities appear but without spatial coupling) and (ii) those where the non-locality enters multiplicatively [4]. For simplicity here, we discuss only the linear class, the so-called neural models [16]. The dynamics of the vegetation-density field, ρ(r,t), is given by
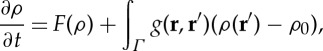 |
2.1 |
where F(ρ) denotes the local dynamics whose steady state is ρ0, and Γ is the spatial domain over which the kernel function g(r,r′) is defined. The term 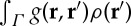 (assuming isotropy and homogeneity it is more commonly expressed as g(|r−r′|)) indicates that spatial interactions positively affect (facilitation) the growth when g>0, and the opposite (competition) happens when g<0. Interaction kernels in these models typically exhibit the shape shown for the one-dimensional case in figure 1a, and are thus positive at short scales and negative at long range. In fact, the way the spatial structure emerges from equation (2.1) is easy to understand: small perturbations larger than the homogeneous state, ρ0, tend to increase locally because of the positive interaction with nearby points, whereas those with ρ<ρ0 decrease in the interaction neighbourhood. Thus, short-range facilitation enhances spatial heterogeneity and the long-range inhibition (the negative part of the kernel) limits the indefinite growth of the perturbation. A justification and deeper analysis of these type of kernels for vegetation models is given in Borgogno et al. [15]. Biologically speaking, the facilitation range is usually assumed to be similar to the crown radius, while the competition range is related to the lateral root length. While negative vegetation densities are mathematically possible under these models, they are biologically nonsensical. Therefore, works using kernel-based models usually set negative densities to zero in numerical simulations [15].
(assuming isotropy and homogeneity it is more commonly expressed as g(|r−r′|)) indicates that spatial interactions positively affect (facilitation) the growth when g>0, and the opposite (competition) happens when g<0. Interaction kernels in these models typically exhibit the shape shown for the one-dimensional case in figure 1a, and are thus positive at short scales and negative at long range. In fact, the way the spatial structure emerges from equation (2.1) is easy to understand: small perturbations larger than the homogeneous state, ρ0, tend to increase locally because of the positive interaction with nearby points, whereas those with ρ<ρ0 decrease in the interaction neighbourhood. Thus, short-range facilitation enhances spatial heterogeneity and the long-range inhibition (the negative part of the kernel) limits the indefinite growth of the perturbation. A justification and deeper analysis of these type of kernels for vegetation models is given in Borgogno et al. [15]. Biologically speaking, the facilitation range is usually assumed to be similar to the crown radius, while the competition range is related to the lateral root length. While negative vegetation densities are mathematically possible under these models, they are biologically nonsensical. Therefore, works using kernel-based models usually set negative densities to zero in numerical simulations [15].
Figure 1.
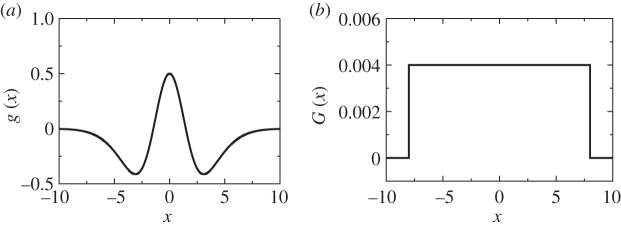
(a) Kernel function of standard one-dimensional kernel-based models considering both competitive and facilitative interactions. It is built with a combination of positive and negative Gaussian functions, 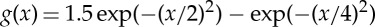 . (b) Competitive-only top-hat kernel G(x) with range R=8.
. (b) Competitive-only top-hat kernel G(x) with range R=8.
(b). A kernel-based model including only competitive interactions
Following previous studies [11,12] suggesting that vegetation patterns could emerge without short-range facilitation, and assuming that competition for water is the unavoidable interaction in arid and semiarid systems, Martínez-García et al. [3] proposed a non-local model with only competitive interactions. The equation for vegetation density is
| 2.2 |
where 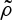 is the mean vegetation density within a neighbourhood, weighted with the kernel G(x), around a given spatial point
is the mean vegetation density within a neighbourhood, weighted with the kernel G(x), around a given spatial point
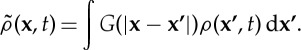 |
2.3 |
The different terms in the model come from considering the growth and death dynamics of vegetation. Population growth follows a sequence of seed production, dispersal and establishment:
Production happens at rate β0 per plant. Assuming local seed dispersion and that all seeds may give rise to new plants, the growth rate is β0ρ. After a seed lands, it has to overcome competition to establish. The following two competing mechanisms are taken into account.
Space availability limits the density to a maximum value
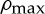 , so the proportion of available space at a point x is
, so the proportion of available space at a point x is 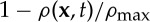 . Density can be scaled such that
. Density can be scaled such that 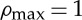 and thus the growth term is limited by a factor (1−ρ(x,t)).
and thus the growth term is limited by a factor (1−ρ(x,t)).Once the seed has germinated, it competes with other plants for water and other resources in the soil. The probability of overcoming this competition is given by
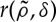 . This function decreases when
. This function decreases when 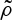 increases, so that
increases, so that 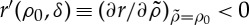 . We assume that plants compete with other plants in their neighbourhood, which is defined by a distance of the order of twice the typical root length.
. We assume that plants compete with other plants in their neighbourhood, which is defined by a distance of the order of twice the typical root length.
It is worth stressing the difference between the function G in this description and the g in §2a. g contains information about the interactions (cooperative when positive and competitive when negative) present in the system [4,17]. Since these are of facilitative and competitive type, the kernels are positive (at short scales) and negative (at long scales). On the contrary, G is strictly positive and defines an influence region of a focal plant which is used to compute an averaged density of other plants around it. Also, non-local competition enters nonlinearly, at variance with equation (2.1), so that negative densities no longer appear.
Performing a linear stability analysis of the stationary solution, ρ0, of equation (2.3) the perturbation growth rate is (see [3] for details)
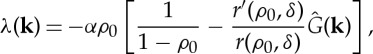 |
2.4 |
where 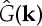 is the Fourier transform of the kernel,
is the Fourier transform of the kernel, 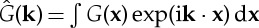 .
.
Since r′<0 equation (2.4) indicates that patterns may appear (λ>0) in the model when 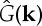 takes negative values, provided that competition is strong enough. This may happen, for example, when the kernel has a finite range (an example is shown in figure 1b), so that it is only different from zero (positive) in a finite domain around x=0. In plant dynamics, this finite range arises naturally from the length of the roots. The model recovers the gapped and striped patterns observed in arid and semiarid landscapes. Figure 2 shows the stationary patterns obtained by integrating equation (2.13) in a patch of 104 m2 with periodic boundary conditions and a competition range of R=8 m. G is a two-dimensional top-hat function (a cut across it will be similar to figure 1b) and the probability of overcoming non-local competition is given by
takes negative values, provided that competition is strong enough. This may happen, for example, when the kernel has a finite range (an example is shown in figure 1b), so that it is only different from zero (positive) in a finite domain around x=0. In plant dynamics, this finite range arises naturally from the length of the roots. The model recovers the gapped and striped patterns observed in arid and semiarid landscapes. Figure 2 shows the stationary patterns obtained by integrating equation (2.13) in a patch of 104 m2 with periodic boundary conditions and a competition range of R=8 m. G is a two-dimensional top-hat function (a cut across it will be similar to figure 1b) and the probability of overcoming non-local competition is given by
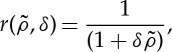 |
2.5 |
which makes ρ0 analytically solvable. The patterns only appear if the Fourier transform of the kernel function has negative values. For the two-dimensional top-hat kernel of width 2R, the Fourier transform is 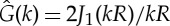 , where J1 is the first-order Bessel function [18], which takes negative values.
, where J1 is the first-order Bessel function [18], which takes negative values.
Figure 2.
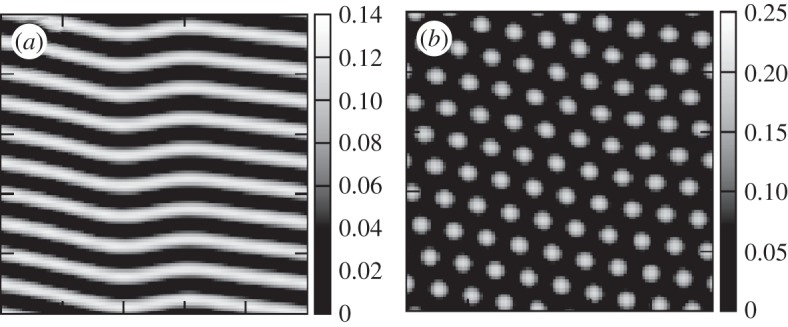
Close-to-stationary spatial structures shown by model (2.2) using 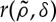 given by equation (2.5). Darker grey levels represent smaller densities. (a) Vegetation stripes, δ=16.0, (b) vegetation spots, δ=17.0, and other parameters: β0=1.0 and α=0.5.
given by equation (2.5). Darker grey levels represent smaller densities. (a) Vegetation stripes, δ=16.0, (b) vegetation spots, δ=17.0, and other parameters: β0=1.0 and α=0.5.
(c). Competition through a non-local nonlinear death term
As a complement to the vegetation dynamics in equation (2.3), we next discuss a system, again without facilitation, where resource competition enters through the death rate. There is now a non-local nonlinear death term resulting in a higher death rate when the surrounding vegetation density increases. This is mathematically expressed as follows:
| 2.6 |
where 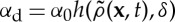 is the non-local death rate (α0 is a constant and h an arbitrary function) and β is the constant birth rate. Non-local competition affecting mortality has been shown to promote clustering in individual-based population models [19].
is the non-local death rate (α0 is a constant and h an arbitrary function) and β is the constant birth rate. Non-local competition affecting mortality has been shown to promote clustering in individual-based population models [19].
As before, 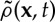 is the non-local density of vegetation at point x, where
is the non-local density of vegetation at point x, where 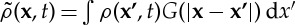 . G is the kernel function that defines an interaction range and modulates its strength with the distance from the focal plant. Space availability for a seed to establish appears in the birth term via 1−ρ(x,t) (local competition).
. G is the kernel function that defines an interaction range and modulates its strength with the distance from the focal plant. Space availability for a seed to establish appears in the birth term via 1−ρ(x,t) (local competition). 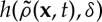 gives the probability that a plant dies as a function of competition for water with the roots of other plants. Since it is a probability, 0<h<1, and it increases with increasing values of the averaged density,
gives the probability that a plant dies as a function of competition for water with the roots of other plants. Since it is a probability, 0<h<1, and it increases with increasing values of the averaged density, 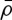 , and the (positive) competition parameter, δ. The stationary solutions of equation (2.6), ρ0, are obtained by solving
, and the (positive) competition parameter, δ. The stationary solutions of equation (2.6), ρ0, are obtained by solving
| 2.7 |
which has a trivial solution, ρ0=0 referring to the bare-ground state, and a vegetated state that is obtained from
| 2.8 |
once the function h has been chosen.
A linear stability analysis of the stationary homogeneous state, ρ0, yields the growth rate
| 2.9 |
where 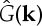 is the Fourier transform of the kernel function.
is the Fourier transform of the kernel function.
The simplest function h that fulfils the above-mentioned properties is a linear function, 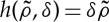 , which limits the values of the competition parameter to 0<δ<1 so that h<1. Then
, which limits the values of the competition parameter to 0<δ<1 so that h<1. Then
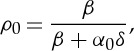 |
2.10 |
while the perturbation growth rate is given by
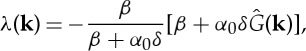 |
2.11 |
from which we obtain a transition to pattern (λ becomes positive) at a competition strength
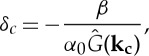 |
2.12 |
where kc is the most unstable mode, which yields the most negative value of 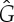 and is the mode with the highest growth rate. First note that again the Fourier transform of G must take negative values for patterns to form. Also, α0 and β have to be chosen properly to have δc≤1. In particular, if we take α0=1, β=0.1 and a top-hat kernel of radius R=8, we get δc≈0.75. It is important to remark that spatial structures result when the maximum death rate, i.e. the death rate in fully vegetated areas, is much higher than the birth rate (α0≫β). Otherwise, the model shows standard logistic growth despite the non-local spatial couplings and the distribution of vegetation is homogeneous. Figure 3 shows the different spatial distributions of vegetation in the stationary state. The homogeneous distribution is stable when δ<δc (3a), while patterns (stripes and spots) exist for δ>δc (3b) and (3c), respectively.
and is the mode with the highest growth rate. First note that again the Fourier transform of G must take negative values for patterns to form. Also, α0 and β have to be chosen properly to have δc≤1. In particular, if we take α0=1, β=0.1 and a top-hat kernel of radius R=8, we get δc≈0.75. It is important to remark that spatial structures result when the maximum death rate, i.e. the death rate in fully vegetated areas, is much higher than the birth rate (α0≫β). Otherwise, the model shows standard logistic growth despite the non-local spatial couplings and the distribution of vegetation is homogeneous. Figure 3 shows the different spatial distributions of vegetation in the stationary state. The homogeneous distribution is stable when δ<δc (3a), while patterns (stripes and spots) exist for δ>δc (3b) and (3c), respectively.
Figure 3.
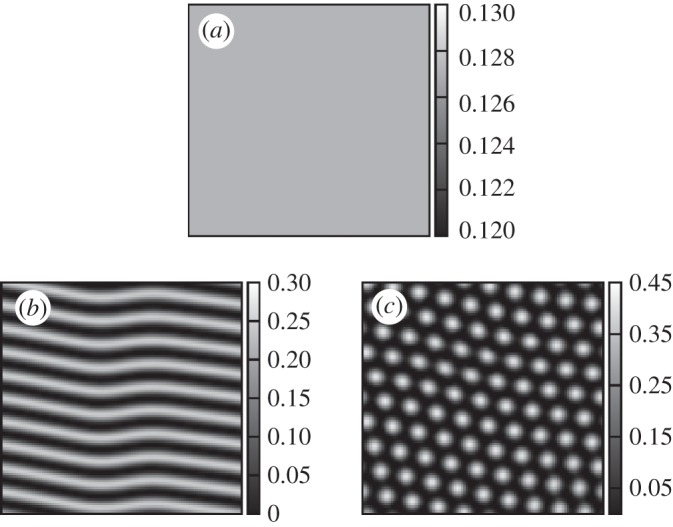
(a−c) Distribution of vegetation produced by model (2.6) with a linear probability h for different values of the competition parameter. δ=0.7 (a), δ=0.8 (b), δ=0.9 (c). α0=1, β=0.1.
(d). Competition through a non-local linear death term
We next study a natural extension of the kernel-based model as presented in equation (2.1) and previous studies [15], but with purely competitive interactions. The local density of vegetation changes with time because of its local dynamics (logistic growth) and the spatial interactions (competition) with other points in the domain
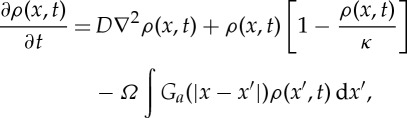 |
2.13 |
where κ is the carrying capacity and Ω is the interaction parameter. We have added a diffusive term modelling seed dispersal. Competitive interactions are determined by considering both the strength of the interactions parameter, Ω, and the kernel function, Ga, always positive. This description is equivalent to considering a non-local linear death term which arises from competition among plants. As mentioned in §2a, the density can take negative values. This is a consequence of the non-local interactions reinforcing the death of vegetation and entering linearly on the model; these models are, therefore, mathematically ill-posed. This is a weakness that these models share with many related kernel-based models (see §2a), but which is absent when non-local competition enters nonlinearly. Negative densities are nonsensical from a biological point of view, so, following Borgogno et al. [15], we set ρ(x,t)=0 in model (2.13) when this occurs. The stationary solutions are ρ0=0 (no vegetated state), and a non-trivial solution,
| 2.14 |
that imposes a constraint on the values of Ω<1.
The growth rate of the perturbations is now
| 2.15 |
and using the expression of the homogeneous steady state, ρ0, given by equation (2.14), it becomes
| 2.16 |
There is, in this model, no restriction on the shape of the Fourier transform of the kernel for the appearance of patterns (note that Ω is always lower than 1). We have numerically integrated equation (2.13) in the regime of patterned solutions and the results are shown in figure 4a,b for two different values of Ω. The same sequence of spatial structures is obtained as in the other models.
Figure 4.
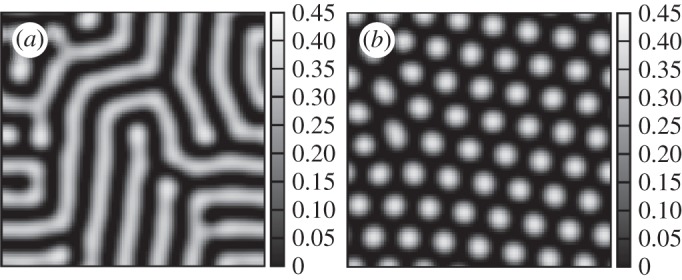
Spatial distribution of vegetation for the model described by equation (2.13). (a) D=1, Ω=0.7 and (b) D=1, Ω=0.9. R=8 in both panels.
3. Derivation of the effective non-local description from tree-water dynamics
The models presented in §2 are all given by a phenomenological evolution equation for vegetation density. An open problem is to infer this type of description from a mechanistic one where the explicit interactive dynamics of vegetation competing for water is considered. This would help, in particular, to unveil the origin and properties of the kernel function. In this section, we present a preliminary (and not fully satisfactory) attempt to derive the model presented by Martínez-García et al. [3] and discussed in §2b (the derivation corresponding to the non-local death model in §2c is a straightforward extension of this calculation).
Let us consider a system involving dimensionless vegetation density, ρ, and soil-water, w. The dynamics is purely local and competitive and takes the form
| 3.1 |
and
| 3.2 |
where the non-dimensional positive parameters are: the seed production rate β; the vegetation death rate α; the consumption rate of water by vegetation μ; the evaporation rate γ; and the rainfall I. Water percolation in the ground is modelled by a diffusion constant Dw. Note that this model is a simplified version, which only includes competitive interactions, of the model presented by Gilad et al. [8].
As the characteristic time scale of the water is much faster than that of the biomass, we can carry out an adiabatic elimination of the variable w (i.e. ∂tw=0) so that
| 3.3 |
and thus
| 3.4 |
whose formal solution can be obtained using Green's functions, Gd,
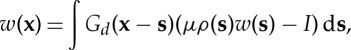 |
3.5 |
with the boundary conditions 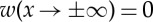 . For simplicity, we now consider a one-dimensional situation, although analogous calculations can be done in two dimensions. Green's function is the solution of
. For simplicity, we now consider a one-dimensional situation, although analogous calculations can be done in two dimensions. Green's function is the solution of
| 3.6 |
and it is given by
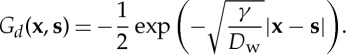 |
3.7 |
Taking the non-dimensional small number μ as the perturbative parameter, we can further obtain an approximate expression for w from equation (3.5)
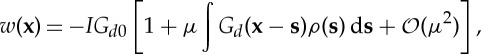 |
3.8 |
where 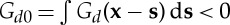 , since Green's function is always negative. Plugging this in the equation for the biomass density (3.1), we obtain the closed expression
, since Green's function is always negative. Plugging this in the equation for the biomass density (3.1), we obtain the closed expression
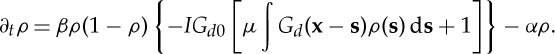 |
3.9 |
Defining the positive non-local density 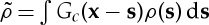 , where Gc=−Gd, we can write equation (3.9) as
, where Gc=−Gd, we can write equation (3.9) as
| 3.10 |
where we have defined 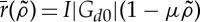 .
.
To have a good agreement with the effective non-local dynamics equation (2.2), 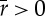 since it represents a probability. This is certainly the case for small μ. Note that some additional conditions on the normalization of Green's function have to be imposed to limit r to values less than 1. Also
since it represents a probability. This is certainly the case for small μ. Note that some additional conditions on the normalization of Green's function have to be imposed to limit r to values less than 1. Also 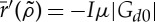 is always negative, as we expected.
is always negative, as we expected.
In this particular example, we obtained an exponential kernel which does not have the finite-range support that would be associated with the finite root extent. As a consequence, the Fourier transform of this kernel has no negative components and so does not lead to pattern formation. The simple modelling of water dispersion by means of a diffusion constant does not contain the additional spatial scale associated with root size and should be replaced by some mechanism implementing root effects. In contrast, the finite range of the kernel is a sufficient but not necessary condition for its Fourier transform to have negative values. It is well known that infinite-range kernels exist whose Fourier transform has negative values. This is the case for all stretched exponentials 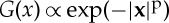 with p>2 [20]. Kernels satisfying this are more platykurtic than the Gaussian function. Work is in progress on this possible route to obtaining pattern-forming kernels.
with p>2 [20]. Kernels satisfying this are more platykurtic than the Gaussian function. Work is in progress on this possible route to obtaining pattern-forming kernels.
4. Conclusion
In this paper, we have reviewed different non-local competitive models of vegetation in water-limited regions where, despite the absence of facilitative interactions, patterns may still appear. The obtained sequence of patterns consists of a striped structure and spots of vegetation interspersed on the bare soil forming a hexagonal lattice. We have not been able to find patterns consisting of spots of bare soil, which are also typical in models with both competition and facilitation among plants. In fact, previous studies [12] in which the range of facilitation was taken to its infinitesimally shortest value (i.e. local) showed these gapped distributions but only in a very narrow parameter region close to the transition to patterns line. This is different from standard models with non-local facilitation in which the whole sequence of patterns (gaps, stripes and spots) appears in a wider parameter interval. This may suggest that facilitative interactions, although not indispensable for the formation of patterns, could be important in order to promote some of the structures that have been reported in field observations. We note in this context that a careful study of the bifurcation sequences in local vegetation models reveals that the standard sequence is not fully robust and depends on nonlinear details of particular models [21].
From a mathematical point of view, non-locality enters through an influence function that determines the number of plants competing within a range with any given plant. A first-order approximation of this distance can be given by (twice) the typical length of the roots, but field measurements are needed in order to determine the range over which individuals of a given plant species can influence their neighbours. A necessary condition for pattern transitions, for the models under study where the non-locality is in the nonlinear term, is the existence of negative values of the Fourier transform of the influence function, which always happens, among other situations, for kernel functions with finite range.
From a biological point of view, competitive interactions alone may give rise to spatial structures because of the development of spatial regions (typically located between maxima of the plant density) where competition is stronger, preventing the growth of more vegetation [3].
An unfortunate consequence of the universal character of these models is that the information it is possible to gain on the underlying biophysical mechanisms operating in the system just by studying the spatial distribution of the vegetation is limited. Many different mechanisms lead to the same patterns. Although patterns are universal, models should be specific to each system. This emphasizes the importance that empirical studies have in developing reasonable models of the behaviour of different systems. Field work may help theoretical efforts by placing biologically reasonable bounds on the shape and extent of the kernel functions used in the models and also by approximations to the probability of overcoming competition, 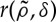 .
.
It is important to note that the type of non-local models presented may have localized solutions. This has been studied, in a different context [22], for a model that reduces to equation (2.6) when the kernels enhance self-interactions, i.e. they are of the type G(x)=F(x)+aδ(x) [23]. In plant ecology, mathematical approaches where the interactions among plants depend on the local biomass density show localized structures as a consequence of the bistable behaviour between the desert state (ρ0=0) and the spatially extended solutions [24]. This result also extends to non-local models either considering the interplay between water and vegetation dynamics [25] or, in more recent studies, using effective equations for the vegetation density [26]. In this latter case, the authors explain the formation of fairy circles (localized barren patches of vegetation) as localized solutions of spatially non-local models.
Finally, with this work we aimed to show that, under certain conditions, non-local competition alone may be responsible for the formation of patterns in semiarid systems. More interestingly, spatially regular distributions of vegetation appear regardless of how competitive interactions are introduced in the different modelling approaches. Certainly, while it may not be possible to unambiguously identify the model that generates an observed pattern, the study of the minimal mechanisms giving rise to pattern formation limits the set of candidate models (and biological mechanisms) that need to be considered. We hope that our results shed light on the task of understanding the fundamental mechanisms—and the possible absence of facilitation—that could be at the origin of pattern formation in semiarid systems.
Acknowledgements
C.L. dedicates this work to the memory of his father.
Funding statement
R.M.-G., C.L. and E.H.-G. acknowledge support from FEDER and MINECO (Spain) through grant nos. FIS2012-30634 INTENSE@COSYP and CTM2012-39025-C02-01 ESCOLA. R.M.-G. is supported by the JAEPredoc program of CSIC.
References
- 1.Dunkerley DL. 2002. Infiltration rates and soil moisture in a groved mulga community near Alice Springs, arid central Australia: evidence for complex internal rainwater redistribution in a runoff–runon landscape. J. Arid Environ. 51, 199–202. ( 10.1006/jare.2001.0941) [DOI] [Google Scholar]
- 2.Barbier N, Couteron P, Lefever R, Deblauwe V, Lejeune O. 2008. Spatial decoupling of facilitation and competition at the origin of gapped vegetation patterns. Ecology 89, 1521–1531. ( 10.1890/07-0365.1) [DOI] [PubMed] [Google Scholar]
- 3.Martínez-García R, Calabrese JM, Hernandez-Garcia E, Lopez C. 2013. Vegetation pattern formation in semiarid systems without facilitative mechanisms. Geophys. Res. Lett. 40, 6143–6147. ( 10.1002/2013GL058797) [DOI] [Google Scholar]
- 4.Lefever R, Lejeune O. 1997. On the origin of tiger bush. Bull. Math. Biol. 59, 263–294. ( 10.1007/BF02462004) [DOI] [Google Scholar]
- 5.D’Odorico P, Laio F, Ridolfi L. 2006. Vegetation patterns induced by random climate fluctuations. Geophys. Res. Lett. 33, L19404 ( 10.1029/2006GL027499) [DOI] [Google Scholar]
- 6.Lefever R, Turner JW. 2012. A quantitative theory of vegetation patterns based on plant structure and the non-local FKPP equation. C. R. Mécanique 340, 818–828. ( 10.1016/j.crme.2012.10.030) [DOI] [Google Scholar]
- 7.von Hardenberg J, Meron E, Shachak M, Zarmi Y. 2001. Diversity of vegetation patterns and desertification. Phys. Rev. Lett. 87, 198101 ( 10.1103/PhysRevLett.87.198101) [DOI] [PubMed] [Google Scholar]
- 8.Gilad E, von Hardenberg J, Provenzale A, Shachak M, Meron E. 2004. Ecosystem engineers: from pattern formation to habitat creation. Phys. Rev. Lett. 93, 098105 ( 10.1103/PhysRevLett.93.098105) [DOI] [PubMed] [Google Scholar]
- 9.Klausmeier CA. 1999. Regular and irregular patterns in semiarid vegetation. Science 284, 1826–1828. ( 10.1126/science.284.5421.1826) [DOI] [PubMed] [Google Scholar]
- 10.Lejeune O, Tlidi M. 1999. A model for the explanation of vegetation stripes (tiger bush). J. Veg. Sci. 10, 201–208. ( 10.2307/3237141) [DOI] [Google Scholar]
- 11.Rietkerk M, van de Koppel J. 2008. Regular pattern formation in real ecosystems. Trends Ecol. Evol. 23, 169–175. ( 10.1016/j.tree.2007.10.013) [DOI] [PubMed] [Google Scholar]
- 12.Martínez-García R, Calabrese JM, López C. 2013. Spatial patterns in mesic savannas: the local facilitation limit and the role of demographic stochasticity. J. Theoret. Biol. 333, 156–165. ( 10.1016/j.jtbi.2013.05.024) [DOI] [PubMed] [Google Scholar]
- 13.Rietkerk M, Boerlijst MC, van Langevelde F, HilleRisLambers R, van de Koppel J, Kumar L, Prins HH, de Roos AM. 2002. Notes and comments: self-organization of vegetation in arid ecosystems. Am. Nat. 160, 534–530. ( 10.1086/342078) [DOI] [PubMed] [Google Scholar]
- 14.D’Odorico P, Laio F, Ridolfi L. 2006. Patterns as indicators of productivity enhancement by facilitation and competition in dryland vegetation. J. Geophys. Res. Biogeosci. 111, 03010 ( 10.1029/2006JG000176) [DOI] [Google Scholar]
- 15.Borgogno F, D’Odorico P, Laio F, Ridolfi L. 2009. Mathematical models of vegetation pattern formation in ecohydrology. Rev. Geophys. 47, 1–36. ( 10.1029/2007RG000256) [DOI] [Google Scholar]
- 16.Murray J. 2002. Mathematical biology. Vol. I. An introduction, 3rd edn Berlin, Germany: Springer. [Google Scholar]
- 17.D’Odorico P, Laio F, Ridolfi L. 2006. Vegetation patterns induced by random climate fluctuations. Geophys. Res. Lett. 33, 19404 ( 10.1029/2006GL027499) [DOI] [Google Scholar]
- 18.Hernández-García E, López C. 2004. Clustering, advection, and patterns in a model of population dynamics with neighborhood-dependent rates. Phys. Rev. E 70, 016216 ( 10.1103/PhysRevE.70.016216) [DOI] [PubMed] [Google Scholar]
- 19.Birch Da, Young WR. 2006. A master equation for a spatial population model with pair interactions. Theoret. Popul. Biol. 70, 26–42. ( 10.1016/j.tpb.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 20.Pigolotti S, López C, Hernández-García E. 2007. Species clustering in competitive Lotka–Volterra models. Phys. Rev. Lett. 98, 258101 ( 10.1103/PhysRevLett.98.258101) [DOI] [PubMed] [Google Scholar]
- 21.Gowda K, Riecke H, Silber M. 2014. Transitions between patterned states in vegetation models for semi-arid ecosystems. Phys. Rev. E 89, 022701 ( 10.1103/PhysRevE.89.022701) [DOI] [PubMed] [Google Scholar]
- 22.Paulau P, Gomila D, López C, Hernández-García E. 2014. Self-localized states in species competition. Phys. Rev. E 89, 032724 ( 10.1103/PhysRevE.89.032724) [DOI] [PubMed] [Google Scholar]
- 23.Hernández-García E, López C, Pigolotti S, Andersen KH. 2009. Species competition: coexistence, exclusion and clustering. Phil. Trans. R. Soc. A 367, 3183–3195. ( 10.1098/rsta.2009.0086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lejeune O, Tlidi M, Couteron P. 2002. Localized vegetation patches: a self-organized response to resource scarcity. Phys. Rev. E 66, 010901 ( 10.1103/PhysRevE.66.010901) [DOI] [PubMed] [Google Scholar]
- 25.Meron E, Yizhaq H, Gilad E. 2007. Localized structures in dryland vegetation: forms and functions. Chaos 17, 037109 ( 10.1063/1.2767246) [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Oto C, Tlidi M, Escaff D, Clerc M. 2013. Strong interaction between plants induces circular barren patches: fairy circles. (http://arxiv.org/abs/1306.4848) [DOI] [PubMed]


