Abstract
Atherosclerosis is an inflammatory disease that is accelerated in human immunodeficiency virus (HIV) infection. Individuals with HIV infection have an activated type I interferon (IFN) monocyte phenotype, which may enhance uptake of modified low-density lipoprotein (LDL) thereby initiating a prefoam cell pathology and recruitment into atherosclerotic plaques. In a sampling of HIV-infected subjects, an increase in monocyte activation genes, MX1 and CXCL10, correlated with monocyte expression of the scavenger receptor A (SR-A), a major receptor for lipid uptake and foam cell formation. Monocytes from HIV-infected subjects accumulated more lipid than control uninfected subjects. We modeled increased activation in HIV infection by priming human monocytes with IFNα followed by exposure to acetylated LDL (acLDL). Exposure to IFNα increased acLDL uptake, which generated increased cellular reactive oxygen species (ROS). We posit that HIV infection augments formation of arterial plaques by triggering monocyte activation with a type I IFN profile, which induces SR-A expression, lipid uptake, and subsequent ROS production. These findings may explain in part why HIV-infected individuals with chronic immune activation have an increased risk of atherosclerosis.
While cardiovascular disease (CVD) is a leading cause of morbidity and mortality with increasing age, individuals with human immunodeficiency virus (HIV) infection have preclinical and more extensive atherosclerosis than would be expected for their age (Grunfeld and others 2009; Hsue and others 2009). Initially thought to be a result of metabolic effects of antiretroviral therapy (ART), new studies show modest lipid changes caused by ART (Grinspoon and others 2008; Patel and others 2012). Atherosclerosis is an inflammatory disease where monocyte/macrophage (M/Mφ) activation plays a major part in the pathogenesis (Crowe and others 2010). Recent studies have established that HIV infection accelerates CVD above age-matched individuals irrespective of ART and other comorbid risk factors (Rasmussen and others 2011; Hsue and others 2012; Lo and Plutzky 2012). The majority of these studies focus on the critical role of inflammation (Baker and others 2011). Further data from HIV-infected elite controllers, who naturally have low viral loads but with immune activation and not on ART, demonstrated a significant increase in coronary atherosclerosis attributed to monocyte activation (Pereyra and others 2012) as determined by an increase in soluble CD163 (sCD163) (Burdo and others 2011; Pereyra and others 2012). With successful ART, peripheral HIV replication is diminished; however, chronic immune activation identified as a type I interferon (IFN) response may persist in many HIV-infected individuals (Rempel and others 2010; Fernandez and others 2011; Pulliam and others 2011; Ries and others 2012).
An initial step in atherosclerosis is increased uptake of low-density lipoproteins (LDL) modified by glycosylation, dyslipidemia, hyperglycemia, and oxidative stress. Monocytes can take up modified lipids such as oxidized low-density lipoprotein (oxLDL) in vivo or acetylated low-density lipoprotein (acLDL) in vitro to form foam cells that are the pathological hallmark of early atherosclerosis (Orso and others 2011). Monocytes take up modified LDL via scavenger receptors SR-A (CD204, MSR1) and CD36 (Greenberg and others 2006; Yu and others 2013), and modified LDL generates reactive oxygen species (ROS) that contribute to the pathological process (Cominacini and others 2000; Pamukcu and others 2010; Shantsila and others 2010; Tavakoli and Asmis 2012). SR-A expressed on monocytes preferentially binds and internalizes oxLDL, but not native LDL (Mitra and others 2011; Nikolic and others 2011).
The overall objective of this study was to examine early events in atherosclerosis to determine how a type I IFN monocyte activation profile might upregulate scavenger receptor expression, acLDL uptake, and increase ROS. We queried our previously collected monocyte gene expression data from an HIV-infected cohort to determine whether or not there was an increase that might lead to increased acLDL uptake. We then sampled a small group of HIV-infected subjects to determine whether increased monocyte SR-A expression correlated with lipid uptake. Finally, we sought to reproduce this in vitro by evaluating whether IFNα priming of monocytes increased modified lipid uptake and ROS production.
Individuals were recruited at the San Francisco Veterans Affairs Medical Center with written consent approved by the University of California, San Francisco Committee on Human Research. For the monocyte gene expression studies, 44 HIV-infected subjects were recruited, 22 with HIV high viral load (HVL, >10,000 RNA copies/mL), 8 with low viral load (LVL, <10,000 RNA copies/mL), and 14 with undetectable viral load (<50 RNA copies/mL). Eleven HIV seronegative controls were included. Subjects' characteristics are listed in Table 1. The cohort was all males between 30 and 66 years of age (mean age for HIV-infected subjects was 50±7.9 years and controls 53±4.1 years). There was no age difference among groups (ANOVA F=0.562, P=0.642). None of the subjects had hepatitis C infection. The cohort was predominantly Caucasian (62%) followed by African American (19%), Hispanic (12%), Asian (4%), and other (3%) individuals (Rempel and others 2010). There was no ethnicity difference among groups (X2=18.97, P=0.0893). All HIV-infected groups had lower mean CD4 counts than controls (ANOVA F=20.66, P=6.75e-9; Tukey post hoc test showed HVL, LVL and UD groups were lower than control, all P values<0.001).
Table 1.
Demographic and Clinical Information
| Control | HIVUD | HIVLVL | HIVHVL | |
|---|---|---|---|---|
| n | 11 | 14 | 8 | 22 |
| Age | 53.0 (4.1) | 51.6 (7.2) | 49.5 (10.5) | 49.9 (7.7) |
| Ethnicity | ||||
| Asian | 1 | 0 | 0 | 1 |
| African American | 1 | 2 | 3 | 6 |
| Caucasian | 8 | 11 | 3 | 12 |
| Hispanic | 1 | 1 | 0 | 3 |
| Other | 0 | 0 | 2 | 0 |
| HIV RNA (log10 copies/mL) | NA | Undetectable | 3.3 (0.8) | 5.0 (0.5) |
| CD4 count | 1,038 (314) | 516 (261) | 456 (392) | 215 (234) |
Mean (± SD) except for ethnicity, which is count.
HIVHVL: HIV plasma viral load ≥10,000 copies/mL.
HIVLVL: HIV plasma viral load <10,000 copies/mL.
HIVUD: HIV plasma viral load undetectable (<50 copies/mL).
NA, not applicable.
Whole blood for monocyte gene expression arrays was drawn into Vacutainer CPT tubes (Becton Dickinson) and peripheral blood mononuclear cells (PBMC) were enriched by centrifugation. To minimize the elapsed time between blood draw and RNA isolation, CD14+ monocytes were positively selected from PBMC by immunomagnetic separation using anti-CD14 monoclonal antibodies conjugated to ferrous beads according to the manufacturer's instructions (Miltenyi Biotech). Monocyte purity exceeded 97% with<1% contaminating T or B cells as determined by flow cytometry (Rempel and others 2010). Total RNA was prepared from monocytes using an RNeasy Micro Kit (Qiagen). Complementary RNA (cRNA) was synthesized and labeled with biotin using iExpress iAmplify kit (Applied Microarrays). cRNA was hybridized to CodeLink Whole Human Genome Bioarrays (55K probes; Applied Microarrays). The hybridization signal was acquired on an Axon GenePix 4000B scanner (Molecular Devices) and image analysis and data extraction were performed by CodeLink Expression Software Kit v4.1 (GE Healthcare) as previously described (Rempel and others 2010).
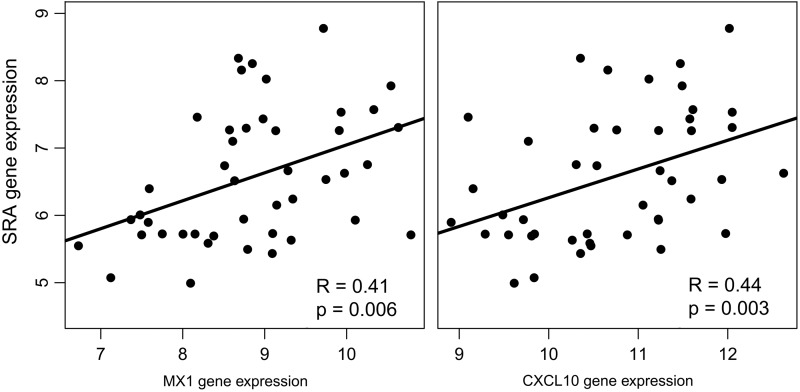
We queried the monocyte gene expression data for genes associated with IFNα activation. We looked at monocyte gene expression for 2 IFNα-induced genes, MX1 and CXCL10, and the scavenger receptors, SR-A and CD36 (Table 2). Our previous study showed that plasma CXCL10 strongly correlated with HIV load (Rempel and others 2010). SR-A expression was significantly increased in subjects with a viral load of >10,000 copies/mL. We did not see any difference in CD36 expression among the HIV-infected groups. Previous reports have shown that protease inhibitors in ART cocktails may promote the upregulation of CD36 and accumulation of lipid in macrophages (Dressman and others 2003). To address the concern that increased SR-A expression may be a result of ART, we looked at a subset of our HIV-infected cohort data (Rempel and others 2010) on (n=8) or off (n=7) ART with comparable viral loads. In this small sample, SR-A gene expression of patients on ART was no different than patients off ART but was significantly higher than controls (P=0.042). We did not see any influence of ART on CD36 expression. The increase in SR-A expression strongly correlated with genes associated with IFNα activation (Fig. 1) and this supports previous evidence that IFN activation increases lipid uptake (Li and others 2011).
Table 2.
Gene Expression Intensities of Selected Genes (Log2 Transformed)
| Control | HIVUD | HIVLVL | HIVHVL | |
|---|---|---|---|---|
| SR-A | 5.9 (0.9) | 6.3 (0.9) | 6.6 (1.2) | 6.7 (0.9)a |
| CD36 | 13.0 (0.3) | 13.0 (0.2) | 12.9 (0.3) | 12.8 (0.5) |
| CXCL10 | 9.4 (0.5) | 9.9 (0.6) | 10.4 (0.8)b | 11.4 (0.6)c |
| MX1 | 9.2 (0.6) | 9.9 (0.4) | 10.3 (1.1)b | 12.1 (1.0)c |
Mean (± SD). Group means were compared using ANOVA with Dunnett post hoc test for comparing infected groups with controls.
P<0.05,bP<0.01, cP<0.001.
HIV, human immunodeficiency virus; SR-A, scavenger receptor A.
FIG. 1.
Monocyte gene expression for MX1 and CXCL10 correlated with scavenger receptor A (SR-A) expression. Monocyte gene expression from HIV-infected subjects (n=44) with viral loads ranging from undetectable to high viral loads. Gene intensity was calculated from cDNA microarray probe signals. There were positive correlations between increasing SR-A and interferon-α (IFNα)-associated genes (Spearman analysis).
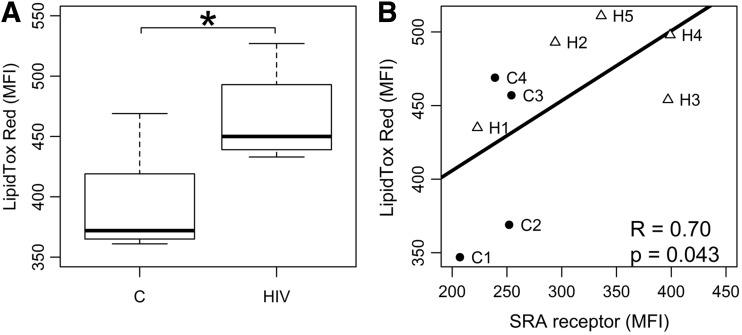
To determine whether monocytes from HIV-infected individuals accumulate more lipids than controls, we sampled a subset of HIV-infected individuals on ART to quantify intracellular lipids. Nine HIV seropositive subjects were recruited. All were men with a mean age of 55.6±11.6 years, with undetectable HIV viral loads and all were taking statins at the time of the blood draw. Four HIV-seronegative age-matched controls were also recruited, mean age 60±5.5 years. One control was taking a statin and 3 were not. Cells were stained with LipidTOX Red (Life Technologies) for 30 min to determine lipid accumulation followed by flow cytometry analysis. Approximately 10,000 events were analyzed using a FACSCalibur from Beckton Dickinson and lipid levels were calculated as mean fluorescent intensity (MFI). Using a neutral LipidTox Red dye (Stoletov and others 2009; Grandl and Schmitz 2010), intracellular lipid in monocytes from HIV-infected subjects was significantly higher compared to controls in spite of statin use (Fig. 2A). To further show an association between lipid uptake and SR-A expression, we measured SR-A protein expression on monocytes and quantified lipid accumulation from 5 of these HIV-infected subjects and 4 HIV seronegative controls using flow cytometry. In this small sample, we demonstrated a strong correlation (R=0.7, P=0.043) between monocyte SR-A expression and LipidTOX Red staining (Fig. 2B). The lipid levels for the subjects shown in Fig. 2B are in Table 3. There were significant differences in total cholesterol and LDL levels between the HIV-infected and control groups. LDL levels were lower in the HIV-infected group, which could be due to the effects of HIV (Grunfeld and others 1992) or the use of statins. We looked at correlations between triglycerides, LDL, and lipid accumulation and they were R=0.3, P=0.43 and R=0.05, P=0.9, respectively, strongly suggesting that the SR-A activation and lipid accumulation is not correlated with circulating lipids. Only 1 control was on a statin (C3) and this subject had the lowest monocyte lipid accumulation and SR-A expression (Fig. 2B). Since monocyte activation upregulates SR-A and this scavenger receptor preferentially takes up modified LDL (Nikolic and others 2011; Orso and others 2011), these results suggest that monocytes from HIV-infected subjects accumulated and retained more modified lipids than controls independent of circulating lipid levels.
FIG. 2.
Monocyte lipid accumulation in HIV-infected subjects. (A) Monocytes from healthy controls (n=5) and HIV-infected subjects on antiretroviral therapy (n=9) were analyzed by flow cytometry for lipid content. Neutral lipids were stained with fluorescent LipidTOX Red and mean fluorescent intensity (MFI) was used to compare the groups. Controlled HIV infection was associated with significantly higher total lipid accumulation in circulating monocytes. *P=0.005. (B) Monocytes from HIV-infected subjects (H1-5, Δ), who were all on statins, and healthy controls (C1-4, •), were stained for SR-A and LipidTOX Red. Controls 1, 2, and 4 were not on statins and C3 was. The lipid profiles for these 9 subjects are shown in Table 3. An increase in monocyte SR-A expression strongly correlated with LipidTOX Red uptake (R=0.70, P=0.04).
Table 3.
Lipid Profiles of Human Immunodeficiency Virus-Infected Subjects and Controls in Fig. 2B
| Tot chol (mg/dL) | LDL (mg/dL) | TG (mg/dL) | HDL (mg/dL) | |
|---|---|---|---|---|
| Controls | ||||
| C1 | 185 | 88 | 74 | 82 |
| C2 | 196 | 116 | 122 | 56 |
| C3 | 223 | 125 | 53 | 87 |
| C4 | 226 | 145 | 168 | 47 |
| Avg | 207.5 | 118.5 | 104.3 | 68.0 |
| SD | 20.17 | 23.67 | 51.38 | 19.51 |
| HIV+ | ||||
| H1 | 133 | 79 | 87 | 36 |
| H2 | 137 | 72 | 53 | 55 |
| H3 | 149 | 61 | 290 | 30 |
| H4 | 185 | 76 | 350 | 39 |
| H5 | 198 | 118 | 126 | 55 |
| Avg | 160.4 | 81.2 | 181.2 | 43.0 |
| SD | 29.36 | 21.67 | 131.04 | 11.42 |
| T-test (P) | 0.025 | 0.049 | 0.279 | 0.077 |
Tot chol, total cholesterol; TG, triglycerides; HDL, high-density lipoproteins; LDL, low-density lipoproteins.
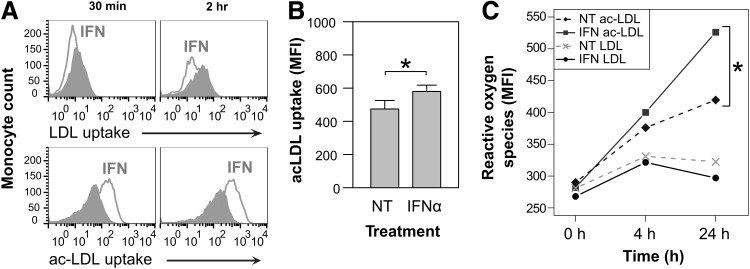
A negative consequence of modified LDL uptake by M/Mø is the production of ROS that leads to further immune activation. To determine whether IFNα would increase uptake of LDL and/or modified LDL, monocytes from leukocyte reduction chambers (LRC) were obtained from the Blood Center of the Pacific (BCP), San Francisco, CA. Monocyte cultures were prepared through sequential enrichment steps using RosetteSep and EasySep (StemCell). Monocyte purity was>90% when cells were assessed by flow cytometry for CD14 expression. Monocytes were cultured in RPMI medium supplemented with 10% fetal bovine serum at 106 cells/mL in 6-well, ultra-low attachment plates positioned on a rotating platform set at 97 rpm. Monocytes isolated from 4 LRCs were primed or not with IFNα (100 U/mL). After 48 h, 106 monocytes were resuspended in 1 mL media with or without labeled 10 μg diI-LDL or diI-acLDL (Life Technologies). Monocytes were assayed for labeled LDL or acLDL uptake by flow cytometry and quantified as MFI. IFNα priming potentiated acLDL (but not native LDL) uptake within 30 min of exposure and continued at 2 h (P=0.018) (Fig. 3A, B). This result is consistent with published findings and those presented here, that IFNα stimulates SR-A expression and SR-A preferentially takes up modified LDL (Nikolic and others 2011; Orso and others 2011). In models of atherosclerosis, modified LDL uptake leads to elevated ROS production (Tavakoli and Asmis 2012).
FIG. 3.
Normal and acetylated low-density lipoprotein (acLDL) uptake and reactive oxygen species (ROS) production in monocytes treated with IFNα. (A) IFNα priming increased acLDL but not normal LDL accumulation in monocytes at 30 min and 2 h (n=4) (representative histogram). Gray peaks are isotype controls. (B) After 2 h, IFNα enhanced acLDL uptake compared with nontreated (NT) monocytes (Student's t-test P=0.029, n=4). Labeled acLDL uptake was quantified using flow cytometry and MFI. (C) ROS were detected using the cell permeable probe 6-carboxy-2′,7′-dichlorodihydro-fluorescein diacetate (DCFDA) and flow cytometry. ROS detection in primed and normal LDL-treated monocytes was comparable. IFNα-primed and acLDL-treated monocytes generated the highest levels of ROS (Student's t-test, P=0.02). NT, untreated.
We used IFNα priming followed by acLDL exposure to determine whether this activation induced oxidative stress by assaying monocytes for cellular ROS accumulation. Enriched monocytes were primed or not with IFNα (100 U/mL) for 48 h. Afterward, cells were washed and exposed to 10 μg/mL acLDL or LDL. Cell-associated ROS were detected with 10 μM 6-carboxy-2′,7′-dichlorodihydro-fluorescein diacetate (DCFDA; Life Technologies) and quantified as MFI by flow cytometry. These results suggest that in the presence of IFNα, normal LDL is not preferentially taken up by monocytes nor is there an increase in ROS production. IFN-primed monocytes plus acLDL produced significant levels of ROS at 24 h compared to no priming (Fig. 3C).
In the era of ART, HIV infection has become a chronic illness with the majority of those treated living longer; however, a new set of issues based on living with a chronic condition and long-term therapy arises. Surprisingly, CVD, which is more common in aging individuals, has become more prevalent in HIV infection even in the presence of reduced viral replication with ART. Whether or not this is a consequence of metabolic changes with long-term ART therapy or the immune dysfunction associated with a chronic illness is under investigation. What is known is that some HIV-infected subjects on therapy or a treatment interruption with viremia have a type I IFN monocyte profile (Rempel and others 2010). While an early IFNα response at the cellular level may be beneficial in retarding viral replication, a sustained type I IFN reaction can lead to chronic immune activation and potentially a higher risk for CVD.
Monocyte response to HIV infection is likely more deleterious to the host than actual infection. Monocyte gene expression from HIV-infected individuals on ART with varying viral loads, showed a type I IFN profile with no lipopolysaccharide (LPS) gene expression profile despite circulating LPS (Rempel and others 2010). Type I IFN signaling has recently been highlighted as a promoter of atherosclerosis by stimulating M/Mø trafficking to lesions (Goossens and others 2010). In a recent study of elite controllers, atherosclerosis and immune activation, as defined by sCD163 levels, were increased over HIV-infected subjects controlled on ART or HIV-negative subjects (Pereyra and others 2012). In addition to sCD163, 2 monocyte-induced markers of immune activation, sCD14 and CXCL10, were also significantly elevated in the elite controllers over the HIV-negative group. Surprisingly, hsIL6 and C-reactive protein were not increased suggesting that the immune activation was likely not driven by LPS. Another chronic condition identified with a monocyte type I IFN signature and an increased incidence of CVD is systemic lupus erythematosus (SLE). IFNα in SLE leads to macrophage recruitment (Goossens and others 2010; Somers and others 2012), foam cell formation (Li and others 2011), disruption in vascular repair (Denny and others 2007), and plaque progression (Thacker and others 2012). IFNα priming caused an increase in monocyte SR-A expression and modified LDL uptake (Li and others 2011). In patients with SLE, an IFNα signature is associated with premature vascular damage (Somers and others 2012). In mouse models of SLE and atherosclerosis, the absence of type I IFN signaling prevented lesion formation and macrophage recruitment to plaques (Thacker and others 2012). Unlike SLE, where circulating plasma levels of IFNα are high, chronically infected HIV subjects do not have high levels of circulating IFNα but rather a monocyte gene expression profile of IFN-activated genes (Rempel and others 2010).
Monocytes from HIV-infected individuals on ART upregulate the scavenger receptor SR-A, which is highly expressed by macrophage foam cells in atherosclerotic lesions (Gough and others 1999). Our data showed no difference in levels of CD36 gene expression between ART treated or not and controls. We found that CD36 is constitutively expressed in all groups. This is contrary to other reports that show an increase in monocyte CD36 expression in HIV infection (Meroni and others 2005) or a decrease in CD36, that is associated with ART (Serghides and others 2002). Both these studies assessed CD36 protein by flow cytometry and did not determine SR-A gene expression. We did not do monocyte protein expression for CD36 or SR-A on our larger HIV cohort. However, we did see differences in SR-A monocyte protein expression in a small set of HIV-positive and negative subjects (Fig. 2B). SR-A facilitates early foam cell formation by preferentially enhancing modified LDL uptake (Horiuchi and others 2003). Circulating oxLDL concentrations are increased in HIV-infected subjects on ART (Duong and others 2006) and are related to those with lipodystrophy. Monocytes from HIV-infected subjects in this study showed an increased ability to accumulate lipids compared with control HIV-negative individuals despite statin use. In another recent study looking at monocyte gene expression in HIV infection, CD36 was noted to be low, or as in our study unchanged, in ART-treated HIV-infected individuals (Feeney and others 2013). In vitro modeling showed that IFNα-primed monocytes take up acLDL to a greater extent than nontreated or HIV-primed monocytes and that this uptake is through SR-A (Nikolic and others 2011; Orso and others 2011).
There are several inflammatory mediators associated with atherosclerosis although the overexpression of SR-A on circulating monocytes preferentially promotes the binding of modified LDL that leads to the generation of ROS (Mitra and others 2011). Modified LDL results in early activation and damage to endothelial cells resulting in expression of adhesion molecules and further recruitment of monocytes to subendothelial layers (Mitra and others 2011). The generation of excess ROS leads to oxidative stress, which is present in all stages of atherosclerosis (Heitzer and others 2001; Tavakoli and Asmis 2012; Voloshyna and others 2013). ROS also induces modified LDL to be recognized by scavenger receptors on M/Mφ suggesting a vicious circle of increased uptake and accumulation of LDL generating excessive ROS, the cycle persisting in the presence of type I IFN activation.
Immune activation is associated with normal aging and explains the increase in atherosclerosis with age. However, HIV-infected individuals have a significantly higher risk of developing CVD earlier and it has been suggested that traditional CVD risk factor be evaluated in HIV-infected individuals under 45 years of age (Boccara and others 2013). Explanations for this include the use of ART, the replication of virus itself and the subsequent proinflammatory state. However, in our studies looking at ART-treated HIV-infected individuals, we do not see increased circulating plasma cytokines compatible with a proinflammatory “cytokine storm,” such as tumor necrosis factor α (TNFα) in IL-6 nor increased gene expression of these cytokines in monocytes despite measurable LPS levels in the plasma (Rempel and others 2010; Pulliam and others 2011). Increasing evidence suggests that it is an indirect immune dysfunction from HIV infection that causes significant comorbidities. Blood monocytes from virally suppressed HIV-infected subjects have monocyte markers and plasma levels of innate immune activation associated with elderly individuals (Pulliam and others 2004; Hearps and others 2012). We and others posit that the chronic immune response required to keep HIV in check may be deleterious to the host by mimicking chronic activation seen in aging.
There are several limitations of this study. Monocyte gene expression data are cross sectional and comes from middle-aged men who are predominantly white. All subjects were on ART or a treatment interruption and did not have significant co-infections including hepatitis C nor did they use drugs of abuse less than 6 months before entering the study. All subjects were veterans and had access to stable medical care. In a recent report from the Women's Interagency HIV Study (WIHS), untreated women show abnormal inflammatory markers such as elevated sCD14, TNFα and monocyte chemoattractant protein (MCP) that were reduced after ART. However, markers of early atherosclerosis such as carotid artery intima-media thickness remained abnormal and TNFα remained elevated post-ART (Kaplan and others 2012). The WIHS cohort included predominantly African American women with a third having HCV coinfection that may influence these results. Another limitation of our study is our small, although significant, numbers. Additional studies need to be performed to confirm that SR-A expression on monocytes from HIV-infected subjects increases lipid accumulation and produces ROS.
In summary, these findings suggest chronic activation observed in HIV-infected subjects can induce SR-A expression and stimulate modified LDL uptake and ROS production that may be a mechanism for the increased atherosclerosis. These results are in spite of statin therapy that put the lipid levels of the HIV-infected subjects in the normal range. Our study includes both in vitro-treated and ex vivo monocytes from ART-treated HIV-infected subjects. Results from this study open new avenues of research in HIV and CVD. Does lowering monocyte IFNα activation lower rates of atherosclerosis? Recent data using a mouse model of lymphocytic choriomeningitis virus demonstrated that a chronic type I IFN response could be safely blocked using an IFN-I-receptor neutralizing antibody. This blockade resulted in reduced viral load and redirection of the immune response (Teijaro and others 2013; Wilson and others 2013). If IFNα activation were reduced, would the immune system be reset with decreases in monocyte SR-A expression and lipid accumulation? Further studies on ways to lower long-term activation are needed. While aging favors an increase in cardiovascular risks, including atherosclerosis, chronic activation from HIV infection may further accelerate the risks.
Acknowledgment
The authors would like to thank Dr. Robert Raffai for helpful discussions.
Author Disclosure Statement
No competing financial interests exist.
References
- Baker JV, Neuhaus J, Duprez D, Cooper DA, Hoy J, Kuller L, Lampe FC, Liappis A, Friis-Moller N, Otvos J, Paton NI, Tracy R, Neaton JD, Group ISS. 2011. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS 25(17):2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, Capeau J, Cohen A. 2013. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 61(5):511–523 [DOI] [PubMed] [Google Scholar]
- Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. 2011. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 204(8):1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. 2000. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 275(17):12633–12638 [DOI] [PubMed] [Google Scholar]
- Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. 2010. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol 87(4):589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, McCune WJ, Kaplan MJ. 2007. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110(8):2907–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, Meade D, Li XA, Zhu W, Uittenbogaard A, Wilson ME, Smart EJ. 2003. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest 111(3):389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong M, Petit JM, Martha B, Galland F, Piroth L, Walldner A, Grappin M, Buisson M, Duvillard L, Chavanet P, Portier H. 2006. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials 7(2):41–47 [DOI] [PubMed] [Google Scholar]
- Feeney ER, McAuley N, O'Halloran JA, Rock C, Low J, Satchell CS, Lambert JS, Sheehan GJ, Mallon PW. 2013. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intracellular cholesterol accumulation. J Infect Dis 207(4):628–637 [DOI] [PubMed] [Google Scholar]
- Fernandez S, Tanaskovic S, Helbig K, Rajasuriar R, Kramski M, Murray JM, Beard M, Purcell D, Lewin SR, Price P, French MA. 2011. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+T cells. J Infect Dis 204(12):1927–1935 [DOI] [PubMed] [Google Scholar]
- Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, Kalinke U, Weber C, Lutgens E, de Winther MP. 2010. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab 12(2):142–153 [DOI] [PubMed] [Google Scholar]
- Gough PJ, Greaves DR, Suzuki H, Hakkinen T, Hiltunen MO, Turunen M, Herttuala SY, Kodama T, Gordon S. 1999. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol 19(3):461–471 [DOI] [PubMed] [Google Scholar]
- Grandl M, Schmitz G. 2010. Fluorescent high-content imaging allows the discrimination and quantitation of E-LDL-induced lipid droplets and Ox-LDL-generated phospholipidosis in human macrophages. Cytometry A 77(3):231–242 [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. 2006. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203(12):2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon SK, Grunfeld C, Kotler DP, Currier JS, Lundgren JD, Dube MP, Lipshultz SE, Hsue PY, Squires K, Schambelan M, Wilson PW, Yarasheski KE, Hadigan CM, Stein JH, Eckel RH. 2008. State of the science conference: initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation 118(2):198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, O'Leary D, Bacchetti P, Kronmal RA. 2009. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 23(14):1841–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. 1992. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 74(5):1045–1052 [DOI] [PubMed] [Google Scholar]
- Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. 2012. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 26(7):843–853 [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. 2001. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104(22):2673–2678 [DOI] [PubMed] [Google Scholar]
- Horiuchi S, Sakamoto Y, Sakai M. 2003. Scavenger receptors for oxidized and glycated proteins. Amino Acids 25(3–4):283–292 [DOI] [PubMed] [Google Scholar]
- Hsue PY, Deeks SG, Hunt PW. 2012. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 205Suppl 3:S375–S382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN, Deeks SG. 2009. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 23(9):1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, Anastos K, Tien PC, Xue X, Lazar J, Parrinello CM, Benning L, Tracy RP. 2012. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr 60(4):359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, Bao C. 2011. Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum 63(2):492–502 [DOI] [PubMed] [Google Scholar]
- Lo J, Plutzky J. 2012. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. J Infect Dis 205Suppl 3:S368–S374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni L, Riva A, Morelli P, Galazzi M, Mologni D, Adorni F, Galli M. 2005. Increased CD36 expression on circulating monocytes during HIV infection. J Acquir Immune Defic Syndr 38(3):310–313 [PubMed] [Google Scholar]
- Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. 2011. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci 342(2):135–142 [DOI] [PubMed] [Google Scholar]
- Nikolic D, Calderon L, Du L, Post SR. 2011. SR-A ligand and M-CSF dynamically regulate SR-A expression and function in primary macrophages via p38 MAPK activation. BMC Immunol 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso E, Grandl M, Schmitz G. 2011. Oxidized LDL-induced endolysosomal phospholipidosis and enzymatically modified LDL-induced foam cell formation determine specific lipid species modulation in human macrophages. Chem Phys Lipids 164(6):479–487 [DOI] [PubMed] [Google Scholar]
- Pamukcu B, Lip GY, Devitt A, Griffiths H, Shantsila E. 2010. The role of monocytes in atherosclerotic coronary artery disease. Ann Med 42(6):394–403 [DOI] [PubMed] [Google Scholar]
- Patel P, Bush T, Overton T, Baker J, Hammer J, Kojic E, Conley L, Henry K, Brooks JT. 2012. Effect of abacavir on acute changes in biomarkers associated with cardiovascular dysfunction. Antivir Ther 17(4):755–761 [DOI] [PubMed] [Google Scholar]
- Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, Abbara S, Grinspoon SK. 2012. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 26(18):2409–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Rempel H, Sun B, Abadjian L, Calosing C, Meyerhoff DJ. 2011. A peripheral monocyte interferon phenotype in HIV infection correlates with a decrease in magnetic resonance spectroscopy metabolite concentrations. AIDS 25(14):1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Rempel H. 2004. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol 157(1–2):93–98 [DOI] [PubMed] [Google Scholar]
- Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, Obel N. 2011. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS 25(13):1637–1646 [DOI] [PubMed] [Google Scholar]
- Rempel H, Sun B, Calosing C, Pillai SK, Pulliam L. 2010. Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS 24(10):1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Pritschet K, Schmidt B. 2012. Blocking type I interferon production: a new therapeutic option to reduce the HIV-1-induced immune activation. Clin Dev Immunol 2012:534929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serghides L, Nathoo S, Walmsley S, Kain KC. 2002. CD36 deficiency induced by antiretroviral therapy. AIDS 16(3):353–358 [DOI] [PubMed] [Google Scholar]
- Shantsila E, Devitt A, Lip GY. 2010. Circulating monocytes and atherogenesis: from animal experiments to human studies. Thromb Haemost 104(2):191–193 [DOI] [PubMed] [Google Scholar]
- Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, Kazerooni EA, McCune WJ, Kaplan MJ. 2012. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One 7(5):e37000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. 2009. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res 104(8):952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli S, Asmis R. 2012. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal 17(12):1785–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340(6129):207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker SG, Zhao W, Smith CK, Luo W, Wang H, Vivekanandan-Giri A, Rabquer BJ, Koch AE, Pennathur S, Davidson A, Eitzman DT, Kaplan MJ. 2012. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum 64(9):2975–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshyna I, Littlefield MJ, Reiss AB. 2013. Atherosclerosis and interferon-gamma: New insights and therapeutic targets. Trends Cardiovasc Med 24(1):45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340(6129):202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. 2013. Foam cells in atherosclerosis. Clin Chim Acta 424C:245–252 [DOI] [PubMed] [Google Scholar]