Abstract
Although homocysteine (Hcy) has been widely implicated in the etiology of various physical health impairments, especially cardiovascular diseases, overwhelming evidence indicates that Hcy is also involved in the pathophysiology of schizophrenia and affective disorders. There are several mechanisms linking Hcy to biological underpinnings of psychiatric disorders. It has been found that Hcy interacts with NMDA receptors, initiates oxidative stress, induces apoptosis, triggers mitochondrial dysfunction and leads to vascular damage. Elevated Hcy levels might also contribute to cognitive impairment that is widely observed among patients with affective disorders and schizophrenia. Supplementation of vitamins B and folic acid has been proved to be effective in lowering Hcy levels. There are also studies showing that this supplementation strategy might be beneficial for schizophrenia patients with respect to alleviating negative symptoms. However, there are no studies addressing the influence of add-on therapies with folate and vitamins B on cognitive performance of patients with schizophrenia and affective disorders. In this article, we provide an overview of Hcy metabolism in psychiatric disorders focusing on cognitive correlates and indicating future directions and perspectives.
Keywords: homocysteine, depression, bipolar disorder, schizophrenia, hyperhomocysteinemia, cognition, brain substrates
Introduction
Homocysteine (Hcy) is one of the non-protein amino acids that is produced in one-carbon metabolism. Two enzymatic pathways are involved in Hcy metabolism—re-mehtylation to methionine and trans-sulfuration to cysteine and taurine. The efficiency of Hcy catabolism depends on the availability of folate, vitamin B12 and vitamin B6. Tans-sulfuration to cysteine, which forms glutathione, is catalyzed by cystathionine beta synthase (CBS) and cystathionase. In turn, conversion from Hcy to methionine is a multistep reaction with a number of enzymes being involved including Hcy methyltransferase, methionine synthase (MS) and methionine synthase reductase (MTRR), as well as the methylenetetrahydrofolate reductase (MTHFR; Scott and Weir, 1998). There are two common polymorphisms located in the MTHFR gene—C677T and A1298C that may lower the activity of MTHFR and lead to increased Hcy levels. The most common one—C677T polymorphism, which is present in 10–12% of population (Gilbody et al., 2007), contributes to the expression of a thermolabile variant of MTHFR. Other factors might also increase Hcy level including higher age, male gender, cigarette smoking, alcohol abuse or dependence, low dietary intake of folate and vitamins B, renal dysfunction and certain medications (e.g., sodium valproate and lamotrigine, diuretics, fibrates) (Frankenburg, 2007). In addition, there is an inverse relationship between Hcy and both folate and vitamin B12 levels (Yoshino et al., 2010).
Several lines of evidence indicate that Hcy serves as an important atherosclerotic factor. It has been found that Hcy may induce vascular damage via initiating oxidative stress and reducing the availability of nitric oxide that is a powerful vasodilator (Perna et al., 2003). These mechanisms underlie well-established links between elevated Hcy levels or MTHFR polymorphisms and cardiovascular diseases including coronary artery disease, myocardial infarction, cerebrovascular disease and peripheral occlusive disease (Mangoni and Jackson, 2002; Trimmer, 2013).
In the recent years, there is a growing interest in the causative links between Hcy and neuropsychiatric disorders. High Hcy levels are increasingly recognized as a risk factor for age-related cognitive deficits together with various types of dementia (Stanger et al., 2009). Studies in this field have provided several links between Hcy and domains of cognitive functioning (Faux et al., 2011; Kim et al., 2013). However, less attention has been paid to cognitive correlates of elevated Hcy level in psychiatric disorders including schizophrenia and affective disorders. In this article, we review the role of Hcy in the pathophysiology of psychiatric disorders including schizophrenia and affective disorders focusing on cognitive correlates.
Mechanisms of homocysteine action—the relevance to psychiatric disorders
The exact neural and behavioral mechanism of Hcy action is not known. It seems that the interaction of Hcy with glutamatergic transmission is the most relevant mechanism explaining the association between Hcy and schizophrenia or affective disorders. Both Hcy and its oxidative metabolite—homocysteic acid—serve as agonists within NMDA receptors (Klancnik et al., 1992; Zhang and Lipton, 1992; Lipton et al., 1997). Stimulation of NMDA receptors by Hcy increases calcium influx that exerts neurotoxic effects (Ho et al., 2002). However, in the presence of low concentrations of glycine, Hcy acts as a partial antagonist within the glycine site of NMDA receptors. Thus, in case of low glycine level Hcy manifests its neuroprotective activity (Lipton et al., 1997) and only high Hcy concentrations may be toxic. On the other hand, when glycine levels are high (after head trauma or stroke), low Hcy levels become toxic (Alam et al., 1998). This dual action of Hcy within NMDA receptors may explain why elevated Hcy levels might be implicated in schizophrenia, in which hypofunction of glutamatergic transmission has been reported and depression that is characterized by up-regulated glutamatergic activity.
Also, various studies have suggested that Hcy might regulate the function of other neuromodulators, such as acetylcholine (Chen et al., 2011) and dopamine, and serotonin (Gao et al., 2011). Specifically, Gao et al. (2011) have reported that rats with hyperhomocysteinemia have lower levels of dopamine and serotonin in the cortex than control rats. Other studies suggest that Hcy regulates synaptic plasticity in the hippocampus (Christie et al., 2005; Algaidi et al., 2006). These prior studies suggest that Hcy has multiple functions in the brain; this can likely explain its links to various psychiatric disorders, including schizophrenia and affective disorders.
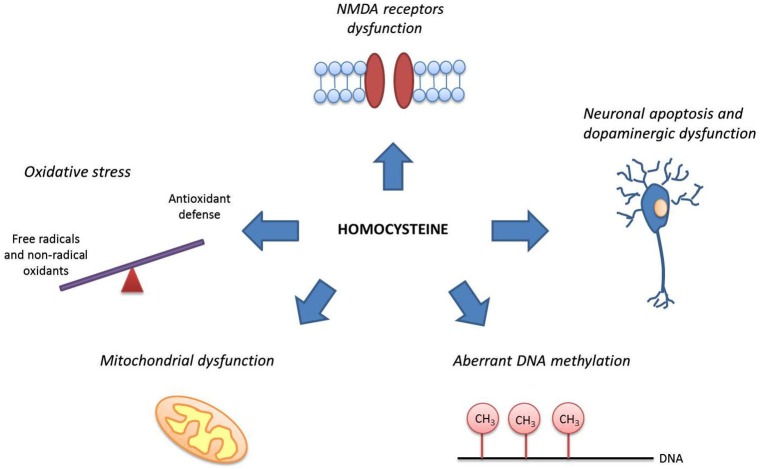
Animals exposed to Hcy exhibit compromised brain energy metabolism (Streck et al., 2003), altered long-term potentiation, disturbances of synaptic plasticity and cognitive impairment in terms of spatial learning (Algaidi et al., 2006) and memory deficits (Streck et al., 2004). Heterozygous and homozygous Mthfr knockout mice are also characterized by neurodevelopmental retardation and altered cerebellar morphology (Chen et al., 2001). Other mechanisms of Hcy toxicity that might be relevant to the pathophysiology of schizophrenia and affective disorders include oxidative stress (Koz et al., 2010; Loureiro et al., 2010; Dietrich-Muszalska et al., 2012), neuronal apoptosis (Wang et al., 2012), vascular damage (Brown et al., 2007) and aberrant DNA methylation (Bromberg et al., 2008, 2009; Kinoshita et al., 2013; Figure 1). Neural studies have shown that Hcy acts on various brain regions, including the hippocampus (den Heijer et al., 2003; Matté et al., 2009; Chen et al., 2011), cortex (den Heijer et al., 2003), and the basal ganglia (Genedani et al., 2010). Higher Hcy levels lead to atrophy in the frontal, parietal, and temporal areas (Rajagopalan et al., 2011).
Figure 1.
Mechanisms of homocysteine action as relevant to neurological and psychiatric disorders. Homocysteine may interact with NMDA receptors altering glutamatergic transmission, exert toxic effects on dopaminergic neurons, initiate neuronal apoptosis, induce oxidative stress, lead to mitochondrial dysfunction and influence DNA methylation altering gene expression.
Homocysteine and cognition in healthy individuals
Homocysteine plays an important role in behavioral and cognitive processes as shown in studies measuring Hcy levels in healthy elderly subjects (Prins et al., 2002; Dufouil et al., 2003; Teunissen et al., 2003; Nurk et al., 2005; Feng et al., 2006; Hooshmand et al., 2012). For example, van den Kommer et al. (2010) reported that higher Hcy levels are associated with slow information processing speed in healthy participants. Further, Nurk et al. (2005) found that impaired episodic memory performance is associated with increased Hcy levels in healthy individuals. Along the same lines, Garcia et al. (2004) revealed that impaired performance in the Stroop test correlates with higher levels of Hcy. Studies on the role of Hcy in cognitive performance in healthy subjects have shown that Hcy is specifically involved in episodic memory (Faux et al., 2011; Narayan et al., 2011), spatial learning (Pirchl et al., 2010), reversal learning (Christie et al., 2005; Algaidi et al., 2006), and executive function (Narayan et al., 2011). However, it is debatable whether Hcy plays a role in working memory processes, as some studies have found they are not related (Narayan et al., 2011), while other studies found that lowering Hcy levels enhances working memory (Macpherson et al., 2012).
Recently published results reveal associations between total Hcy levels and cognitive functions in healthy subjects. It has been found that lower overall cognitive performance measured by Cambridge Cognitive Examination (CAMCOG) are associated with higher Hcy levels (Budge et al., 2002). This study also revealed an inverse correlation between hippocampal volume and Hcy levels (Budge et al., 2002). Other studies have found a positive correlation between total Hcy levels and ventricle-brain ratios in the anterior and middle ventricular regions in elderly participants (Sachdev et al., 2002). It has also been reported that higher Hcy levels are associated with lower scores in Mini Mental State Examination (MMSE; Kalmijn et al., 1999). It has been demonstrated that impaired cognition in elderly participants correlates with Hcy levels, especially for psychomotor speed and memory functions (Prins et al., 2002).
Recent data show that higher Hcy levels are associated with silent brain infarctions and subcortical white matter lesions in older adults (Vermeer et al., 2002). Higher Hcy levels have been associated with increased prevalence of silent brain infarction and decreased brain volume in comparison with subjects having lower total Hcy (Morris, 2003).
Homocysteine in psychiatric disorders
Total Hcy level changes have also been shown to be associated with many psychiatric disorders, including schizophrenia and affective disorders. These observations stimulated further studies on the association between elevated Hcy levels and neuropsychiatric symptoms and disorders.
Patients having cognitive disorders and depression have been reported in many studies to have low vitamin B12 and folate levels. In 1980, an important finding by Shorvon et al. (1980) was published on the neuropsychiatric manifestations in megaloblastic anemia that occurred due to low folate or vitamin B12 levels. Their study revealed that up to 56% of patients with affective disorders have serum folate deficiency (Shorvon et al., 1980). Below, we describe the relationship between changes in Hcy levels and schizophrenia, depression, and bipolar disorder.
Schizophrenia
In 1975, Freeman et al. (1975) described a case of homocystinuria, caused by a deficient MTHFR activity, accompanied by psychotic-like behavior that responded to folate treatment. More recently, a new hypothesis for the development of schizophrenia has been proposed—the DNA polymorphism-diet-cofactor-development (DDCD) hypothesis (Johnson, 1999). This hypothesis states that mutations of genes related to folate and vitamins B metabolism potentiated by maternal dietary vitamin deficiencies contribute to the development of schizophrenia. Total Hcy serum levels in schizophrenia were first measured by Regland et al. (1995). In this study, elevated Hcy levels were found in 9 out of 20 schizophrenic patients (Regland et al., 1995).
Subsequently, elevated total Hcy levels have been widely described in various subgroups of schizophrenia patients (Muntjewerff et al., 2006; Nishi et al., 2014) including drug-naïve first-episode psychosis subjects (Kale et al., 2010; Ayesa-Arriola et al., 2012; García-Bueno et al., 2013) and chronic schizophrenia patients (Eren et al., 2010). Total Hcy level has been found to negatively correlate with folate and vitamin B12 levels in this group of patients (Bouaziz et al., 2010). In addition, some authors have found that Hcy levels are higher especially in young male schizophrenia patients (Levine et al., 2002). It has also been estimated that a 5-μmol increase in plasma Hcy level may increase the risk of schizophrenia by 70% (Muntjewerff et al., 2006). Several studies have proved a positive correlation between Hcy levels and the severity of schizophrenia negative symptoms (Goff et al., 2004; Petronijević et al., 2008; Bouaziz et al., 2010; Misiak et al., 2014). These studies are in concordance with the studies showing a negative correlation between duration of untreated psychosis (DUP) and Hcy levels (Ayesa-Arriola et al., 2012; Misiak et al., 2014). The association of increased Hcy levels with schizophrenia psychopathology has provided grounds for add-on therapies with vitamin supplementation (Hill et al., 2011; Roffman et al., 2013). The largest randomized, double-blind and placebo-controlled study of folic acid and vitamin B12 supplementation revealed the improvement of negative symptoms in schizophrenia patients. However, this supplementation strategy was effective only in patients being homozygotes of the 484T > C polymorphism in the FOLH1 gene that encodes folate hydrolase involved in intestinal folate transport (Roffman et al., 2013).
Elevated Hcy levels found in first-episode psychosis patients suggest that one-carbon metabolism alterations may share common genetic underpinnings with schizophrenia. Another proof for this assumption is that siblings of schizophrenia patients are also characterized by increased plasma Hcy levels (Geller et al., 2013) and schizophrenia patients with positive family history of schizophrenia in first or second degree relatives have significantly higher Hcy levels compared to those with negative family history of schizophrenia (Misiak et al., 2014). Several studies have reported an association of two common polymorphisms in the MTHFR gene (C677T and A1298C) with schizophrenia (Lewis et al., 2005; Muntjewerff et al., 2006; Gilbody et al., 2007; Shi et al., 2008). Furthermore, these polymorphisms have been found to predict the development of metabolic syndrome following the treatment with antipsychotics or at least might be associated with increased incidence of metabolic disturbances, such as visceral obesity, impaired metabolism of glucose and lipids (Misiak et al., 2013). Furthermore, schizophrenia patients with the comorbid metabolic syndrome are characterized by higher Hcy levels in comparison with those, who do not meet the criteria of metabolic syndrome (Vuksan-Ćusa et al., 2011, 2013).
Although the MTHFR gene polymorphisms are known to influence the risk of metabolic adverse effects of antipsychotics, the influence of antipsychotic treatment on Hcy requires further investigation due to scarcity of well-designed studies. There is only one observational study on drug-naïve first episode schizophrenia patients showing the lack of significant changes in Hcy levels in the course of antipsychotic pharmacotherapy (Bicikova et al., 2011). Another study on acutely relapsed schizophrenia patients has revealed significantly higher Hcy levels during symptomatic exacerbation than during the remission phase (Petronijević et al., 2008). In turn, the cross-sectional study by Eren et al. (2010) on chronic schizophrenia patients revealed significantly lower levels of plasma folate, but not Hcy or vitamin B12, in patients receiving higher doses of typical antipsychotics (chlorpromazine equivalent >400 mg). Another cross-sectional study revealed no significant difference in Hcy level between schizophrenia patients receiving clozapine in monotherapy and healthy controls (Wysokiński and Kłoszewska, 2013). There is also one study showing a positive relationship between Hcy levels and N-desmethyl-olanzapine concentration that is one of the main olanzapine metabolites (Lu et al., 2013). These inconsistent results might be attributed to heterogenous methodology such as the recruitment of different patients defined by illness duration or symptomatic presentation, as well as the lack of adjustment for possible confounders including the MTHFR genotype, dietary habits, cigarette smoking or other known factors influencing Hcy metabolism.
Several studies have established direct links between the MTHFR gene polymorphisms and cognitive dysfunction in terms of executive function and blunted response to errors in schizophrenia. It has been found that the 677T variant of the MTHFR gene induces a dose-dependent blunting of dorsal anterior cingulate cortex activation in response to errors using the antisaccade paradigm (Roffman et al., 2011b), positively correlates with impairments of verbal fluency (Roffman et al., 2007) and interacts with the 108Val allele in the COMT gene increasing the number of perseverative errors on the Wisconsin Card Sorting Task (WCST; Roffman et al., 2008b). Although the MTHFR gene variants have been reported to influence certain domains of cognitive functioning in schizophrenia patients, Hcy levels have not been found to correlate with cognitive impairment in first-episode schizophrenia spectrum disorders patients (Ayesa-Arriola et al., 2012). These discrepancies suggest that other Hcy-independent consequences of one-carbon metabolism dysfunction due to genetic factors are implicated in the occurrence of cognitive impairment in schizophrenia. Given that the 677T allele in the MTHFR gene is associated with lower genomic DNA methylation (Friso et al., 2002), it might be hypothesized that epigenetic phenomena are involved in cognitive impairment in schizophrenia. Furthermore, the 677T variant enhances dopamine metabolism (Roffman et al., 2008a,b), which is linked to schizophrenia pathophysiology and is implicated in the activation of dorsal anterior cingulate cortex in response to errors (Holroyd and Coles, 2002) and influences prefrontally-mediated executive functioning (Tan et al., 2007).
Depression
Several studies have established that depressive episodes may predict the development of cardiovascular diseases (de Jonge et al., 2014). These findings suggest that depression is linked to co-occurring metabolic deregulation increasing cardiovascular risk. Indeed, elevated Hcy levels have been shown in major depression (Folstein et al., 2007; Yapislar et al., 2012; Delport et al., 2014; Lok et al., 2014). Notably, it has been found that Hcy level negatively correlates with vitamin B12 and folate levels in depressed patients (Ebesunun et al., 2012). There are also studies showing that the MTHFR C677T polymorphism may increase the susceptibility to major depression (Wu et al., 2013; Delport et al., 2014; Lok et al., 2014; Shen et al., 2014). Interestingly, it has been found that the MTHFR 677T allele may interact with childhood traumatic events influencing the time to recurrence in major depressive disorder (Lok et al., 2013). Indeed, the carriers of the MTHFR 677T allele with childhood traumatic events had shorter time to recurrence of major depressive disorder in comparison with those without such events. These findings corroborate emerging evidence indicating that posttraumatic stress disorder (PTSD) patients are also characterized by elevated Hcy levels (Levine et al., 2008; Jendricko et al., 2009).
In the recent study with older adults, it was found that serum folate levels correlate with the severity of depressive symptoms (Ebly et al., 1998). In studies that failed to prove an association between low serum folic acid and depression, there was a negative correlation between folate level and the duration of the depressive episode, or a negative correlation between folate level and length of hospitalization and therefore with treatment outcome. Regarding the severity of depression, patients with lower folate levels were more severely depressed than those with normal folate levels (Alpert et al., 2000). In the Womens Health and Aging Study, low vitamin B12 levels were reported in elderly disabled community participants and significant vitamin B12 deficiency was more common among depressed than healthy participants. Significant vitamin B12 deficiency was associated with a two-fold higher risk of developing severe depression (Penninx et al., 2000). Interestingly, in the study by Gabryelewicz et al. (2007), depression and higher baseline Hcy levels were the strongest predictors of conversion from mild cognitive impairment (MCI) to dementia. Elevated total Hcy levels were also observed in the study of 213 patients with major depression compared with controls (Fava et al., 1997). S-Adenosyl Methionine (SAM), a precursor of Hcy, is used in some countries as an effective adjuvant therapy in the treatment of depression. On the basis of a meta-analysis, Bressa (1994) also suggested that SAM can act as an antidepressive agent. S-Adenosyl Methionine has also been found to be effective in the treatment of depression related to Parkinsons disease (Di Rocco et al., 2000). Studies on patients with geriatric depression have revealed correlations between Hcy and cognitive performance. In this group of patients, Hcy level positively correlated with language processing and processing speed (Alexopoulos et al., 2010).
Bipolar disorder
Although elevated Hcy levels have been repeatedly reported in bipolar disorder patients (Baek et al., 2013), no significant differences have been found across various mood states (Chiarani et al., 2013). Studies on bipolar disorder indicate that high Hcy levels are significantly more frequent among males than females with bipolar depressive episode (Permoda-Osip et al., 2013b, 2014b). Similarly to schizophrenia and major depression patients, an inverse relationship between Hcy and both folate vitamin B12 levels has been demonstrated in bipolar disorder subjects (Permoda-Osip et al., 2013b). However, it has been found that Hcy level negatively correlates with the level of endothelial damage markers including E-selectin and intracellular adhesion molecule-1 (ICAM-1) in bipolar depression subjects suggesting that the pathways of cardiovascular risk are not associated with Hcy metabolism in this group of patients (Permoda-Osip et al., 2013b).
As similar to schizophrenia, two common polymorphisms in the MTHFR gene (C677T and A1298C) might increase the risk of bipolar disorder and predict the development of comorbid metabolic syndrome suggesting the existence of common genetic underpinnings (Peerbooms et al., 2011; Ellingrod et al., 2012). There is also one study showing an association between the T833C polymorphism in the CBS gene and bipolar disorder risk (Permoda-Osip et al., 2014a).
However, in contrast to studies on schizophrenia, evidence for the influence of Hcy on cognition is more convincing. There are studies showing an inverse relationship between plasma Hcy and verbal learning, executive function or immediate memory in euthymic bipolar disorder patients (Dittmann et al., 2007, 2008; Osher et al., 2008). It should be noted that two studies consistently reported the correlation between Hcy levels and executive functioning measured in terms of cognitive flexibility tapped by Trail Making Test subtest B (Osher et al., 2008) and perseverative errors assessed on WCST (Dittmann et al., 2007). Notably, these findings overlap with the influence of MTHFR polymorphisms on cognitive performance reported in schizophrenia patients (Roffman et al., 2007, 2008a,b, 2011a,b). As mentioned above, the C677T polymorphism in the MTHFR gene has been associated with greater deficits of executive functioning assessed on WCST in schizophrenia subjects (Roffman et al., 2007).
Cognitive deficits due to elevated Hcy level might be particularly prominent among older bipolar disorder patients or those with a delayed onset of the disorder (Dias et al., 2009). However, it should be kept in mind that aging increases Hcy levels and some cognitive deficits due to hyperhomocysteinemia may occur regardless of depression. It has been shown that hyperhomocysteinemia worsens cognitive performance in tests of immediate or delayed memory, as well as global cognitive functioning in older subjects (Ford et al., 2013). It should also be noted that patients with bipolar disorder might exhibit higher Hcy levels due to the treatment with mood stabilizers. Indeed, experimental studies have revealed that sodium valproate inhibits methionine adenosyltransferase, while lamotrigine serves as a weak dihydrofolate reductase inhibitor leading to lower functional folate levels despite of normal blood levels of folate (Baek et al., 2013).
There are two randomized placebo controlled trials investigating the efficacy of folic acid supplementation in bipolar depression. These studies revealed that folic acid may enhance lithium prophylaxis (Coppen et al., 1986) and antidepressant action of fluoxetine in females (Coppen and Bailey, 2000). Furthermore, it has been found that the augmentation of sodium valproate with folic acid might be beneficial in terms of reducing manic symptoms (Behzadi et al., 2009). Inconsistent results also indicate that higher vitamin B12 level may predict favorable response to single ketamine infusion in bipolar depression patients (Permoda-Osip et al., 2013a; Lundin et al., 2014). Ketamine is an NMDA receptor antagonist, emerging as a therapeutic strategy in treatment-resistant depression (Naughton et al., 2014).
Future directions and conclusion
Undoubtedly, elevated Hcy levels are associated with a wide spectrum of psychiatric disorders including particularly schizophrenia and affective disorders. It might be assumed that the dual action of Hcy (as agonist or antagonist) within NMDA receptors (Lipton et al., 1997) explains why elevated Hcy levels are involved in the pathophysiology of both schizophrenia and affective disorders. This association is probably strengthened by high prevalence of metabolic syndrome and its single components, which is a consequence of antipsychotic treatment. Emerging evidence indicates that high Hcy levels may, to some extent, account for cognitive deficits among these groups of patients. It seems that the influence of Hcy on executive functioning occurs regardless of a psychiatric diagnosis since this correlation has been found both in schizophrenia and bipolar disorder patients. In this regard, it is also recommended to investigate the influence of Hcy on cognition in healthy adults in order to determine the extent of cognitive deficits that are the consequence of elevated Hcy levels. Further, future studies should investigate the relationship between Hcy levels in these patient populations on and off their medications to tease apart the relationship between Hcy, psychiatric disorders, and treatment duration or type of medications.
There is still a scarcity of studies investigating the relationship between Hcy and cognitive deficits in drug-naïve first-episode patients and high-risk populations. These studies are warranted as they may indicate the correlation between Hcy levels and early cognitive deficits that are strictly associated with schizophrenia and affective disorders regardless of medication and disease duration. Irrespective of a diagnostic subgroup, future studies should take into account the confounding effect of such variables as body weight, dietary habits, smoking or alcohol consumption that are less frequently controlled, as previous studies have shown that these variables are correlated with Hcy levels and may thus confound findings in the relationship between psychiatric disorders and Hcy levels. Given the largely known contribution of Hcy to the etiology of various types of dementia, it might be also beneficial to address the role of Hcy in the neuroprogression of cognitive deficits that is widely observed particularly in affective disorders and remains the matter of dispute in schizophrenia. Longitudinal measurements of Hcy along with assessment of cognitive functioning that take into account the effects of age as an confounding factor are required to initiate this vein of research.
It should be noted that supplementation of folic acid and vitamins B may normalize Hcy levels. However, we are not aware of any studies addressing the efficacy of supplementation strategies with respect to alleviating cognitive deficits among patients with schizophrenia or affective disorders. Similarly, a gap exists in addressing the influence of antipsychotic treatment on Hcy metabolism, and its correlations with cognitive processes, which should be the focus in future work. As mentioned above, there is only one cross-sectional study revealing a negative correlation between folate levels and high chlorpromazine equivalents (>400 mg/day) (Eren et al., 2010) in chronic schizophrenia patients and one observational study performed in a small sample of drug-naïve first-episode schizophrenia patients reporting no significant alterations in Hcy levels in the course of antipsychotic treatment (Bicikova et al., 2011). Another study revealed a decrease in Hcy levels during the treatment of acute relapse of schizophrenia (Petronijević et al., 2008). This issue is important due to the known influence of certain antipsychotics on the development of obesity and its metabolic consequences, such as dyslipidemia, diabetes or hypertension that have been found to influence cognitive performance in schizophrenia patients (Lancon et al., 2012; Lindenmayer et al., 2012; Boyer et al., 2013; Li et al., 2014).
Results of studies based on candidate gene approach and investigating genetic variation within the Hcy metabolism enzymes should be interpreted with caution. Previous genome-wide association studies (GWAS) have not confirmed the association between polymorphisms in the MTHFR gene or other genes implicated in Hcy metabolism and schizophrenia (Yoshimi et al., 2010; Lencz et al., 2013; Ripke et al., 2013, 2014; Ivorra et al., 2014; Saito et al., 2014) or bipolar disorder (Sklar et al., 2011; Kuo et al., 2014; Mühleisen et al., 2014; Xu et al., 2014) risk. There is only one genome-wide linkage analysis of recurrent depressive disorder providing evidence for linkage on chromosome region 1p36 including the MTHFR gene with the LOD score for female-female pairs estimated at 2.73 (McGuffin et al., 2005). In this regard and taking into account the involvement of Hcy pathway in several physical health impairments, it might be hypothesized that discordant results of GWAS and candidate gene approach studies may originate from genetic heterogeneity across studied populations and various clinical phenotypes including distinct somatic comorbidities that have also been attributed to polymorphisms in the MTHFR gene.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the research grant “The role of genetic variation in one-carbon metabolic cycle in the etiology of metabolic syndrome in patients with schizophrenia” awarded by National Science Center (decision number: DEC-2011/03/N/NZ5/0024). Błażej Misiak is supported by the START scholarship provided by the Foundation for Polish Science.
References
- Alam Z., Coombes N., Waring R. H., Williams A. C., Steventon G. B. (1998). Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J. Neurol. Sci. 156, 102–106 10.1016/s0022-510x(98)00023-9 [DOI] [PubMed] [Google Scholar]
- Alexopoulos P., Topalidis S., Irmisch G., Prehn K., Jung S. U., Poppe K., et al. (2010). Homocysteine and cognitive function in geriatric depression. Neuropsychobiology 61, 97–104 10.1159/000275821 [DOI] [PubMed] [Google Scholar]
- Algaidi S. A., Christie L. A., Jenkinson A. M., Whalley L., Riedel G., Platt B. (2006). Long-term homocysteine exposure induces alterations in spatial learning, hippocampal signalling and synaptic plasticity. Exp. Neurol. 197, 8–21 10.1016/j.expneurol.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Alpert J. E., Mischoulon D., Nierenberg A. A., Fava M. (2000). Nutrition and depression: focus on folate. Nutrition 16, 544–546 10.1016/s0899-9007(00)00327-0 [DOI] [PubMed] [Google Scholar]
- Ayesa-Arriola R., Pérez-Iglesias R., Rodríguez-Sánchez J. M., Mata I., Gómez-Ruiz E., García-Unzueta M., et al. (2012). Homocysteine and cognition in first-episode psychosis patients. Eur. Arch. Psychiatry Clin. Neurosci. 262, 557–564 10.1007/s00406-012-0302-2 [DOI] [PubMed] [Google Scholar]
- Baek J. H., Bernstein E. E., Nierenberg A. A. (2013). One-carbon metabolism and bipolar disorder. Aust. N Z J. Psychiatry 47, 1013–1018 10.1177/0004867413502091 [DOI] [PubMed] [Google Scholar]
- Behzadi A. H., Omrani Z., Chalian M., Asadi S., Ghadiri M. (2009). Folic acid efficacy as an alternative drug added to sodium valproate in the treatment of acute phase of mania in bipolar disorder: a double-blind randomized controlled trial. Acta Psychiatr. Scand. 120, 441–445 10.1111/j.1600-0447.2009.01368.x [DOI] [PubMed] [Google Scholar]
- Bicikova M., Hampl R., Hill M., Ripova D., Mohr P., Putz Z. (2011). Neuro- and immunomodulatory steroids and other biochemical markers in drug-naive schizophrenia patients and the effect of treatment with atypical antipsychotics. Neuro Endocrinol. Lett. 32, 141–147 [PubMed] [Google Scholar]
- Bouaziz N., Ayedi I., Sidhom O., Kallel A., Rafrafi R., Jomaa R., et al. (2010). Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 179, 24–29 10.1016/j.psychres.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Boyer L., Richieri R., Dassa D., Boucekine M., Fernandez J., Vaillant F., et al. (2013). Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Res. 210, 381–386 10.1016/j.psychres.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Bressa G. M. (1994). S-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol. Scand. Suppl. 154, 7–14 10.1111/j.1600-0404.1994.tb05403.x [DOI] [PubMed] [Google Scholar]
- Bromberg A., Bersudsky Y., Levine J., Agam G. (2009). Global leukocyte DNA methylation is not altered in euthymic bipolar patients. J. Affect. Disord. 118, 234–239 10.1016/j.jad.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Bromberg A., Levine J., Nemetz B., Belmaker R. H., Agam G. (2008). No association between global leukocyte DNA methylation and homocysteine levels in schizophrenia patients. Schizophr. Res. 101, 50–57 10.1016/j.schres.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Bottiglieri T., Schaefer C. A., Quesenberry C. P., Jr., Liu L., Bresnahan M., et al. (2007). Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch. Gen. Psychiatry 64, 31–39 10.1001/archpsyc.64.1.31 [DOI] [PubMed] [Google Scholar]
- Budge M. M., de Jager C., Hogervorst E., Smith A. D., Oxford Project To Investigate Memory and Ageing (OPTIMA) (2002). Total plasma homocysteine, age, systolic blood pressure and cognitive performance in older people. J. Am. Geriatr. Soc. 50, 2014–2018 10.1046/j.1532-5415.2002.50614.x [DOI] [PubMed] [Google Scholar]
- Chen Z., Karaplis A. C., Ackerman S. L., Pogribny I. P., Melnyk S., Lussier-Cacan S., et al. (2001). Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 10, 433–443 10.1093/hmg/10.5.433 [DOI] [PubMed] [Google Scholar]
- Chen C. S., Kuo Y. T., Tsai H. Y., Li C. W., Lee C. C., Yen C. F., et al. (2011). Brain biochemical correlates of the plasma homocysteine level: a proton magnetic resonance spectroscopy study in the elderly subjects. Am. J. Geriatr. Psychiatry 19, 618–626 10.1097/JGP.0b013e318209ddf1 [DOI] [PubMed] [Google Scholar]
- Chiarani F., Tramontina J. F., Ceresér K. M., Kunz M., Paim L., Vargas C. R., et al. (2013). Homocysteine and other markers of cardiovascular risk during a manic episode in patients with bipolar disorder. Rev. Bras. Psiquiatr. 35, 157–160 10.1590/1516-4446-2012-0797 [DOI] [PubMed] [Google Scholar]
- Christie L. A., Riedel G., Algaidi S. A., Whalley L. J., Platt B. (2005). Enhanced hippocampal long-term potentiation in rats after chronic exposure to homocysteine. Neurosci. Lett. 373, 119–124 10.1016/j.neulet.2004.09.072 [DOI] [PubMed] [Google Scholar]
- Coppen A., Bailey J. (2000). Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J. Affect. Disord. 60, 121–130 10.1016/s0165-0327(00)00153-1 [DOI] [PubMed] [Google Scholar]
- Coppen A., Chaudhry S., Swade C. (1986). Folic acid enhances lithium prophylaxis. J. Affect. Disord. 10, 9–13 10.1016/0165-0327(86)90043-1 [DOI] [PubMed] [Google Scholar]
- de Jonge P., Alonso J., Stein D. J., Kiejna A., Aguilar-Gaxiola S., Viana M. C., et al. (2014). Associations between DSM-IV mental disorders and diabetes mellitus: a role for impulse control disorders and depression. Diabetologia 57, 699–709 10.1007/s00125-013-3157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delport D., Schoeman R., van der Merwe N., van der Merwe L., Fisher L. R., Geiger D., et al. (2014). Significance of dietary folate intake, homocysteine levels and MTHFR 677 C>T genotyping in South African patients diagnosed with depression: test development for clinical application. Metab. Brain Dis. 29, 377–384 10.1007/s11011-014-9506-7 [DOI] [PubMed] [Google Scholar]
- den Heijer T., Vermeer S. E., Clarke R., Oudkerk M., Koudstaal P. J., Hofman A., et al. (2003). Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126, 170–175 10.1093/brain/awg006 [DOI] [PubMed] [Google Scholar]
- Dias V. V., Brissos S., Cardoso C., Andreazza A. C., Kapczinski F. (2009). Serum homocysteine levels and cognitive functioning in euthymic bipolar patients. J. Affect. Disord. 113, 285–290 10.1016/j.jad.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A., Malinowska J., Olas B., Głowacki R., Bald E., Wachowicz B., et al. (2012). The oxidative stress may be induced by the elevated homocysteine in schizophrenic patients. Neurochem. Res. 37, 1057–1062 10.1007/s11064-012-0707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rocco A., Rogers J. D., Brown R., Werner P., Bottiglieri T. (2000). S-Adenosyl-Methionine improves depression in patients with Parkinson’s disease in an open-label clinical trial. Mov. Disord. 15, 1225–1229 [DOI] [PubMed] [Google Scholar]
- Dittmann S., Seemüller F., Grunze H. C., Schwarz M. J., Zach J., Fast K., et al. (2008). The impact of homocysteine levels on cognition in euthymic bipolar patients: a cross-sectional study. J. Clin. Psychiatry 69, 899–906 10.4088/jcp.v69n0603 [DOI] [PubMed] [Google Scholar]
- Dittmann S., Seemüller F., Schwarz M. J., Kleindienst N., Stampfer R., Zach J., et al. (2007). Association of cognitive deficits with elevated homocysteine levels in euthymic bipolar patients and its impact on psychosocial functioning: preliminary results. Bipolar Disord. 9, 63–70 10.1111/j.1399-5618.2007.00412.x [DOI] [PubMed] [Google Scholar]
- Dufouil C., Alpérovitch A., Ducros V., Tzourio C. (2003). Homocysteine, white matter hyperintensities and cognition in healthy elderly people. Ann. Neurol. 53, 214–221 10.1002/ana.10440 [DOI] [PubMed] [Google Scholar]
- Ebesunun M. O., Eruvulobi H. U., Olagunju T., Owoeye O. A. (2012). Elevated plasma homocysteine in association with decreased vitamin B(12), folate, serotonin, lipids and lipoproteins in depressed patients. Afr. J. Psychiatry (Johannesbg) 15, 25–29 10.4314/ajpsy.v15i1.3 [DOI] [PubMed] [Google Scholar]
- Ebly E. M., Schaefer J. P., Campbell N. R., Hogan D. B. (1998). Folate status, vascular disease and cognition in elderly Canadians. Age Ageing 27, 485–491 10.1093/ageing/27.4.485 [DOI] [PubMed] [Google Scholar]
- Ellingrod V. L., Taylor S. F., Dalack G., Grove T. B., Bly M. J., Brook R. D., et al. (2012). Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J. Clin. Psychopharmacol. 32, 261–265 10.1097/JCP.0b013e3182485888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E., Yeğin A., Yilmaz N., Herken H. (2010). Serum total homocystein, folate and vitamin B12 levels and their correlation with antipsychotic drug doses in adult male patients with chronic schizophrenia. Clin. Lab. 56, 513–518 [PubMed] [Google Scholar]
- Faux N. G., Ellis K. A., Porter L., Fowler C. J., Laws S. M., Martins R. N., et al. (2011). Homocysteine, vitamin B12 and folic acid levels in Alzheimer’s disease, mild cognitive impairment and healthy elderly: baseline characteristics in subjects of the Australian Imaging Biomarker Lifestyle study. J. Alzheimers Dis. 27, 909–922 10.3233/JAD-2011-110752 [DOI] [PubMed] [Google Scholar]
- Fava M., Borus J. S., Alpert J. E., Nierenberg A. A., Rosenbaum J. F., Bottiglieri T. (1997). Folate, vitamin B12 and homocysteine in major depressive disorder. Am. J. Psychiatry 154, 426–428 [DOI] [PubMed] [Google Scholar]
- Feng L., Ng T. P., Chuah L., Niti M., Kua E. H. (2006). Homocysteine, folate and vitamin B-12 and cognitive performance in older Chinese adults: findings from the Singapore Longitudinal ageing study. Am. J. Clin. Nutr. 84, 1506–1512 [DOI] [PubMed] [Google Scholar]
- Folstein M., Liu T., Peter I., Buell J., Arsenault L., Scott T., et al. (2007). The homocysteine hypothesis of depression. Am. J. Psychiatry 164, 861–867 10.1176/appi.ajp.164.6.861 [DOI] [PubMed] [Google Scholar]
- Ford A. H., Flicker L., Singh U., Hirani V., Almeida O. P. (2013). Homocysteine, depression and cognitive function in older adults. J. Affect. Disord. 151, 646–651 10.1016/j.jad.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Frankenburg F. R. (2007). The role of one-carbon metabolism in schizophrenia and depression. Harv. Rev. Psychiatry 15, 146–160 10.1080/10673220701551136 [DOI] [PubMed] [Google Scholar]
- Freeman J. M., Finkelstein J. D., Mudd S. H. (1975). Folate-responsive homocystinuria and “schizophrenia”. A defect in methylation due to deficient 5,10-methylenetetrahydrofolate reductase activity. N. Engl. J. Med. 292, 491–496 10.1056/nejm197503062921001 [DOI] [PubMed] [Google Scholar]
- Friso S., Choi S. W., Girelli D., Mason J. B., Dolnikowski G. G., Bagley P. J., et al. (2002). A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. U S A 99, 5606–5611 10.1073/pnas.062066299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryelewicz T., Styczynska M., Luczywek E., Barczak A., Pfeffer A., Androsiuk W., et al. (2007). The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int. J. Geriatr. Psychiatry 22, 563–567 10.1002/gps.1716 [DOI] [PubMed] [Google Scholar]
- Gao L., Zeng X. N., Guo H. M., Wu X. M., Chen H. J., Di R. K., et al. (2011). Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol. Sci. 33, 39–43 10.1007/s10072-011-0645-x [DOI] [PubMed] [Google Scholar]
- Garcia A., Haron Y., Pulman K., Hua L., Freedman M. (2004). Increases in homocysteine are related to worsening of stroop scores in healthy elderly persons: a prospective follow-up study. J. Gerontol. A Biol. Sci. Med. Sci. 59, 1323–1327 10.1093/gerona/59.12.1323 [DOI] [PubMed] [Google Scholar]
- García-Bueno B., Bioque M., Mac-Dowell K. S., Barcones M. F., Martínez-Cengotitabengoa M., Pina-Camacho L., et al. (2013). Pro-/Anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr. Bull. 40, 376–387 10.1093/schbul/sbt001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller V., Friger M., Sela B. A., Levine J. (2013). Elevated homocysteine level in siblings of patients with schizophrenia. Psychiatry Res. 210, 769–772 10.1016/j.psychres.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Genedani S., Agnati L. F., Leo G., Buzzega D., Maccari F., Carone C., et al. (2010). beta-Amyloid fibrillation and/or hyperhomocysteinemia modify striatal patterns of hyaluronic acid and dermatan sulfate: possible role in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 7, 150–157 10.2174/156720510790691074 [DOI] [PubMed] [Google Scholar]
- Gilbody S., Lewis S., Lightfoot T. (2007). Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am. J. Epidemiol. 165, 1–13 10.1093/aje/kwj347 [DOI] [PubMed] [Google Scholar]
- Goff D. C., Bottiglieri T., Arning E., Shih V., Freudenreich O., Evins A. E., et al. (2004). Folate, homocysteine and negative symptoms in schizophrenia. Am. J. Psychiatry 161, 1705–1708 10.1176/appi.ajp.161.9.1705 [DOI] [PubMed] [Google Scholar]
- Hill M., Shannahan K., Jasinski S., Macklin E. A., Raeke L., Roffman J. L., et al. (2011). Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr. Res. 127, 41–45 10.1016/j.schres.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Ho P. I., Ortiz D., Rogers E., Shea T. B. (2002). Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 70, 694–702 10.1002/jnr.10416 [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Coles M. G. (2002). The neural basis of human error processing: reinforcement learning, dopamine and the error-related negativity. Psychol. Rev. 109, 679–709 10.1037/0033-295x.109.4.679 [DOI] [PubMed] [Google Scholar]
- Hooshmand B., Solomon A., Kåreholt I., Rusanen M., Hänninen T., Leiviskä J., et al. (2012). Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. J. Intern. Med. 271, 204–212 10.1111/j.1365-2796.2011.02484.x [DOI] [PubMed] [Google Scholar]
- Ivorra J. L., Rivero O., Costas J., Iniesta R., Arrojo M., Ramos-Ríos R., et al. (2014). Replication of previous genome-wide association studies of psychiatric diseases in a large schizophrenia case-control sample from Spain. Schizophr. Res. [Epub ahead of print]. 10.1016/j.schres.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Jendricko T., Vidović A., Grubisić-Ilić M., Romić Z., Kovacić Z., Kozarić-Kovacić D. (2009). Homocysteine and serum lipids concentration in male war veterans with posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 134–140 10.1016/j.pnpbp.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Johnson W. G. (1999). DNA polymorphism-diet-cofactor-development hypothesis and the gene-teratogen model for schizophrenia and other developmental disorders. Am. J. Med. Genet. 88, 311–323 [DOI] [PubMed] [Google Scholar]
- Kale A., Naphade N., Sapkale S., Kamaraju M., Pillai A., Joshi S., et al. (2010). Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 175, 47–53 10.1016/j.psychres.2009.01.013 [DOI] [PubMed] [Google Scholar]
- Kalmijn S., Launer L. J., Lindemans J., Bots M. L., Hofman A., Breteler M. M. (1999). Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am. J. Epidemiol. 150, 283–289 10.1093/oxfordjournals.aje.a010000 [DOI] [PubMed] [Google Scholar]
- Kim G., Kim H., Kim K. N., Son J. I., Kim S. Y., Tamura T., et al. (2013). Relationship of cognitive function with B vitamin status, homocysteine and tissue factor pathway inhibitor in cognitively impaired elderly: a cross-sectional survey. J. Alzheimers Dis. 33, 853–862 10.3233/JAD-2012-121345 [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Numata S., Tajima A., Shimodera S., Imoto I., Ohmori T. (2013). Plasma total homocysteine is associated with DNA methylation in patients with schizophrenia. Epigenetics 8, 584–590 10.4161/epi.24621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klancnik J. M., Cuénod M., Gähwiler B. H., Jiang Z. P., Do K. Q. (1992). Release of endogenous amino acids, including homocysteic acid and cysteine sulphinic acid, from rat hippocampal slices evoked by electrical stimulation of Schaffer collateral-commissural fibres. Neuroscience 49, 557–570 10.1016/0306-4522(92)90226-r [DOI] [PubMed] [Google Scholar]
- Koz S. T., Gouwy N. T., Demir N., Nedzvetsky V. S., Etem E., Baydas G. (2010). Effects of maternal hyperhomocysteinemia induced by methionine intake on oxidative stress and apoptosis in pup rat brain. Int. J. Dev. Neurosci. 28, 325–329 10.1016/j.ijdevneu.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Kuo P. H., Chuang L. C., Liu J. R., Liu C. M., Huang M. C., Lin S. K., et al. (2014). Identification of novel loci for bipolar I disorder in a multi-stage genome-wide association study. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 58–64 10.1016/j.pnpbp.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Lancon C., Dassa D., Fernandez J., Richieri R., Padovani R., Boyer L. (2012). Are cardiovascular risk factors associated with verbal learning and memory impairment in patients with schizophrenia? A cross-sectional study. Cardiovasc. Psychiatry Neurol. 2012:204043 10.1155/2012/204043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T., Guha S., Liu C., Rosenfeld J., Mukherjee S., DeRosse P., et al. (2013). Genome-wide association study implicates NDST3 in schizophrenia and bipolar disorder. Nat. Commun. 4:2739 10.1038/ncomms3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Stahl Z., Sela B. A., Gavendo S., Ruderman V., Belmaker R. H. (2002). Elevated homocysteine levels in young male patients with schizophrenia. Am. J. Psychiatry 159, 1790–1792 10.1176/appi.ajp.159.10.1790 [DOI] [PubMed] [Google Scholar]
- Levine J., Timinsky I., Vishne T., Dwolatzky T., Roitman S., Kaplan Z., et al. (2008). Elevated serum homocysteine levels in male patients with PTSD. Depress. Anxiety 25, E154–E157 10.1002/da.20400 [DOI] [PubMed] [Google Scholar]
- Lewis S. J., Zammit S., Gunnell D., Smith G. D. (2005). A meta-analysis of the MTHFR C677T polymorphism and schizophrenia risk. Am. J. Med. Genet. B Neuropsychiatr. Genet. 135B, 2–4 10.1002/ajmg.b.30170 [DOI] [PubMed] [Google Scholar]
- Li C., Zhan G., Rao S., Zhang H. (2014). Metabolic syndrome and its factors affect cognitive function in chronic schizophrenia complicated by metabolic syndrome. J. Nerv. Ment. Dis. 202, 313–318 10.1097/nmd.0000000000000124 [DOI] [PubMed] [Google Scholar]
- Lindenmayer J. P., Khan A., Kaushik S., Thanju A., Praveen R., Hoffman L., et al. (2012). Relationship between metabolic syndrome and cognition in patients with schizophrenia. Schizophr. Res. 142, 171–176 10.1016/j.schres.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Kim W. K., Choi Y. B., Kumar S., D’Emilia D. M., Rayudu P. V., et al. (1997). Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. U S A 94, 5923–5928 10.1073/pnas.94.11.5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok A., Bockting C. L., Koeter M. W., Snieder H., Assies J., Mocking R. J., et al. (2013). Interaction between the MTHFR C677T polymorphism and traumatic childhood events predicts depression. Transl. Psychiatry 3:e288 10.1038/tp.2013.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok A., Mocking R. J., Assies J., Koeter M. W., Bockting C. L., de Vries G. J., et al. (2014). The one-carbon-cycle and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in recurrent major depressive disorder; influence of antidepressant use and depressive state? J. Affect. Disord. 166, 115–123 10.1016/j.jad.2014.04.048 [DOI] [PubMed] [Google Scholar]
- Loureiro S. O., Romão L., Alves T., Fonseca A., Heimfarth L., Moura Neto V., et al. (2010). Homocysteine induces cytoskeletal remodeling and production of reactive oxygen species in cultured cortical astrocytes. Brain Res. 1355, 151–164 10.1016/j.brainres.2010.07.071 [DOI] [PubMed] [Google Scholar]
- Lu M. L., Lin C. H., Chen Y. C., Yang H. C., Wu T. H. (2013). Determination of olanzapine and N-desmethyl-olanzapine in plasma using a reversed-phase HPLC coupled with coulochemical detection: correlation of olanzapine or N-desmethyl-olanzapine concentration with metabolic parameters. PLoS One 8:e65719 10.1371/journal.pone.0065719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin N. B., Niciu M. J., Luckenbaugh D. A., Ionescu D. F., Richards E. M., Vande Voort J. L., et al. (2014). Baseline vitamin B12 and folate levels do not predict improvement in depression after a single infusion of ketamine. Pharmacopsychiatry 47, 141–144 10.1055/s-0034-1377042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson H., Ellis K. A., Sali A., Pipingas A. (2012). Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: a randomized controlled trial. Psychopharmacology (Berl) 220, 351–365 10.1007/s00213-011-2481-3 [DOI] [PubMed] [Google Scholar]
- Mangoni A. A., Jackson S. H. (2002). Homocysteine and cardiovascular disease: current evidence and future prospects. Am. J. Med. 112, 556–565 10.1016/S0002-9343(02)01021-5 [DOI] [PubMed] [Google Scholar]
- Matté C., Pereira L. O., Dos Santos T. M., Mackedanz V., Cunha A. A., Netto C. A., et al. (2009). Acute homocysteine administration impairs memory consolidation on inhibitory avoidance task and decreases hippocampal brain-derived neurotrophic factor immunocontent: prevention by folic acid treatment. Neuroscience 163, 1039–1045 10.1016/j.neuroscience.2009.07.023 [DOI] [PubMed] [Google Scholar]
- McGuffin P., Knight J., Breen G., Brewster S., Boyd P. R., Craddock N., et al. (2005). Whole genome linkage scan of recurrent depressive disorder from the depression network study. Hum. Mol. Genet. 14, 3337–3345 10.1093/hmg/ddi363 [DOI] [PubMed] [Google Scholar]
- Misiak B., Frydecka D., Piotrowski P., Kiejna A. (2013). The multidimensional nature of metabolic syndrome in schizophrenia: lessons from studies of one-carbon metabolism and DNA methylation. Epigenomics 5, 317–329 10.2217/epi.13.22 [DOI] [PubMed] [Google Scholar]
- Misiak B., Frydecka D., Slezak R., Piotrowski P., Kiejna A. (2014). Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab. Brain Dis. 29, 671 10.1007/s11011-014-9567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. S. (2003). Homocysteine and Alzheimer’s disease. Lancet Neurol. 2, 425–428 10.1016/s1474-4422(03)00438-1 [DOI] [PubMed] [Google Scholar]
- Mühleisen T. W., Leber M., Schulze T. G., Strohmaier J., Degenhardt F., Treutlein J., et al. (2014). Genome-wide association study reveals two new risk loci for bipolar disorder. Nat. Commun. 5:3339 10.1038/ncomms4339 [DOI] [PubMed] [Google Scholar]
- Muntjewerff J. W., Kahn R. S., Blom H. J., den Heijer M. (2006). Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol. Psychiatry 11, 143–149 10.1038/sj.mp.4001746 [DOI] [PubMed] [Google Scholar]
- Narayan S. K., Saxby B. K., Firbank M. J., O’Brien J. T., Harrington F., Mckeith I. G., et al. (2011). Plasma homocysteine and cognitive decline in older hypertensive subjects. Int. Psychogeriatr. 23, 1607–1615 10.1017/s1041610211000779 [DOI] [PubMed] [Google Scholar]
- Naughton M., Clarke G., O’Leary O. F., Cryan J. F., Dinan T. G. (2014). A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J. Affect. Disord. 156, 24–35 10.1016/j.jad.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Nishi A., Numata S., Tajima A., Kinoshita M., Kikuchi K., Shimodera S., et al. (2014). Meta-analyses of blood homocysteine levels for gender and genetic association studies of the MTHFR C677T polymorphism in schizophrenia. Schizophr. Bull. 40, 1154–1163 10.1093/schbul/sbt154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk E., Refsum H., Tell G. S., Engedal K., Vollset S. E., Ueland P. M., et al. (2005). Plasma total homocysteine and memory in the elderly: the Hordaland Homocysteine Study. Ann. Neurol. 58, 847–857 10.1002/ana.20645 [DOI] [PubMed] [Google Scholar]
- Osher Y., Bersudsky Y., Silver H., Sela B. A., Belmaker R. H. (2008). Neuropsychological correlates of homocysteine levels in euthymic bipolar patients. J. Affect. Disord. 105, 229–233 10.1016/j.jad.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Peerbooms O. L., Van Os J., Drukker M., Kenis G., Hoogveld L., De Hert M., et al. (2011). Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav. Immun. 25, 1530–1543 10.1016/j.bbi.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Penninx B. W., Guralnik J. M., Ferrucci L., Fried L. P., Allen R. H., Stabler S. P. (2000). Vitamin B(12) deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. Am. J. Psychiatry 157, 715–721 10.1176/appi.ajp.157.5.715 [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A., Dmitrzak-Weglarz M., Hauser J., Rybakowski J. K. (2014a). Are genes connected with homocysteine metabolism associated with bipolar disorder? Neuropsychobiology 69, 107–111 10.1159/000358091 [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A., Dorszewska J., Bartkowska-Sniatkowska A., Chlopocka-Wozniak M., Rybakowski J. K. (2013a). Vitamin B12 level may be related to the efficacy of single ketamine infusion in bipolar depression. Pharmacopsychiatry 46, 227–228 10.1055/s-0033-1349861 [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A., Dorszewska J., Rybakowski J. (2014b). The concentration of homocysteine and treatment of depression in affective disorders. Farmakoter Psychiatr. Neurol. 1, 15–20 [Google Scholar]
- Permoda-Osip A., Dorszewska J., Skibinska M., Chlopocka-Wozniak M., Rybakowski J. K. (2013b). Hyperhomocysteinemia in bipolar depression: clinical and biochemical correlates. Neuropsychobiology 68, 193–196 10.1159/000355292 [DOI] [PubMed] [Google Scholar]
- Perna A. F., Ingrosso D., De Santo N. G. (2003). Homocysteine and oxidative stress. Amino Acids 25, 409–417 10.1007/s00726-003-0026-8 [DOI] [PubMed] [Google Scholar]
- Petronijević N. D., Radonjić N. V., Ivković M. D., Marinković D., Piperski V. D., Duricić B. M., et al. (2008). Plasma homocysteine levels in young male patients in the exacerbation and remission phase of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1921–1926 10.1016/j.pnpbp.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Pirchl M., Ullrich C., Humpel C. (2010). Differential effects of short- and long-term hyperhomocysteinaemia on cholinergic neurons, spatial memory and microbleedings in vivo in rats. Eur. J. Neurosci. 32, 1516–1527 10.1111/j.1460-9568.2010.07434.x [DOI] [PubMed] [Google Scholar]
- Prins N. D., Den Heijer T., Hofman A., Koudstaal P. J., Jolles J., Clarke R., et al. (2002). Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology 59, 1375–1380 10.1212/01.wnl.0000032494.05619.93 [DOI] [PubMed] [Google Scholar]
- Rajagopalan P., Hua X., Toga A. W., Jack C. R., Jr., Weiner M. W., Thompson P. M. (2011). Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport 22, 391–395 10.1097/wnr.0b013e328346bf85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regland B., Johansson B. V., Grenfeldt B., Hjelmgren L. T., Medhus M. (1995). Homocysteinemia is a common feature of schizophrenia. J. Neural Transm. Gen. Sect. 100, 165–169 10.1007/bf01271539 [DOI] [PubMed] [Google Scholar]
- Ripke S., Neale B. M., Corvin A., Walters J. T. R., Farh K.-H., Holmans P. A., et al. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S., O’dushlaine C., Chambert K., Moran J. L., Kähler A. K., Akterin S., et al. (2013). Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 45, 1150–1159 10.1038/ng.2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J. L., Brohawn D. G., Friedman J. S., Dyckman K. A., Thakkar K. N., Agam Y., et al. (2011a). MTHFR 677C>T effects on anterior cingulate structure and function during response monitoring in schizophrenia: a preliminary study. Brain Imaging Behav. 5, 65–75 10.1007/s11682-010-9111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J. L., Gollub R. L., Calhoun V. D., Wassink T. H., Weiss A. P., Ho B. C., et al. (2008a). MTHFR 677C –> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val –> Met. Proc. Natl. Acad. Sci. U S A 105, 17573–17578 10.1073/pnas.0803727105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J. L., Lamberti J. S., Achtyes E., Macklin E. A., Galendez G. C., Raeke L. H., et al. (2013). Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry 70, 481–489 10.1001/jamapsychiatry.2013.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J. L., Nitenson A. Z., Agam Y., Isom M., Friedman J. S., Dyckman K. A., et al. (2011b). A hypomethylating variant of MTHFR, 677C>T, blunts the neural response to errors in patients with schizophrenia and healthy individuals. PLoS One 6:e25253 10.1371/journal.pone.0025253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J. L., Weiss A. P., Deckersbach T., Freudenreich O., Henderson D. C., Purcell S., et al. (2007). Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr. Res. 92, 181–188 10.1016/j.schres.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Roffman J. L., Weiss A. P., Deckersbach T., Freudenreich O., Henderson D. C., Wong D. H., et al. (2008b). Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 990–995 10.1002/ajmg.b.30684 [DOI] [PubMed] [Google Scholar]
- Sachdev P. S., Valenzuela M., Wang X. L., Looi J. C., Brodaty H. (2002). Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology 58, 1539–1541 10.1212/wnl.58.10.1539 [DOI] [PubMed] [Google Scholar]
- Saito T., Kondo K., Iwayama Y., Shimasaki A., Aleksic B., Yamada K., et al. (2014). Replication and cross-phenotype study based upon schizophrenia GWASs data in the Japanese population: support for association of MHC region with psychosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 165B, 421–427 10.1002/ajmg.b.32246 [DOI] [PubMed] [Google Scholar]
- Scott J. M., Weir D. G. (1998). Folic acid, homocysteine and one-carbon metabolism: a review of the essential biochemistry. J. Cardiovasc. Risk 5, 223–227 10.1177/174182679800500403 [DOI] [PubMed] [Google Scholar]
- Shen X., Wu Y., Guan T., Wang X., Qian M., Lin M., et al. (2014). Association analysis of COMT/MTHFR polymorphisms and major depressive disorder in Chinese Han population. J. Affect. Disord. 161, 73–78 10.1016/j.jad.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Shi J., Gershon E. S., Liu C. (2008). Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr. Res. 104, 96–107 10.1016/j.schres.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S. D., Carney M. W., Chanarin I., Reynolds E. H. (1980). The neuropsychiatry of megaloblastic anaemia. Br. Med. J. 281, 1036–1038 10.1136/bmj.281.6247.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P., Ripke S., Scott L. J., Andreassen O. A., Cichon S., Craddock N., et al. (2011). Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977–983 10.1038/ng.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger O., Fowler B., Piertzik K., Huemer M., Haschke-Becher E., Semmler A., et al. (2009). Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev. Neurother. 9, 1393–1412 10.1586/ern.09.75 [DOI] [PubMed] [Google Scholar]
- Streck E. L., Bavaresco C. S., Netto C. A., Wyse A. T. (2004). Chronic hyperhomocysteinemia provokes a memory deficit in rats in the Morris water maze task. Behav. Brain Res. 153, 377–381 10.1016/j.bbr.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Streck E. L., Delwing D., Tagliari B., Matte C., Wannmacher C. M., Wajner M., et al. (2003). Brain energy metabolism is compromised by the metabolites accumulating in homocystinuria. Neurochem. Int. 43, 597–602 10.1016/s0197-0186(02)00230-9 [DOI] [PubMed] [Google Scholar]
- Tan H. Y., Callicott J. H., Weinberger D. R. (2007). Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb. Cortex 17(Suppl 1), i171–181 10.1093/cercor/bhm069 [DOI] [PubMed] [Google Scholar]
- Teunissen C. E., Blom A. H., Van Boxtel M. P., Bosma H., De Bruijn C., Jolles J., et al. (2003). Homocysteine: a marker for cognitive performance? A longitudinal follow-up study. J. Nutr. Health Aging 7, 153–159 [PubMed] [Google Scholar]
- Trimmer E. E. (2013). Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Curr. Pharm. Des. 19, 2574–2593 10.2174/1381612811319140008 [DOI] [PubMed] [Google Scholar]
- van den Kommer T. N., Dik M. G., Comijs H. C., Jonker C., Deeg D. J. (2010). Homocysteine and inflammation: predictors of cognitive decline in older persons? Neurobiol. Aging 31, 1700–1709 10.1016/j.neurobiolaging.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Vermeer S. E., Van Dijk E. J., Koudstaal P. J., Oudkerk M., Hofman A., Clarke R., et al. (2002). Homocysteine, silent brain infarcts and white matter lesions: the Rotterdam scan study. Ann. Neurol. 51, 285–289 10.1002/ana.10111 [DOI] [PubMed] [Google Scholar]
- Vuksan-Ćusa B., Jakovljević M., Šagud M., Mihaljević Peleš A., Marčinko D., Topić R., et al. (2011). Metabolic syndrome and serum homocysteine in patients with bipolar disorder and schizophrenia treated with second generation antipsychotics. Psychiatry Res. 189, 21–25 10.1016/j.psychres.2010.11.021 [DOI] [PubMed] [Google Scholar]
- Vuksan-Ćusa B., Šagud M., Jakovljević M., Peleš A. M., Jaksic N., Mihaljević S., et al. (2013). Association between C-reactive protein and homocysteine with the subcomponents of metabolic syndrome in stable patients with bipolar disorder and schizophrenia. Nord. J. Psychiatry 67, 320–325 10.3109/08039488.2012.745601 [DOI] [PubMed] [Google Scholar]
- Wang J., Bai X., Chen Y., Zhao Y., Liu X. (2012). Homocysteine induces apoptosis of rat hippocampal neurons by inhibiting 14–3-3epsilon expression and activating calcineurin. PLoS One 7:e48247 10.1371/journal.pone.0048247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. L., Ding X. X., Sun Y. H., Yang H. Y., Chen J., Zhao X., et al. (2013). Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 78–85 10.1016/j.pnpbp.2013.06.015 [DOI] [PubMed] [Google Scholar]
- Wysokiński A., Kłoszewska I. (2013). Homocysteine levels in patients with schizophrenia on clozapine monotherapy. Neurochem. Res. 38, 2056–2062 10.1007/s11064-013-1113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Cohen-Woods S., Chen Q., Noor A., Knight J., Hosang G., et al. (2014). Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med. Genet. 15:2 10.1186/1471-2350-15-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapislar H., Aydogan S., Ozüm Ü. (2012). Biological understanding of the cardiovascular risk associated with major depression and panic disorder is important. Int. J. Psychiatry Clin. Pract. 16, 27–32 10.3109/13651501.2011.620127 [DOI] [PubMed] [Google Scholar]
- Yoshimi A., Aleksic B., Kawamura Y., Takahashi N., Yamada S., Usui H., et al. (2010). Gene-wide association study between the methylenetetrahydrofolate reductase gene (MTHFR) and schizophrenia in the Japanese population, with an updated meta-analysis on currently available data. Schizophr. Res. 124, 216–222 10.1016/j.schres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Yoshino K., Nishide M., Sankai T., Inagawa M., Yokota K., Moriyama Y., et al. (2010). Validity of brief food frequency questionnaire for estimation of dietary intakes of folate, vitamins B6 and B12 and their associations with plasma homocysteine concentrations. Int. J. Food Sci. Nutr. 61, 61–67 10.3109/09637480903286363 [DOI] [PubMed] [Google Scholar]
- Zhang D., Lipton S. A. (1992). L-homocysteic acid selectively activates N-methyl-D-aspartate receptors of rat retinal ganglion cells. Neurosci. Lett. 139, 173–177 10.1016/0304-3940(92)90545-i [DOI] [PubMed] [Google Scholar]