Abstract
Background:
Implant-based reconstruction is the most frequently performed breast reconstruction procedure. A persistent issue with this approach is optimizing outcomes in the setting of radiotherapy. Experimental evidence suggests that acellular dermal matrix use may provide a protective benefit, but clinical evidence is lacking. The purpose of this study was to assess postoperative complications and the effect of radiotherapy on complications and outcomes in women who underwent immediate, porcine acellular dermal matrix (PADM, Strattice)-assisted, implant-based breast reconstruction postmastectomy.
Methods:
Patients with at least 1 year of follow-up were included in this retrospective study. Patient charts were reviewed for demographic data, adjunctive therapy use, duration of follow-up, and type and incidence of complications during follow-up.
Results:
A total of 158 reconstructions were performed in 103 patients. Adjuvant therapy included chemotherapy in 51% of patients and radiotherapy in 25% of breasts. Mean follow-up was 36.2 months. Complications occurred in 17 breasts (10.8%): implant/expander loss (8.2%); infection (5.7%); dehiscence (3.8%); eschar (1.9%); and ischemia, hematoma, and seroma (0.6% each). Nine breasts with complications had been irradiated; all were irradiated prereconstruction. Rate of total complications, implant/expander loss, and dehiscence was significantly higher in irradiated breasts. Breasts irradiated postreconstruction had no complications.
Conclusions:
Addition of PADM to implant-based reconstruction is associated with acceptable complication rates comparable to those observed with standard submuscular reconstructions. Complications are increased in the setting of radiotherapy; but PADM use may protect against the adverse effects of postreconstruction radiotherapy.
Implant-based reconstruction, either a 2-stage expander/implant or a single-stage direct-to-implant approach, is currently the most frequently performed breast reconstruction procedure; almost 70% of breast reconstructions are performed using this approach.1 Advances in surgical techniques (skin-sparing and nipple-sparing mastectomy as well as the dual-plane technique) and implant technology have made excellent outcomes possible with implant-based reconstruction in appropriately selected patients. The shorter operative procedure without donor site morbidity and a quicker recovery have also made this procedure an attractive alternative to autologous procedures.
The introduction of acellular dermal matrices (ADMs) has further facilitated implant-based breast reconstruction. Placement of ADM at the inferolateral pole to provide reinforcement of the expander or implant pocket contributes to defining the inframammary and lateral mammary folds, prevents window shading of the pectoralis major muscle, and allows for improved implant positioning as compared with the complete submuscular coverage technique.2–5 In 2-stage procedures, the use of ADMs also allows for greater intraoperative expansion, thereby reducing the total number of fills and the time to complete tissue expansion.5–7 The technique of using ADM for lower pole reinforcement was first conceived with the use of AlloDerm (LifeCell Corporation, Branchburg, N.J.), a human ADM, about a decade ago2–5; since then, a variety of ADMs have become available and are used for this purpose. About half of all implant-based reconstructions are now being performed with ADM assistance.8
Despite the advances in implant-based breast reconstruction, a persistent issue with this approach is the optimization of outcomes when performed in conjunction with radiotherapy. Both pre- and postreconstruction radiotherapy can lead to significant increases in complications (infection, skin necrosis, and capsular contracture) and poor outcomes (including reconstructive failure).9–12 When the indication for radiotherapy is known preoperatively, the tendency is to delay reconstruction to after completion of radiotherapy and to use an autologous flap procedure as the reconstructive option. Patients, on the contrary, may not be amenable to delaying reconstruction or undergoing a flap procedure. Thus, there is a need to ameliorate the outcome of implant-based reconstruction in the setting of radiotherapy. Experimental evidence suggests that ADMs may provide a protective effect on breasts with implants that are irradiated after implantation.13 Furthermore, the deleterious effect of irradiation does not seem to affect the viability and integration of ADM implanted in an irradiated field or when irradiated after implantation.14,15 Clinical data on the use of ADM in conjunction with radiotherapy is, however, sparse16,17, and no definitive conclusions can be made regarding the protective effect of ADM, if any.
The purpose of this study was to assess the postoperative complications associated with the use of a porcine acellular dermal matrix (PADM, Strattice; LifeCell Corporation) in implant-based breast reconstruction and, in particular, to assess the complications and outcomes of patients who had received radiotherapy. The effect of the timing of radiotherapy on outcomes was also assessed.
PATIENTS AND METHODS
Patients who underwent immediate, PADM-assisted, implant-based breast reconstruction (2-stage or single-stage) postmastectomy in the author’s practice between January 2009 and May 2013 with at least 1 year of follow-up (from the date of surgery) were included in this study. Patients who had prior breast reconstructive surgery or augmentation mammaplasty or those who had implant-based flap procedures were excluded. This is a single-center, single-surgeon study that was approved by the local institutional review board.
Single-stage or 2-stage breast reconstructive surgery with PADM assistance was performed in a similar manner to those described with the use of human ADM.2–5 In the author’s opinion, the technical details of importance for a successful outcome are aggressive antibiotic irrigation of the mastectomy pocket, adequate intraoperative expansion, and adequate drainage. After creation of a subpectoral pocket for the implant or tissue expander, copious antibiotic (bacitracin in saline) irrigation of the breast pocket was performed with a triple antibiotic solution. The implant or expander was also rinsed with the antibiotic solution before introduction into the breast pocket. This strict antibiotic protocol is important to minimize the risk of postoperative infection and the chronic inflammatory response that infection might cause.18 When tissue expanders were used, care was exercised not to overexpand intraoperatively as this would place undue stress on the overlying upper mastectomy flap and increase the risk of ischemia and skin necrosis. At the same time, adequate intraoperative expansion was ensured such that PADM placed at the lower breast pole was in direct contact with the lower mastectomy flap to allow for recellularization and revascularization of the matrix. In both single-stage and 2-stage procedures, one drain each was placed in the retropectoral and subcutaneous spaces to ensure adequate drainage to reduce the risk of seroma formation.
Patient records were reviewed retrospectively. Preoperative demographic data, including age, body mass index, and comorbid conditions known to influence postoperative outcomes (obesity and tobacco use),19 were collected. The duration of follow-up and adjunctive therapy use (chemotherapy or radiotherapy) and its timing (prereconstruction or postreconstruction) were recorded. All postoperative complications (type and incidence) that were recorded during the follow-up period were analyzed. Comparisons of complications in irradiated vs nonirradiated breasts were performed using Fisher’s exact test. Statistical significance was set at a P-value of <0.05. Prereconstruction radiotherapy was performed before mastectomy as part of breast conserving surgery. Postreconstruction radiotherapy was performed after tissue expansion in those undergoing a 2-stage procedure or after implant placement in those undergoing a single-stage procedure.
RESULTS
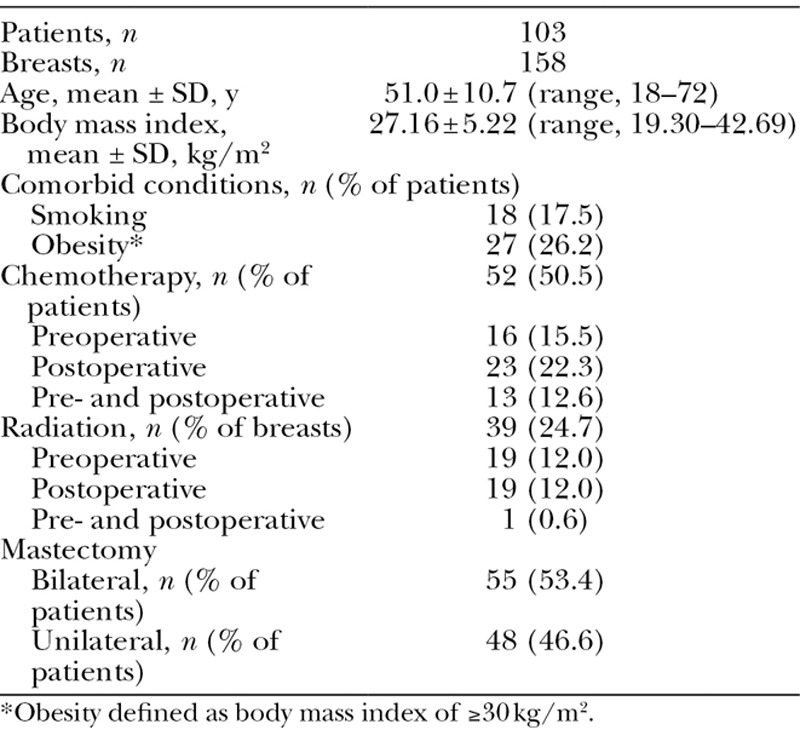
A total of 103 patients met the inclusion criteria. Baseline demographic and clinical characteristics of these patients are listed in Table 1. Of note, 27% of the patients were obese and 18% used tobacco. A total of 158 reconstructions, 48 unilateral and 55 bilateral, were performed. About half of the patients received chemotherapy (16% preoperatively, 22% postoperatively, and 13% both pre- and postoperatively) and a quarter of the breasts had been irradiated (12% preoperatively, 12% postoperatively, and 1% pre- and postoperatively).
Table 1.
Patient Demographics, Adjuvant Therapy, and Mastectomy Procedures Performed
Patients were followed for a minimum of 1 year (mean, 36.2 ± 7.6 mo; range, 23.0–50.4 mo) from initial surgery. During this period, complications occurred in 17 breasts for a total complication rate of 10.8% in this series. The clinical characteristics of the patients and their complications are described in Table 2. The incidence of individual complications and the overall rate of complications in the study population are provided in Table 3. Complications included 13 cases of implant/expander loss (8.2%), 9 cases of infection (5.7%), 6 cases of dehiscence (3.8%), 3 cases of incision-site eschar (1.9%), and 1 case each of ischemia, hematoma, and seroma (0.6% each) (Table 3). Of the 13 cases of implant/expander loss, 8 were secondary to infection (7 with methicillin-resistant Staphylococcus aureus and 1 with Pseudomonas aeruginosa), 3 were secondary to dehiscence, 1 was secondary to infection (with methicillin-resistant S. aureus) and dehiscence, and 1 was secondary to multiple ischemic episodes. Two cases of dehiscence were successfully reoperated and repaired without further issues. Of the 17 breasts with complications, 9 had been irradiated. Compared with nonirradiated breasts, the rate of total complications and implant/expander loss and dehiscence were significantly higher in irradiated breasts (Table 3). All 9 irradiated breasts with complications were irradiated before reconstruction. There were no complications in breasts that had been irradiated postreconstruction (Table 4). Interestingly, the patient who was irradiated both prereconstruction and postreconstruction had no complications.
Table 2.
Clinical Characteristics and Complications of Patients
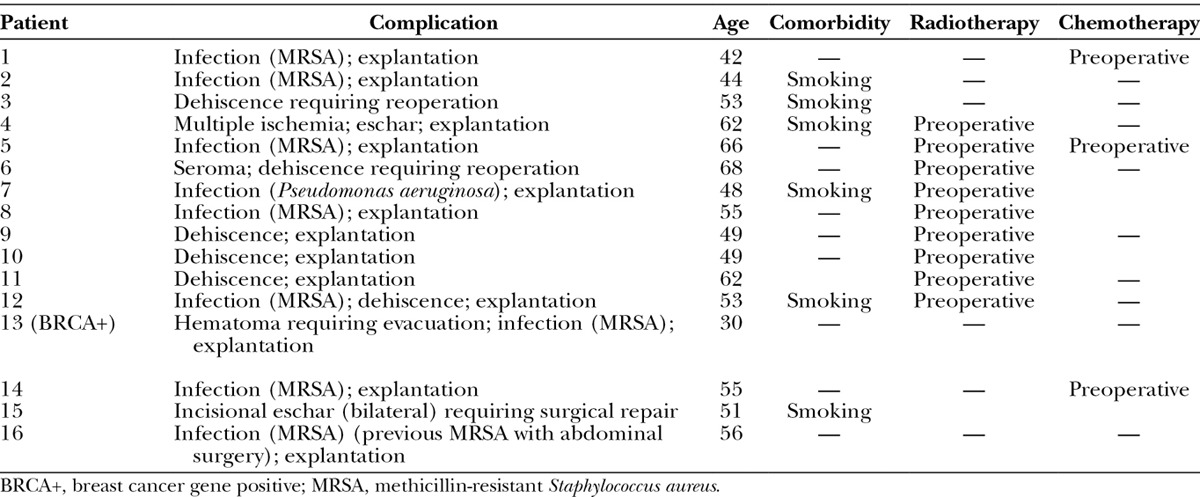
Table 3.
Complications Stratified by Radiation Status
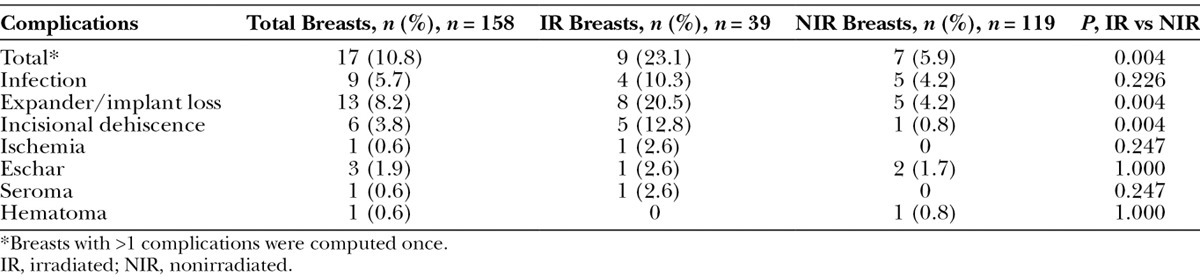
Table 4.
Complications Stratified by Timing of Radiotherapy

DISCUSSION
PADM is a relatively recent addition to the growing list of ADM products available for use in breast surgery. It offers ease of use after a 2-minute soak in saline solution with no polarity and is available in large sizes. Clinical experience with its use in breast reconstruction includes 4 dedicated series in the published literature.20–23 Israeli and Feingold20 performed PADM-assisted 2-stage reconstructions in 44 patients and reported an overall complication rate of 16.9% after stage 1 reconstruction (77 reconstructions) and 10.4% after stage 2 reconstruction (67 reconstructions). Stage 1 complications included tissue expander loss (13%), infection (10.4%), skin necrosis (7.8%), seroma (5.2%), and hematoma (1.3%). Stage 2 complications during a follow-up period of 8–345 days included infection (3%), seroma (3%), implant loss (3%), and capsular contracture (grade III/IV, 4.5%). In 27 single-stage breast reconstructions, Himsl et al21 reported that the use of PADM was associated with predictable and acceptable esthetic and haptic outcomes with reduced risks of fibrosis and reconstructive failure. Complications in this series included 2 cases each of skin complications and fibrosis (capsular contracture ranking was no higher than Baker grade I) and 1 case each of implant malposition and soft-tissue thinning at 6 months of follow-up. Glasberg and Light22 used PADM in 144 2-stage reconstructions and reported consistent outcomes with its use. The overall complication rate in this series was 6.3% (including infection/cellulitis 2.1%, expander loss 1.4%, flap necrosis 1.4%, and seroma 1.4%) with an absence of clinically significant capsular contracture (Baker grade III/IV) at a mean follow-up of 14 months.22 Salzberg et al23 used PADM in 105 predominantly single-stage reconstructions and reported low complication rates (total complication, 8.6%) and good outcomes. Furthermore, with a mean follow-up of 41.4 months, they reported no incidence of long-term complications, such as capsular contracture or implant malposition.
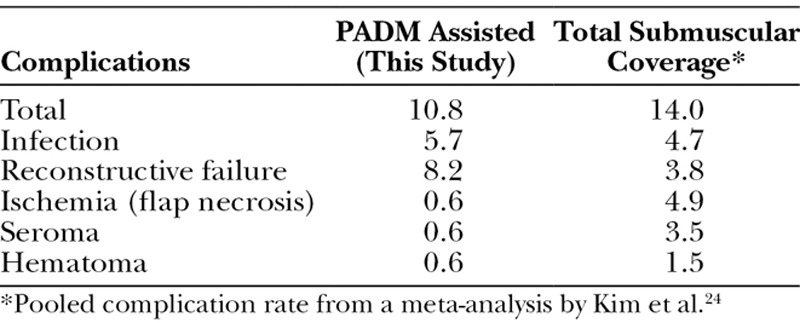
In this study, PADM was used in 158 reconstructions (single- and 2-stage). The overall complication rate of 10.8% is comparable to those reported by Glasberg and Light22 and Israeli and Feingold20 (second stage) in their series of PADM-assisted reconstructions, thus supporting the observation that consistent outcomes can be obtained with the use of PADM. Furthermore, the complication rates in the present series also compare favorably with those reported with standard breast reconstruction using the total submuscular coverage technique24 (Table 5). The comparable complication rate between these 2 approaches is reassuring and indicates that the use of Strattice does not elevate complications beyond those that are to be expected with implant-based breast reconstruction. The only exception was reconstructive failure which was about 8.2% in this series; the high rate seems to be associated with the use of adjuvant radiotherapy. In nonirradiated breasts, reconstructive failure was 4.2%.
Table 5.
Complications Associated with PADM-Assisted Reconstructions Compared with Standard Reconstructions via the Total Submuscular Coverage Technique
The adjunctive use of radiotherapy in implant-based breast reconstruction has consistently been shown to be associated with increased postoperative complications and poor outcomes, both historically and in the current era, despite improvements in radiotherapy delivery methods and protocols and in implant designs.9–12,25 Radiotherapy adversely affects the skin causing thickening and fibrosis and alters hair, sweat, and sebaceous gland function required for reepithelialization and wound repair, thus compromising wound healing.26,27 Consequently, complications of wound dehiscence, infection, reconstructive failure, and capsular contracture are elevated in the setting of radiotherapy. Whether the use of ADM is able to minimize the effects of radiotherapy is unclear given the lack of clinical data. However, experimental data suggest that human ADM decreases radiation-related inflammation and delays or diminishes pseudoepithelium formation around capsules, which is a precursor to the formation of a fibrotic capsule in humans.13
In this study, wound dehiscence and expander/implant loss were found to be significantly higher in irradiated vs nonirradiated breasts, which is consistent with the adverse effects of radiotherapy on skin and wound healing. In addition, the infection rate was also elevated in irradiated breasts although this did not reach statistical significance. Others have also reported higher complications in breasts reconstructed with ADMs in the setting of radiotherapy compared with no radiotherapy.5,16,20,28–32
Analysis of the data by timing of radiotherapy suggests that PADM may protect against postreconstruction radiotherapy. None of the breasts that were irradiated postreconstruction were found to have complications in this series. Of most significance, physical examination of these patients revealed irradiated reconstructed breasts that seemed symmetrical to the nonirradiated reconstructed contralateral breasts and similar in pliability and texture. In contrast to postreconstruction irradiated breasts, half of breasts (9 of 19) that had been irradiated prereconstruction had complications. The dehiscence rate of 26% and failure rate of 42% in breast irradiated prereconstruction in the present study are comparable to those reported in breasts that had been irradiated prereconstruction and then underwent standard submuscular reconstructions (dehiscence 22% and reconstructive failure 40%).9,10 Albeit a small sample, these data support the paradigm that implant-based breast reconstruction is better avoided in patients who have undergone premastectomy radiotherapy and that an autologous procedure would be more appropriate.33
The absence of complications in patients who had received postreconstruction radiotherapy is a significant finding in this study because previous studies suggested that the timing of radiotherapy with respect to ADM placement did not impact the complication rate and that in general complications were higher in irradiated vs nonirradiated patients.16,31 However, similar to our study, a recent study by Seth et al34 also noted that ADM may be advantageous in patients undergoing postoperative radiation therapy. They reported no significance difference in complication rates between nonirradiated and irradiated patients (irradiated during tissue expansion stage) who underwent breast reconstruction with ADM assistance, but complication rates were significantly higher in irradiated patients who underwent standard reconstruction without ADM assistance compared with nonirradiated patients. Because we did not have non-PADM control groups, which is a limitation of this study, we reviewed published studies to assess the complications in prereconstruction and postreconstruction irradiated breasts that had been reconstructed using the standard submuscular approach. Nava et al11 noted a 40% failure rate with postreconstruction radiation therapy on tissue expanders and a 6.4% failure rate with postreconstruction radiation therapy on permanent implants vs a 2.3% failure rate in nonirradiated breasts. Lin et al10 reported a 43.8% complication rate with prereconstruction irradiation, a 41.2% complication rate with irradiation during reconstruction, and a 13.8% complication rate with no radiation exposure. Both of these recent studies indicate a high incidence of complications and reconstructive failure when radiotherapy is delivered postreconstruction, which is in contrast to the absence of complication with the use of PADM in this study and further supports the observation of a possible protective benefit with the use of PADM with postreconstruction radiation. The reason for the protective effect of PADM in postreconstruction but not prereconstruction radiotherapy remains to be elucidated, but it could be related to differences in the extent of revascularization and repopulation of PADM in the 2 settings. This, however, cannot be verified because histologic analyses of implanted PADM were not performed, which is another limitation of this study.
CONCLUSIONS
This study demonstrates that the addition of porcine acellular dermal matrix to implant-based reconstructions is associated with acceptable complication rates that are comparable to those observed in standard reconstructions using the submuscular approach. In the setting of adjunctive radiotherapy, complications, especially infection, dehiscence, and reconstructive failure, are increased in accordance with the adverse effects of radiotherapy on the skin and wound healing. However, it seems that porcine acellular dermal matrix may protect against the adverse effects of postreconstruction radiotherapy. There were no complications in breasts that had been irradiated postreconstruction in this series. Further controlled studies are needed to elucidate this finding.
Footnotes
Disclosure: Dr. Mitchell has received noncompensatory research support from LifeCell Corporation. The Article Processing Charge was paid for by LifeCell Corporation.
REFERENCES
- 1.American Society of Plastic Surgeons. 2012 Plastic Surgery Statistics Report Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2012-Plastic-Surgery-Statistics/full-plastic-surgery-statistics-report.pdf [Google Scholar]
- 2.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. doi: 10.1097/01.sap.0000168527.52472.3c. [DOI] [PubMed] [Google Scholar]
- 3.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 4.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 6.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 7.Hanna KR, DeGeorge BR, Jr, Mericli AF, et al. Comparison study of two types of expander-based breast reconstruction: acellular dermal matrix-assisted versus total submuscular placement. Ann Plast Surg. 2013;70:10–15. doi: 10.1097/SAP.0b013e31822f6765. [DOI] [PubMed] [Google Scholar]
- 8.Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg. 2013;70:103–110. doi: 10.1097/SAP.0b013e31822ed5ce. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plast Reconstr Surg. 2012;129:354–361. doi: 10.1097/PRS.0b013e31823ae8b1. [DOI] [PubMed] [Google Scholar]
- 10.Lin KY, Blechman AB, Brenin DR. Implant-based, two-stage breast reconstruction in the setting of radiation injury: an outcome study. Plast Reconstr Surg. 2012;129:817–823. doi: 10.1097/PRS.0b013e31824421d0. [DOI] [PubMed] [Google Scholar]
- 11.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 12.Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130:513e–523e. doi: 10.1097/PRS.0b013e318262f059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg. 2009;123:807–816. doi: 10.1097/PRS.0b013e318199eef3. [DOI] [PubMed] [Google Scholar]
- 14.Dubin MG, Feldman M, Ibrahim HZ, et al. Allograft dermal implant (AlloDerm) in a previously irradiated field. Laryngoscope. 2000;110:934–937. doi: 10.1097/00005537-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim HZ, Kwiatkowski TJ, Montone KT, et al. Effects of external beam radiation on the allograft dermal implant. Otolaryngol Head Neck Surg. 2000;122:189–194. doi: 10.1016/S0194-5998(00)70237-3. [DOI] [PubMed] [Google Scholar]
- 16.Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg. 2012;130:1–9. doi: 10.1097/PRS.0b013e3182547a45. [DOI] [PubMed] [Google Scholar]
- 17.Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg. 2012;130(5 Suppl 2):27S–34S. doi: 10.1097/PRS.0b013e318265f690. [DOI] [PubMed] [Google Scholar]
- 18.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. doi: 10.1097/PRS.0b013e3181e3b456. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 20.Israeli R, Feingold RS. Acellular dermal matrix in breast reconstruction in the setting of radiotherapy. Aesthet Surg J. 2011;31(7 Suppl):51S–64S. doi: 10.1177/1090820X11418089. [DOI] [PubMed] [Google Scholar]
- 21.Himsl I, Drinovac V, Lenhard M, et al. The use of porcine acellular dermal matrix in silicone implant-based breast reconstruction. Arch Gynecol Obstet. 2012;286:187–192. doi: 10.1007/s00404-012-2266-x. [DOI] [PubMed] [Google Scholar]
- 22.Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg. 2012;129:1223–1233. doi: 10.1097/PRS.0b013e31824ec429. [DOI] [PubMed] [Google Scholar]
- 23.Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg. 2013;66:323–328. doi: 10.1016/j.bjps.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 25.Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930–942. doi: 10.1097/00006534-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein EF, Sullivan FJ, Mitchell JB, et al. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993;20:435–453. [PubMed] [Google Scholar]
- 27.Burns JL, Mancoll JS, Phillips LG. Impairments to wound healing. Clin Plast Surg. 2003;30:47–56. doi: 10.1016/s0094-1298(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg. 2006;56:22–25. doi: 10.1097/01.sap.0000185460.31188.c1. [DOI] [PubMed] [Google Scholar]
- 29.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. doi: 10.1097/SAP.0b013e31802f8426. [DOI] [PubMed] [Google Scholar]
- 31.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen M-D, Chen C, Colakoğlu S, et al. Infectious complications leading to explantation in implant-based breast reconstruction with AlloDerm. Eplasty. 2010;10:404–411. [PMC free article] [PubMed] [Google Scholar]
- 33.Roostaeian J, Crisera C. Current options in breast reconstruction with or without radiotherapy. Curr Opin Obstet Gynecol. 2011;23:44–50. doi: 10.1097/GCO.0b013e328340e18a. [DOI] [PubMed] [Google Scholar]
- 34.Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg. 2012;130:750–758. doi: 10.1097/PRS.0b013e318262f009. [DOI] [PubMed] [Google Scholar]


