Abstract
Summary:
Fat graft breast reconstruction following a mastectomy is always limited by the size of the skin envelope, which affects the amount of graft that can be injected in 1 session. Because the fat graft naturally resorbs in all patients, several sessions of fat grafting are necessary. BRAVA’s negative pressure causes a “reverse” expansion of the skin envelope, thus permitting more space for the fat graft. This allows decreasing number of required procedures for an adequate breast reconstruction. We operated on a 38-year-old patient 4 years after bilateral mastectomy without irradiation for breast cancer. Before the procedure, the patient was instructed to wear the BRAVA system for 12 hours daily for 2 months before the first session, at all times between the sessions and for 1 month following the last fat grafting session. We performed 3 fat grafting sessions, as planned. Altogether, we injected 840 cm3 of fat on the right side and 790 cm3 of fat on the left side. Four months after the last operation, the patient was very satisfied with her new breasts. The breasts were soft, with good sensation and a natural feel. Using the BRAVA external expansion system for the enhancement of fat grafting is a suitable technique for breast reconstruction after a mastectomy. This technique produces soft and natural feeling breasts in fewer operative sessions, with a minimal risk of complications. Patient compliance, however, is greatly needed to achieve the desired results.
For many years, we have known that a mastectomy significantly influences the quality of life.1 In recent years, an increasing number of patients are seeking reconstructive procedures following a mastectomy. Depending on the local findings in the breast area after a mastectomy, we can offer these patients a variety of procedures. All of these procedures attempt to make the breasts appear as natural as possible when the patient is unclothed and normal while wearing clothes. The most common approaches to breast reconstruction are the expander/implant, the latissimus/implant, the free transverse rectus abdominis muscle, and the deep inferior epigastric artery perforator reconstruction.2 However, these procedures are associated with either a risk of a reaction to a foreign biomaterial or a risk of nonnegligible donor-site morbidity.
Fat grafting (autologous fat transfer) has experienced a tremendous boom in recent years. Similar techniques have been used for more than 100 years. In 1908, Eugen Holander published his findings on the use of autologous fat mixed with ram fat.3 There has been a negative attitude toward this method, which includes suspicions that it causes necrosis, colliquation, and mainly that the whole graft may be quickly absorbed.4 Since 1990, however, together with the improvement of techniques and extensive experimental and clinical studies, an increasing number of plastic surgeons have begun to add this method to their armamentarium. The fat represents a filler with ideal properties because it naturally integrates into tissues and because it is autologous; therefore, the fat is 100% biocompatible. Additionally, the operations are noninvasive because there is no incision or suturing, and the scars are quite small.
The popularity of fat graft breast reconstruction has increased in recent years. Fat grafting is the best approach for breast reconstruction following a lumpectomy because of its regenerative properties in irradiated tissue and the desirability of filling only part of the breast. Fewer plastic surgeons, however, have used fat grafting for breast reconstruction following a total mastectomy.
Some authors advocate enriching fat grafts with stem cells to decrease graft absorption during the postoperative period.5 We have attempted this method on several patients who elected for a breast reconstruction procedure after a mastectomy, and we have achieved nice outcomes. The downside of this technique is the high price of the equipment, the length of time required for stem cell separation, and the fact that the amount of injected fat is always limited by the skin envelope.
Khouri and Del Vecchio6 started to promote the use of BRAVA (Brava Breast Enhancement and Shaping System, BRAVA) in conjunction with fat graft breast reconstruction and breast augmentation several years ago. BRAVA is a special bra consisting of plastic domes with silicon rims that is worn by a patient for several weeks before a fat grafting session. This technique has several benefits. Negative pressure from the BRAVA system causes a “reverse” expansion of the skin envelope, thus preparing more space for the fat graft. It has been proven that this negative pressure induces increased vascularization in the breast tissue. In addition, some studies suggest that local mediators and stem cells are increased in the breast tissue after an external expansion.7
The most important issue for patients who have had a mastectomy and are being considered for a fat graft breast reconstruction is the availability of a sufficient amount of skin envelope to apply the fat graft to. By using a BRAVA expansion bra, this problem may be partially resolved.
To our knowledge, there are not much data in the literature describing a fat graft breast reconstruction after a mastectomy that was potentiated by an external expansion. The only clinical study was reported by Khouri et al.8
In this article, we describe a case of a breast reconstruction after a mastectomy using an autologous fat transfer combined with the BRAVA external tissue expansion system.
METHODS
The principles outlined in the Declaration of Helsinki have been followed. We operated on a 38-year-old patient 4 years after bilateral modified radical mastectomy without irradiation for breast cancer. She was generally healthy without any active disease. Before the procedure, the patient was instructed to wear the BRAVA system for 12 hours daily (during night) for 2 months before first session, at all times between the sessions and for 1 month after the last fat grafting session. During the preoperative counseling, the plan for 3 consecutive operations in 3-month increments was explained to the patient.
The operation consisted of 3 phases: fat harvesting, fat processing, and fat graft injection. The procedure was performed under general anesthesia. Before the liposuction, we infiltrated the subcutaneous tissue with a tumescent solution containing 1 L of saline and 1 mL of adrenaline. Fat was harvested from the abdominal wall, the flanks, or the lateral thighs using handheld 60-mL Toomey syringe and a 3-mm Mercedes cannula (Mentor, Santa Barbara, Calif.). We processed the fat in 3 parts using 250 cm3 PureGraft bags (Cytori Therapeutics). Each part was rinsed 2 times with 150 mL of Ringer’s solution. We found that the use of the PureGraft bags was superior to centrifugation in a large-volume fat grafting procedure because it saved time. We find the most important consideration for a favorable fat graft outcome, that is, the utilization of a correct graft injection technique. The graft was injected into many spots around the reconstructed area using a 9-cm type III Coleman cannula (Mentor). The injection was performed very slowly with a fan-shaped technique during the withdrawal of the cannula. We injected the graft into subcutaneous plane and partly into pectoral muscle. We attempted to prevent accumulation of the fat graft as much as possible and the overfilling of a tissue to prevent ischemia, necrosis, colliquation, or calcification. Therefore, we stopped filling when there was no resistance of tissue while injecting the cannula or when tissues started to be tight. The patient began wearing the BRAVA system 1 day after each fat grafting session.
RESULTS
We performed 3 fat grafting sessions, as planned, and the patient’s cooperation was excellent. During the first fat grafting session, we injected 210 cm3 on the left side and 260 cm3 on the right side. After 3 months, during the second session, we injected 280 cm3 on the left side and 280 cm3 on the right side. Six months after the first session, during the third session, we injected 300 cm3 on both sides. Altogether, we injected 840 cm3 on the right side and 790 cm3 on the left side. The patient began wearing the BRAVA system 2 months before the first operation and finished wearing the system 1 month after the last operation, as planned. Thanks to the external reverse expansion, excess pendulous skin was present before each fat grafting session. Four months after the last operation, the patient was very satisfied with her new breasts. The breasts were soft, with good sensation and a natural feel (Fig. 1). We did not experience any complication during these operations. In addition, there was no complication in postoperative course, such as cysts, fat necrosis, palpable lumps, or ultrasonography abnormalities.
Fig. 1.
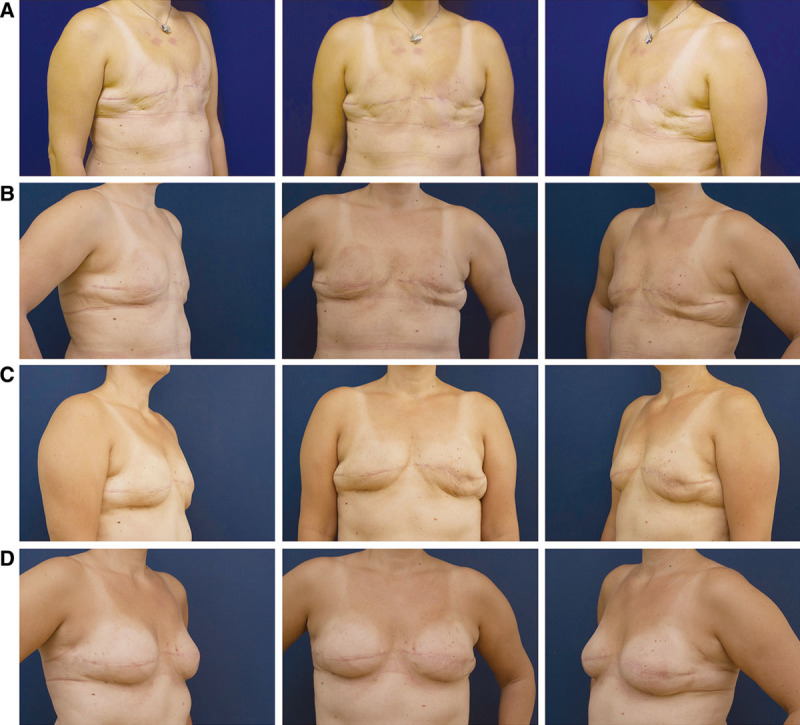
A, The patient before the first session of fat grafting, after 2 mo of wearing the BRAVA bra; B, The patient before the second session of fat grafting, 3 mo after the first session; C, The patient before the third session of fat grafting, 3 mo after the second session; D, The patient 4 mo after the last session of fat grafting.
DISCUSSION
The first attempts of grafting fat into the breast, which occurred around 1990, elicited negative reactions from plastic surgeons who feared that the development of microcalcifications could hinder breast cancer detection. Subsequently, this technique was not utilized for several years, without substantial underlying scientific data to backup those concerns. Microcalcifications also occur following different breast operations, such as breast reduction. In addition, calcifications after fat grafting are localized in different areas and have a different radiological appearance than breast cancer calcifications.9,10 Therefore, expert radiologists should not have problems distinguishing between tumor and post-fat grafting calcifications.
The use of fat grafting in patients with a history of breast cancer had been, until recently, a controversial topic. This belief was caused by several studies that described an increasing tumor reactivation by mesenchymal stem cells.11–13 However, there are data suggesting that these in vitro studies were not conducted using normal tumor cells but with tumor lineage cells, and therefore, they did not have an in vivo correlate.14 On the other hand, there are clinical studies that do not show increased locoregional relapses of breast cancer.15 Petit et al16 described a series of 646 patients who underwent fat grafting after breast cancer therapy. They were followed up for a mean of 19.2 months, and the locoregional relapse rate was 2.4%. This number was not significantly different from the risk of a locoregional relapse in the group without lipofilling. However, as in other types of breast reconstructions, regular screening following breast reconstruction by fat grafting is necessary.
Considering its flexibility, excellent postoperative results, and low incidence of complications, fat grafting is well appreciated by patients after breast cancer therapy. However, fat graft breast reconstruction after a mastectomy is always limited by the size of the skin envelope, which affects the amount of graft that can be injected in 1 session. Because the fat naturally resorbs in all patients, several fat grafting sessions are required. External expansion before and after the operation increases the size of the skin envelope for fat grafting and allows for a decreased number of procedures necessary for an adequate breast reconstruction.
Wearing an external expansion system lasts for several months and is quite challenging. A very important point in considering this technique for a patient is the thorough explanation of all of the demands. It is a consideration for motivated patients who are willing to endure the regular BRAVA bra use.
CONCLUSIONS
Using an external expansion for the enhancement of fat grafting is a suitable technique for breast reconstruction after a mastectomy. It produces soft and natural feeling breasts in fewer operative sessions, with a minimal risk of complications. This technique has many advantages compared with other reconstructive techniques; however, patient compliance is greatly needed to achieve the desired results.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was entirely supported by research grant of Grant Agency of Charles University in Prague No. 268011. The Article Processing Charge was paid for by the research grant.
REFERENCES
- 1.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106:1014–1025. doi: 10.1097/00006534-200010000-00010. discussion 1026–1027. [DOI] [PubMed] [Google Scholar]
- 2.Nahabedian MY. Breast reconstruction: a review and rationale for patient selection. Plast Reconstr Surg. 2009;124:55–62. doi: 10.1097/PRS.0b013e31818b8c23. [DOI] [PubMed] [Google Scholar]
- 3.Joseph M. Handbuch der kosmetik. Leipzig: Veit & Co; 1912. [Google Scholar]
- 4.Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219–227. discussion 228. [PubMed] [Google Scholar]
- 5.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273. doi: 10.2217/17460751.4.2.265. [DOI] [PubMed] [Google Scholar]
- 6.Khouri R, Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;36:269–280, viii. doi: 10.1016/j.cps.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Suga H, Eto H, et al. Reversible adipose tissue enlargement induced by external tissue suspension: possible contribution of basic fibroblast growth factor in the preservation of enlarged tissue. Tissue Eng Part A. 2010;16:2029–2040. doi: 10.1089/ten.TEA.2009.0551. [DOI] [PubMed] [Google Scholar]
- 8.Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava and autologous fat transfer is a safe and effective breast augmentation alternative: results of a 6-year, 81-patient, prospective multicenter study. Plast Reconstr Surg. 2012;129:1173–1187. doi: 10.1097/PRS.0b013e31824a2db6. [DOI] [PubMed] [Google Scholar]
- 9.Veber M, Tourasse C, Toussoun G, et al. Radiographic findings after breast augmentation by autologous fat transfer. Plast Reconstr Surg. 2011;127:1289–1299. doi: 10.1097/PRS.0b013e318205f38f. [DOI] [PubMed] [Google Scholar]
- 10.Rubin JP, Coon D, Zuley M, et al. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: a blinded study. Plast Reconstr Surg. 2012;129:1029–1038. doi: 10.1097/PRS.0b013e31824a2a8e. [DOI] [PubMed] [Google Scholar]
- 11.Albarenque SM, Zwacka RM, Mohr A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011;7:163–171. doi: 10.1016/j.scr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Kucerova L, Kovacovicova M, Polak S, et al. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 2011;58:361–370. doi: 10.4149/neo_2011_05_361. [DOI] [PubMed] [Google Scholar]
- 13.Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg. 2012;65:283–288. doi: 10.1016/j.bjps.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerlin L, Donnenberg AD, Rubin JP, et al. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigotti G, Marchi A, Stringhini P, et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;34:475–480. doi: 10.1007/s00266-010-9481-2. [DOI] [PubMed] [Google Scholar]
- 16.Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. 2012;23:582–588. doi: 10.1093/annonc/mdr158. [DOI] [PubMed] [Google Scholar]


