Abstract
The ARVO 2014 minisymposium on “Novel Approaches for Retinal Drug and Gene Delivery” was held on May 6, 2014 in Orlando, FL. The main intent of the symposium was to review recent advances in retinal drug and gene delivery with specific emphasis on novel approaches that address current limitations and have the potential to translate into clinical practice. The symposium was sponsored by Translational Vision Science and Technology.
Keywords: retinal gene delivery, intraocular drug delivery, nonsteroidal anti-inflammatory drugs, nanotechnology
Background: The Problem
The world's population is predicted to grow to approximately 7.9 billion by 2020 and it is estimated that 76 million people will suffer from blindness. Age-related retinal disorders including age-related macular degeneration (AMD) and diabetic retinopathy (DR) are two of the leading causes of blindness in the world. AMD is the leading cause of blindness in the United States and will affect nearly 8 million Americans by 2020 and cost the US $30 billion per year.1 Many patients with AMD have moderate vision loss (20/50 to 20/100) in the better eye, which results in quality-of-life measurements that are 32% below normal and similar to patients with severe angina or hip fractures.2 An increasing percentage of patients with AMD suffer severe vision loss (20/800), which results in a 60% reduction in quality of life and is similar to a patient who is bedridden due to a catastrophic stroke. Similarly, nearly 300 million individuals have diabetes worldwide and prevalence is rising.3 Approximately 35% of diabetic patients will have some form of DR and 5% to 10% of these individuals will suffer from vision-threatening complications, most commonly macular edema.4 Proven preventable measures include lowering of high blood pressure and strict control of blood glucose, but despite these measures and other available treatments, DR remains the leading cause of legal blindness among working-age adults.
One approach to deliver drug to treat exudative AMD and diabetic macular edema (DME) is to introduce a needle that penetrates the globe, thereby permitting the direct placement of a therapeutic substance into the vitreous. Although invasive, this route achieves a high intraocular bioavailability by bypassing several anatomic and dynamic barriers of the posterior segment and results in relatively predictable drug delivery. While intravitreal administration of vascular endothelial growth factor (VEGF) inhibitors have been an important advance in treating both diseases, they require frequent and repeated injections and are invasive. Furthermore, this method of drug delivery is of short duration because dynamic clearance mechanisms, such as anterograde aqueous flow and posterior vitreoretinal-choroidal flow rapidly eliminate current VEGF formulations from the vitreous.
Due to the eye's unique anatomy and physiology, developing a potentially less invasive retinal drug delivery system is a challenge.5 Systemic administration exposes the entire body to unwanted side effects in addition to facing the hurdle of the blood–retinal barrier. Additionally, large complex molecules such as proteins may not be stable for long periods at physiologic temperatures. Topical drugs have to overcome lacrimatory wash off and corneal permeability issues with long diffusion distance through the vitreous to reach the retina. Transscleral drug delivery of drugs is feasible because of persistent high permeability of the sclera for large molecules, but both topical and periocular approaches have the disadvantage of systemic absorption via nasolacrimal passage and less predictable intraocular drug levels when compared with direct intraocular injection.
Consequently intravitreal injections have rapidly become the current standard to treat several retinal diseases but repeated injections are currently needed to maintain adequate intraocular concentration, which places a burden on patients, the healthcare system, and increases the cumulative risk of adverse events such as endophthalmitis. Consequently, novel mechanisms and new treatments that allow sustained retinal drug delivery with less frequent treatment and greater focus on prevention would be advantageous. Strategies and therapies to achieve sustained therapeutic delivery of drug to the retina discussed in this minisymposium include: nanotechnology, hydrogels, nonsteroidal anti-inflammatory drugs (NSAIDs), suprachoroidal drug delivery, and topical delivery.
On the other spectrum of age-related disorders are inherited retinal disorders, which preferentially effect children and young adults. They are a common cause of blindness affecting approximately 1 in 4000 persons. In particular, Leber's congential amaurosis (LCA), which was first described in 1867, is a heterogeneous group of autosomal recessive diseases characterized by early onset rod-cone dystrophy and severe vision loss affecting 2 to 3 per 100,000 live births. It constitutes 5% of all cases of congenital blindness. There are a variety of genes that produce the LCA phenotype but particular attention has focused on RPE65. In diseases where the gene defect is known, introduction of a wild-type gene could replace the functional gene product and restore function and/or prevent cell death. After encouraging animal studies, three separate human phase 1 trials have demonstrated the safety and efficacy of gene therapy replacement of RPE65 via adeno-associated virus vector by subretinal injection. The early success of these trials have promoted application of gene replacement therapy to other diseases such as choroideremia, autosomal recessive retinitis pigmentosa (RP), Stargardt's disease, and exudative macular degeneration. Recent advances in retinal gene therapy and preliminary results from a phase 1 trial in patients with RP were discussed.
Nanotechnology
Nanotechnology is a current hot topic issue because of its potential to significantly impact several fields related to chemistry, engineering, biology, and medicine.6 Nanoscience refers to the study of material characteristics (physical, chemical, and biological) at the nanometer scale. Nanotechnology encompasses the design, fabrication, or application of material with at least one dimension in the nanometer scale. Mammalian cells typically have a diameter of a few microns and their organelles are within the nanometer range. A nanometer is a dimension that is one billionth of a micron. It is therefore intuitive that cells can be better targeted with nanoparticles, as opposed to micron size delivery systems, if the intent is for cellular uptake and inhibition of molecular targets. The use of nanotechnology is being investigated for several different ophthalmic applications for retinal disease. These applications focus on improved drug and gene delivery to target tissue in the retina and/or choroid.
In his talk entitled, “Targeted Nanocarriers for Therapy of Retinal Vascular Diseases,” Ashwath Jayagopal, PhD, Assistant Professor of Ophthalmology and Visual Sciences, at Vanderbilt University, Nashville, TN discussed the application of nanotechonology for targeting pathologic ocular neovascularization with small interfering RNA (siRNA). His overall objective is to clinically translate molecularly targeted therapeutic agents for retinal vascular disease.
Jayagopal discussed the advantages of siRNA as a candidate therapy for ocular neovascularization. siRNA is a class of double-stranded RNA molecules, typically 20 to 25 base pairs in length. They act to interfere in the expression of specific genes with complementary nucleotide sequences by causing mRNA to be broken down after transcription, and thereby preventing translation. siRNA, as therapeutic agents, offer several key advantages including sequence specific targeting of virtually any molecular target, access to currently “undruggable” targets, and relatively easy design at low cost. Unfortunately, these key advantages are offset by barriers to clinical application of siRNA technology, which include poor stability in vivo, potential for adverse off-target effects on tissue, and the unmet need for effective and safe strategies to selectively deliver siRNA exclusively within diseased but not healthy cells. Jayagopal summarized that siRNA is a very promising therapy in search of an effective delivery vehicle. Consequently his lab has turned to nanotechnology to address this need.
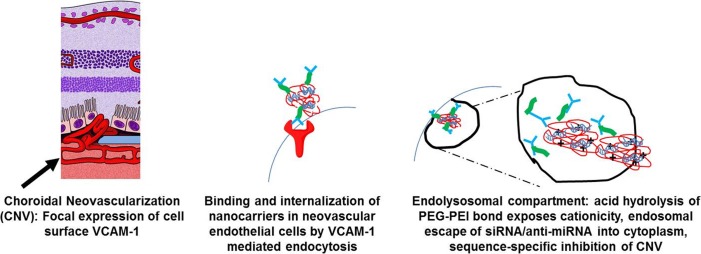
To accomplish this, siRNA is encapsulated within a core and then condensed with a cationic polymer to compact the siRNA and facilitate cytoplasmic release. This polymer is then covered with a coating such as polyethylene glycol to protect the cargo in vivo until its triggered release within the cytoplasm of a targeted cell. The polymer is then functionalized with a disease specific recognition element such as an antibody or aptamer that allows the drug to interface with targeted disease tissue. In his talk, polyethylene coating was functionalized by an antibody fragment to allow vascular cell adhesion molecule 1 (VCAM-1) binding.
Jayagopal's lab specifically looked at endothelial VCAM-1, which is expressed on dysfunctional choroidal endothelial cells, and therefore allows it to be a potential biomarker for selective drug delivery of nanotechnology to this tissue site. Mouse models of laser-induced choroidal neovascularization (CNV) demonstrated increased expression of VCAM-1 on choroidal endothelial cells both early and late in the disease course. Jayagopal's lab further demonstrated that quantom dot nanoparticles, which are optical nanocrystals functionalized with VCAM-1 antibodies were capable of homing to and binding CNV lesions in a mouse model. His lab also demonstrated that dye-labeled VCAM-1 targeted nanocarriers were internalized in choroidal endothelial cells that had been stimulated to increase VCAM-1 expression by exposure to tumor necrosis factor-α.
Jayagopal hypothesized that intravenously administered VCAM-1 targeted nanocarriers in mouse models of laser-induced CNV could home to CNV lesions focally expressing VCAM-1, bind, and then internalize nanocarriers in endothelial cells by VCAM-1 mediated endocytosis (Fig. 1). Subsequent hydrolysis of the polyethylene glycol coating would then allow escape of siRNA into cytoplasm resulting in sequence specific inhibition of the molecular target (VEGR2). Their experiments demonstrated that intravenously-injected VCAM-1 dye-labelled nanocarriers successfully colocalized with laser-induced CNV complexes and that intravenous injection of VCAM-1 nonocarriers with VEGF receptor (R)2 siRNA significantly inhibited laser-induced CNV growth in mice. There were no electroretinographic signs of retinal toxicity. Furthermore, they have been able to switch the cargo of nanocarriers from siRNA to anti-miRNA.
Figure 1.
Targeted nanocarriers address barriers to clinical siRNA administration.
Jayagopal summarized his talk by saying that targeted nanocarriers address key barriers to clinical translation of nucleic acid therapies. They allow specific intracellular delivery of siRNA within dysfunctional cells, are biocompatible, allow multiple administration routes, and provide for targeted and flexible modular design (swap targeting antibody, nucleic acid cargo, anti-miRNA).
Hydrogels and Nano/Microparticles
Jennifer Kang-Mieler, PhD, Associate Professor of Biomedical Engineering at Illinois Institute of Technology, Chicago, IL spoke about the potential for hydrogels to be a novel platform to deliver drug to the posterior segment. The overall objective of her lab is to develop an optimal drug delivery platform that would release anti-VEGF agents in a controlled manner in order to replace the conventional method of intravitreal injection and reduce the frequency of injections needed.
Hydrogels are polymers that have the ability to swell in water or aqueous solvent systems and hold the solvents (intended drugs) in a swollen cross-linked gel phase for delivery. Through manipulation of permeation and diffusion characteristics, they are able to retain hydrophobic and hydrophilic agents, small molecules, and macromolecules. Depending on the specific structure, they can be nondegradable or degradable in application. Hydrogels have the ability to retain hydrophobic, hydrophilic, small molecules, and macromolecules. They can be designed to be temperature and pH-sensitive.
Kang-Mieler's lab has been focusing on Poly (N-isopropylacrylamide) (PNIPAAm), which is one of the best-known thermo-sensitive materials. As a thermo-responsive hydrogel, PNIPAAm is sensitive to temperature change. At room temperature the material is swollen and exists as hydrated polymer chains. At body temperature, however, the PNIPAAm collapses, becomes hydrophobic, and releases water and potentially any bound drug. The change in physical state is rapid and reversible, which grants PNIPAAm attractive characteristics for drug delivery.
Advantages of hydrogels are that they can be designed to be biodegradable or nonbiodegradable by cross-linking them with other agents. They can be designed to encapsulate various ocular pharmaceutical agents such as antibiotics, corticosteroids, or anti-VEGF agents. They also have the potential to encapsulate nano/micro particles for localized delivery.
Intravitreal injection of a thermo-sensitive hydrogel appeared to be safe in an animal model with no observed changes in retinal thickness or alternations in retinal blood flow or function. Pharmacokinetic studies evaluating the protein release profiles of radioactive-labelled immunoglobulin G (IgG) at 37°C encapsulated by hydrogel demonstrated a rapid drug release (burst) of 50% to 80% of drug in the initial 2 days and then subsequent slow release of drug for approximately 14 days.
To prolong duration of drug release, Kang-Mieler's lab used polylactic-co-glycolic acid (PLGA) to fabricate nano/micro particles in order to create a longer lasting drug delivery system. The advantages of this delivery system is that nana/micro particles can encapsulate various ocular pharmacological agents and as the particles degrade, the system slowly releases the encapsulated agent. Degradation rate also can be manipulated to prolong release of the encapsulated agent.
A microparticle drug delivery system was created using ovalbumin as the encapsulated agent. Ovalbumin was chosen because its molecular size (44.3 kDA) was similar to ranibizumab (48.4 kDA). Release of ovalbumin from the microparticle was recorded over 150 days with an initial burst of only 18% of drug compared with greater than 50% with hydrogels. The microparticles were then encapsulated with hydrogels to protect the microparticles from rapid clearance by the retinal circulation after intravitreal injection as well as localize the material to the delivery site. Studies demonstrated that the combined delivery system allowed sustained release of ovalbumin for over 170 days and lessoned the initial burst of drug release (∼5% initial burst) seen with microparticles alone. Preliminary data presented by Kang-Mieler using ranibizumab encapsulated by microparticles and hydrogel demonstrated sustained release of ranibizumab for over 3 months.
Kang-Mieler concluded by saying that thermo-responsive hydrogels can be delivered intravitreally and appear to be safe. Its combination with nano/micro particles may extend release of anti-VEGF agents for approximately 4 to 6 months. Currently, bioactivity of released anti-VEGF drugs are under investigation.
Nonsteroidal Anti-Inflammatory Drugs for Retinal Disease
Stephen Kim, MD, Associate Professor of Ophthalmology and Visual Sciences at Vanderbilt, Nashville, TN, discussed the pathologic role of prostaglandins in AMD and DR and the therapeutic application of NSAIDs.
NSAIDS are one of the most commonly prescribed classes of medications worldwide due to their established analgesic, antipyretic, and anti-inflammatory therapeutic effects. Approximately 1 in 6 individuals in the United States are prescribed a NSAID and many more use over the counter ones. NSAIDs are potent inhibitors of cyclooxygenase (COX) enzyme, and thereby all downstream production of pro-inflammatory prostaglandins. Within the eye, it is well established that prostaglandins cause vasodilation, increase vascular permeability, facilitate leukocyte migration, and promote angiogenesis.7 Consequently, their inhibition has favorable clinical effects on ocular inflammation and cystoid macular edema (CME).
In addition to their established therapeutic effects, a growing body of scientific evidence indicates that prostaglandins play a pathologic role in the progression of AMD and recent years have seen more studies examining a therapeutic role of NSAIDs in slowing progression.8 While anti-VEGF inhibitors have been an important advance in the treatment of exudative AMD, they do not slow down the underlying disease process. Moreover, VEGF is essential for normal homeostasis of retinal cells and its chronic inhibition may be undesirable. In addition, solely inhibiting VEGF does not address the underlying cause of VEGF induction. In this regard, COX can be detected in human choroidal neovascular membranes and considerable scientific evidence indicates that COX is a promoter of angiogenesis. Pharmacologic inhibition of COX appears to reduce VEGF expression in cultured human cells and suppresses VEGF in animal models of retinal angiogenesis. Published work from Kim's lab has demonstrated that both pharmacologic inhibition and genetic deletion of COX reduces laser-induced CNV in animal models.9,10 While results from clinical studies investigating a therapeutic benefit of NSAIDs for AMD have been inconsistent, two recent prospective, randomized, controlled clinical studies reported favorable effects of topical bromfenac with respect to retinal thickness and reduced number of anti-VEGF treatments in patients with exudative AMD.8
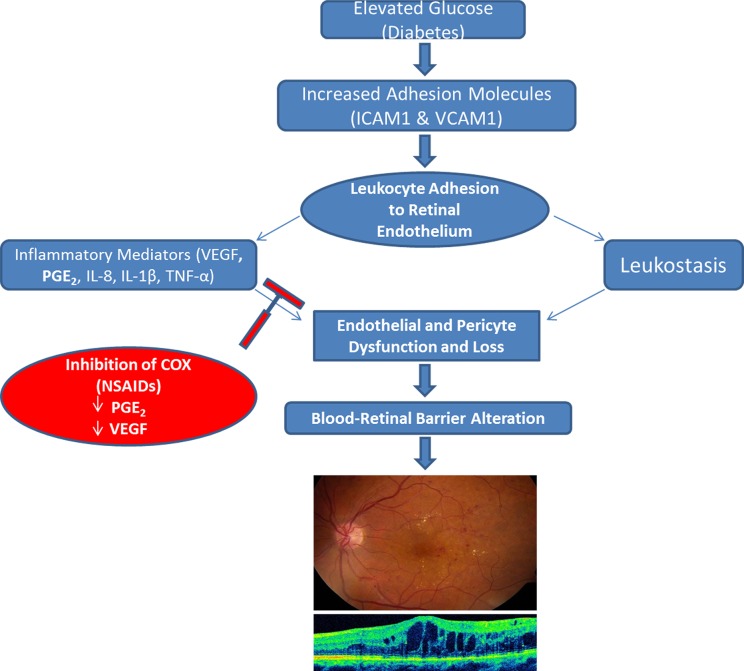
DR is the most frequent cause of legal blindness among working-aged individuals in developed countries.3 DME is the most common cause of vision loss in diabetic patients, affecting about 75,000 new patients in the United States every year.4 Proven preventable measures for DR include lowering of high blood pressure and strict control of blood glucose, but a growing body of scientific evidence supports a pathogenic role of inflammation.8 In support of this, a number of pro-inflammatory cytokines, including prostaglandin E2 (PGE2) and VEGF, are consistently elevated in the vitreous of patients with advanced stages of DR and DME and treatment with NSAIDs prevents or delays DR progression in animal models. Kim presented work from his group that demonstrated elevated levels of prostaglandin PGE2 in vitreous samples taken from patients with advanced DR that correlated with vitreous levels of VEGF.11 Kim also presented results from a prospective, randomized clinical study that demonstrated significant intraocular reduction of interleukin 8 (IL-8) and platelet-derived growth factor (PDGF)-AA levels in eyes with advanced DR treated with topical ketorolac 0.45%.12 Kim reported that meaningful inhibition of PGE2 and other inflammatory cytokines implicated in the pathogenesis of DR may prevent or delay progression of DME (Fig. 2).
Figure 2.
Potential application of NSAIDs to slow progression of diabetic macular edema. Inhibition of COX enzyme directly reduces PGE2 and prevents upregulation of VEGF, which may slow compromise of the blood–retinal barrier.
Despite scientific evidence that inhibition of prostaglandins may delay the progression of AMD and DR, Kim emphasized that topical or oral application of NSAIDs does not result in sufficient retinal drug levels to meaningfully reduce retinal prostaglandin levels. Intraocular injection, on the other hand, provides considerably higher retinal drug levels while minimizing systemic exposure. Previous work from Kim's lab has demonstrated that intravitreal ketorolac results in therapeutic retinal drug levels 100 to 1000 times greater than after topical application and significantly reduces intraocular inflammation and PGE2 levels in an animal model of uveitis.13,14 However, ketorolac has a short intraocular half-life, which greatly limits its therapeutic effect.
Kim presented new data on the safety, pharmacokinetics, and efficacy of intraocular celecoxib. Celecoxib is a selective COX-2 inhibitor used in the treatment of inflammatory arthritis. It is more than 300 times more selective for COX-2 than COX-1. COX-2 is the inducible isoform of COX and predominately involved in inflammatory responses and its preferential inhibition may minimize toxicity. Celecoxib is also relatively insoluble in vitreous, which may translate into more sustained delivery after intraocular injection. Escalating doses of celecoxib were intravitreally injected into rabbit eyes and were found to be nontoxic to the retina and resulted in sustained retinal drug levels out to 8 weeks. Intraocular celecoxib significantly reduced intraocular inflammation and PGE2 in an animal model of uveitis. Despite the observation of cataract formation at higher doses, there was no increase in intraocular pressure and the drug was well tolerated with no other evident adverse effects.
Kim concluded that despite increasing rationale to test the efficacy of NSAIDs to slow progression and prevent vision-threatening complications of AMD and DR, there is no convincing evidence that topical or systemic NSAIDs achieve measurable retinal drug levels. In contrast, intravitreal administration of celecoxib results in considerably higher retinal drug levels and sustained intraocular delivery.
Suprachoroidal Drug Delivery
Development of safe and efficacious local drug delivery technology for treating posterior segment disorders of the eye remains a major challenge in vision research. Local delivery minimizes systemic levels and decreases side effects.
Timothy Olsen, MD, Chairman of the Department of Ophthalmology at Emory University, Atlanta, GA discussed the implications and therapeutic potential of suprachoroidal drug delivery. The sclera has a large and accessible surface area and a high degree of hydration that renders it conducive to water-soluble substances. Human sclera is permeable to hydrophilic compounds with a broad range of molecular weights. There is an inverse relationship between permeability and molecular weight and permeability is enhanced with greater thinness of sclera. Transscleral drug delivery offers a safe route for delivery by avoiding intraocular manipulation and taking advantage of simple diffusion kinetics through the sclera. Previous work suggests that smaller hydrophilic molecules delivered in thinner equatorial regions of the eye may be optimal choices for transscleral systems. However, larger, hydrophobic molecules would likely demonstrate suboptimal drug diffusion kinetics.
The suprachoroidal space (SCS) is a potential space, limited anteriorly in the region of the scleral spur and posteriorly by the transscleral connections of the short posterior ciliary vessels to the choroid. Olsen focused his talk on a novel drug delivery method that used the unique anatomy of the SCS in concert with a microcannulation system.15 This novel microcannulation system featured a flexible, small-diameter cannula (325-μm outer diameter and 175-μm inner diameter cannula channel), a stainless steel shaft for optimizing the transition properties (flexibility with enough stiffness to be pushable), a fiber-optic light source for localizing a red, flashing fiber-optic tip, with contoured design for maneuvering safely in the SCS.
Olsen was able to demonstrate that assessing the suprachoroidal space by the microcannulation system could be performed safety and in a reproducible manner in a pig and primate model. Sustained release agents in the suprachoroidal space also demonstrated prolonged local tissue levels, but biologic agents such as bevacizumab in this same space were removed rapidly. Intravitreal injection of bevacizumab demonstrated a more sustained pharmacologic profile than a similar dose delivered to the suprachoroidal space.16 Intravitreal injection of bevacizumab also distributed drug more to the inner retina, whereas suprachoroidal delivery occurred primarily at the choroid, retinal pigment epithelium, and photoreceptor outer segments.
Olsen concluded that a sustained release formulation of larger biological molecules may optimize suprachoroidal delivery. A method of using this same suprachoroidal delivery cannula to deliver cells into the subretinal space is possible, yet has many technical issues that currently limit its usefulness.
Topical Drug Delivery for Retinal Disease
Topical instillation of ophthalmic drops is the most common method used to administer treatments for ocular disease. This route is noninvasive and can be self-administered by patients. However, due to the eye's unique anatomy and physiology, developing a useful retinal drug-delivery system presents several challenges.
Baruch Kuppermann, MD, PhD, Professor of Ophthalmology and Biomedical Engineering at University of California, Irvine, CA, discussed current advances in topical drug delivery for retinal disease and stated that the most significant challenge for effective topical therapy is finding molecules that are small, potent, and have charge characteristics suitable for enhanced ocular penetrance. Long diffusional path lengths, counter-directional intraocular convection, tearing, and corneal impermeability to large molecules all contribute to limited delivery of topically applied drugs to the posterior segment. Consequently, less than 5% of a topically applied drug permeates the cornea and reaches intraocular tissues. The major portion of the instilled dose is absorbed systemically by way of the conjunctiva via the highly vascular conjunctival stroma and through the lid margin vessels. Significant systemic absorption also occurs when the solution enters the nasolacrimal duct and is absorbed by the nasal and nasopharyngeal mucosa.
Kuppermann explained that there are two general pathways whereby a drug can reach the posterior tissue and blood vessels from a drop. One pathway is via the cornea into the anterior chamber and then through the lens and pupil. The other pathway is via the conjunctiva either directly across the sclera, choroid, choriocapillaris, and RPE into the retina or indirectly into the retrobulbar space. The cornea is a unique tissue, which has an aqueous phase (stroma) sandwiched by two lipid layers (epithelium and endothelium). As a result a drug that is both hydrophobic and hydrophilic can penetrate corneal tissue more freely. Mechanically blocking off the corneal surface has little effect on drug penetration into posterior tissues, which suggests that the conjunctival route is more important for drug delivery. The sclera has a large and accessible surface area and a high degree of hydration that renders it conducive to water-soluble substances.
Kuppermann reviewed a number of topical therapies that have been or are currently under investigation. Mecamylamine, a potent nonselective nicotinic acetylcholine receptor antagonist reduced angiogenesis in animal models. A phase II trial showed no benefit in patients with neovascular AMD. OcuCure OC-10X selectively inhibits tubulin and possesses antiangiogenic and angiolytic activity. It is lipid soluble and crosses the human cornea and achieves therapeutic concentrations in retina and choroid. Preclinical data has demonstrated safety and efficacy in animal models and reduction in laser-induced CNV formation. OT-551 inhibits inflammation and angiogenesis but a benefit was not observed in dry or wet AMD. TG100801 is a targeted inhibitor of VEGFR/SRC kinase with reported anti-inflammatory and anti-angiogenic activity. Clinical development halted after a phase II trial demonstrated corneal impregnation with pigment. Topical Tandospirone (AL-8309B) is a selective serotonin 1A agonist and neuroprotective in animal models preventing photoreceptor damage from oxidative stress. Clinical trials are ongoing. GlaxoSmithKline (GSK) pazopanib is a potent multitargeted tyrosine kinase inhibitor that inhibits angiogenesis and tumor growth and may reduce inflammation and fibrosis. The drug showed efficacy in reducing neovascularization in animal models, but a phase IIb study did not demonstrate clinical benefit.
Kupperman concluded by saying that poor ocular penetrance has limited use of topical therapy for posterior segment diseases in the past. Smaller molecules with higher potency have been developed to overcome this challenge. A number of topical ocular drugs have been or are currently being evaluated for treatment of AMD, but despite robust preclinical data, no benefit to date has been shown in clinical studies.
Adeno-Associated Virus Gene Therapy
Shannon Boye, PhD, Assistant Professor at the University of Florida, Gainesville, FL discussed recent advances in adeno-associated virus (AAV) gene therapy. Gene therapy vectors based on AAV are currently in clinical trials for several retinal diseases including LCA, choroideremia, and AMD. Despite its considerable promise and emerging clinical success, several challenges impede the broader implementation of AAV gene therapy, including the prevalence of neutralizing antibodies in the human population, low transduction of a number of therapeutically relevant cell and tissue types, an inability to overcome physical and cellular barriers in vivo and a relatively limited carrying capacity. Newer methods are needed to try to address the current limitations of AAV therapeutic gene delivery.
Boye focused her talk on “what's new in the field of retinal gene therapy.” Specifically, Boye reviewed modifications that are being made to both the outside (capsid) and inside (genome) of the AAV vector in order to generate novel variants with unique transduction profiles. AAV is a nonpathogenic parvovirus composed of a 4.7-kb single-stranded DNA genome within a nonenveloped, icosahedral capsid. The virus's capsid governs its ability to transduce cells, from initial cell surface receptor binding to gaining entry into the nucleus. Large interstrand loops or variable regions makeup the outside of the AAV capsid and sequence differences in these regions account for the functional differences seen between serotypes 1 to 9 (their receptor biology, immunological profile, etc.). Modification of these variable regions on the AAV capsid may allow design of new AAVs with improved transduction profiles into desired targeted cells such as retina pigment epithelium cells.
Two methods are being used to modify these variable regions: rational design and directed evolution. Rational design involves using current knowledge of vector biology to make structure-informed mutations to the capsid sequence to generate novel variants. An example of rational design is the generation of tyrosine-phenylalanine mutants. Outside of the AAV capsid there are surface exposed tyrosine residues that are phosphorylated once the capsid enters the cell, which then target the capsid for ubiquination and degradation in the cell proteasome. Mutation of tyrosine residues to prevent phosphorylation may prevent targeted degradation of the capsid and improve transduction efficiency. Recognition of this, formed the basis for generation of the tyrosine-phenylalanine (Y-F) mutants and eventually serine, threonine, and lysine mutants.
Directed evolution is a second method by which novel variants can be generated. This method relies on nature to create capsid sequences that confer vectors with the ability to best overcome a selective pressure. Two variants mined via this methodology were “7m8” and “shh10.” The former shows increased transduction of photoreceptors following intravitreal injection. Despite improvements, transduction remains limited to regions where the internal limiting membrane (ILM) is thin (peripapillary regions, over vasculature, foveal pit). The shh10 variant demonstrates preferential transduction of Müller cells following intravitreal injection. A unique feature of these novel variants as well as those rationally designed is their differential affinity for heparan sulfate proteoglycan (HSPG) relative to their unmodified parent serotypes. HSPG is an abundant component of the ILM. HSPG binding appears necessary for transducing neural retina via the vitreous but not when directly injected in the subretinal space.
Boye then discussed work related to the genome and selective gene expression by using cellular promoters that restrict transgene expression to specific retinal cell types. For example, newer promoters have activity specifically in retinal ganglion and ON bipolar cells. Boye's lab is currently exploring an ON bipolar specific promoter based on the Purkinje cell protein 2 (PCP2) in combination with a rationally designed variant for its ability to drive therapeutic nyctalopin (NYX) expression following intravitreal injection in the nob mouse, a model of congenital stationary night blindness.
On the horizon, more complex, structure-informed libraries are being developed and screened. Emphasis will be placed on variants that can: transduce photoreceptors via the vitreous, avoid immunological detection, and package larger payloads. Specifically, Boye's lab has developed several discretely characterized dual vector platforms that have the capability of delivering larger genes. They demonstrated for the first time that the sequence fidelity of mRNA transcript emanating from recombined genomes was 100% accurate. This has implications for regulatory approval of such dual vector platforms.
Simian Immunodeficiency Virus (SIV)-based Gene Therapy for Retinitis Pigmentosa
Yasuhiro Ikeda, DVM, PhD, discussed the preliminary results of a phase 1 clinical study of a third-generation SIV-based lentiviral vector carrying human pigment epithelium-derived factor (PEDF) gene for patients with RP.
RP is a group of inherited retinal degenerative diseases caused by several gene abnormalities that effects approximately 1 in 5000 individuals worldwide. The progressive loss of photoreceptors, especially rod photoreceptors, leads to impaired night vision and a gradual loss of visual fields. More than 50 responsible genes have been implicated. Although extensive effort has been made to treat or cure RP, it remains intractable and there is no effective treatment.
Gene transfer of neurotrophic factors in animal models has shown to be safe and can delay photoreceptor cell degeneration. Ikeda and colleagues used a recently developed novel lentiviral vector derived from the nonpathogenic SIV to transfer a human pigment epithelium-derived factor (hPEDF) gene, one of the most potent neurotrophic factors found in humans, in patients with RP. The SIV vector was developed originally by DNAVEC Corporation in Japan and has a number of favorable characteristics for gene therapy including the potential for stable gene expression due to integration into the host genome and was designed with the added safety of self-inactivation.
hPEDF is a 50-kDa secreted glycoprotein from the serine protease inhibitor superfamily. It is a survival factor for several neural cells. SIV vector–mediated gene transfer of hPEDF protected retinal degeneration and functional loss in an animal model of RP. This provides the rationale for hPEDF gene transfer in patients with RP for neuroprotection and delay in photoreceptor cell loss. Stable retinal gene expression was demonstrated in the retinal pigment epithelium after subretinal injection of the SIV vector in monkeys for over 4 years. Preclinical work demonstrated no acute toxicity after intraocular injection in monkeys or long-term safety issues with the SIV vector after an observation period of 5 years.
Ikeda presented preliminary results from a phase 1 clinical study in patients with RP. The primary purpose of this study was to assess the safety of subretinal administration of SIV-hPEDF in 20 consecutive RP patients in an open-label, dose-escalation study. The first trial subjects were enrolled on March 2013. The first five patients injected with the low titer have been enrolled. The procedure consisted of a pars plana vitrectomy with removal of the vitreous body and subretinal injection of SIV-hPEDF using a 41-G needle. No toxic or systemic side effects have been observed up to date. The next 15 patients enrolled will be injected with a higher titer.
In summary, Ikeda presented the preliminary results of the first clinical trial of a SIV-mediated hPEDF gene therapy for patients with RP. The study is ongoing. SIV-mediated hPEDF gene transfer may ultimately be a potential treatment to slow vision loss in patients with RP.
Conclusions
Effective treatment of retinal disease remains a formidable challenge due to both anatomic and physiologic barriers that can impede agents from reaching target tissue. Advances in nanotechnology and biomaterials along with greater understanding of the role of inflammation in disease pathogenesis, holds promise for providing better solutions to the challenge of ocular drug delivery. Advances in gene therapy continue to progress at a rapid rate and despite its current limitations, offers the possibility of cure of several important inherited retinal dystrophies.
Acknowledgments
Minisymposium presented at the annual ARVO meeting on Tuesday May 6th 2014.
Disclosure: S.J. Kim, None
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, Beauchamp GR. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005;103:173–184. [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti DA, Klein R, Gardner TW. Diabetic Retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 4.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kuppermann BD. Drug delivery strategies for combination ophthalmic treatments. Retina. 2009;29:S24–S26. doi: 10.1097/IAE.0b013e3181ad2463. [DOI] [PubMed] [Google Scholar]
- 6.Kompella UB, Amrite AC. Pacha Ravi R, Durazo SA. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Retin Eye Res. 2013;36:172–198. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SJ, Flach AJ, Jampol LE. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–133. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Schoenberger SD, Kim SJ. Nonsteroidal anti-inflammatory drugs for retinal disease. Int J Inflam. 2013;2013:281981. doi: 10.1155/2013/281981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezaei KA, Toma HS, Cai J, Penn JS, Sternberg P, Kim SJ. Reduced choroidal neovascular membrane formation in cyclooxygenase-2 deficient mice. Invest Ophthalmol Vis Sci. 2011;52:701–707. doi: 10.1167/iovs.10-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SJ, Toma HS. Inhibition of choroidal neovascularization by intravitreal ketorolac. Arch Ophthalmol. 2010;128:596–600. doi: 10.1001/archophthalmol.2010.69. [DOI] [PubMed] [Google Scholar]
- 11.Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906–5911. doi: 10.1167/iovs.12-10410. [DOI] [PubMed] [Google Scholar]
- 12.Schoenberger SD, Kim SJ, Shah R, Sheng J, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac 0.45% in patients with diabetic retinopathy. JAMA Ophthamol. 2014;132:32–37. doi: 10.1001/jamaophthalmol.2013.6203. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Adams NA, Toma HS, Belair M, Thorne JE, Green WR, Jabs DA. Safety of intravitreal ketorolac and diclofenac: an electroretinographic and histopathologic study. Retina. 2008;28:595–605. doi: 10.1097/IAE.0b013e31815e98a5. [DOI] [PubMed] [Google Scholar]
- 14.Baranano DE, Kim SJ, Edelhauser HF, Durairaj C, Kompella UB, Handa JT. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br J Ophthalmol. 2009;93:1387–1390. doi: 10.1136/bjo.2009.157297. [DOI] [PubMed] [Google Scholar]
- 15.Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, Smith ME, Cameron JD. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–787. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749–4756. doi: 10.1167/iovs.10-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]