Abstract
Omega-3 polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid have beneficial effects in many inflammatory disorders. Although the mechanism of eicosapentaenoic acid and docosahexaenoic acid action is still not fully defined in molecular terms, recent studies have revealed that, during the course of acute inflammation, omega-3 polyunsaturated fatty acid-derived anti-inflammatory mediators including resolvins and protectins are produced. This review presents recent advances in understanding the formation and action of these mediators, especially focusing on the LC-MS/MS-based lipidomics approach and recently identified bioactive products with potent anti-inflammatory property.
Keywords: omega-3 fatty acid, anti-inflammation, metabolomics, lipid mediator
Introduction
Lipids are recognized as extremely diversified molecules. Precise determination of molecular lipid species becomes a prerequisite not only to understand their biological functions in physiology and disease, but also to discover the novel link between lipid metabolisms and biological phenotypes. We have developed a liquid chromatography tandem mass spectrometry (LC-MS/MS)-based lipidomics system geared to the comprehensive analyses of polyunsaturated fatty acid (PUFA)-derived lipid mediators with simultaneous and quantitative measurements.
PUFAs exhibit a range of biological effects, many of which are mediated through the formation and actions of bioactive metabolites such as prostaglandins (PGs), leukotrienes (LTs), lipoxins (LXs), resolvins (Rvs) and protectins. The lipid mediators are potent endogenous regulators of inflammation and related diseases. Dietary supplementation of omega-3 PUFAs including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) display beneficial impact in a wide range of human diseases in which unresolved inflammation is suspected as a key component of pathogenesis.(1,2) Also, elevated tissue omega-3 PUFA levels in fat-1 transgenic mice (mice that endogenously biosynthesize omega-3 PUFAs from omega-6 PUFAs due to the presence of an omega-3 desaturase fat-1 gene),(3) led to effective protection against a series of inflammation and cancer models.(4) Omega-3 PUFAs may act via several mechanisms: by preventing conversion of omega-6 arachidonic acid (AA) to the pro-inflammatory eicosanoids (e.g., LTs and PGs), by serving as an alternative substrate producing less potent products,(5) or by being converted to potent anti-inflammatory mediators such as Rvs and protectins (Fig. 1).(6,7) In this review, we provide an overview of the formation and actions of novel omega-3 PUFA-derived anti-inflammatory lipid mediators.
Fig. 1.
Possible mechanisms of omega-3 PUFAs’ anti-inflammatory actions. Omega-3 PUFAs are widely held to act via several possible mechanisms, such as preventing conversion of arachidonic acid (AA) into proinflammatory eicosanoids such as LTs and PGs via substrate competition, or serving as an alternative substrate to produce less potent products. In addition, EPA and DHA are converted to bioactive metabolites such as resolvins and protectins with anti-inflammatory and pro-resolving properties.
Novel Omega-3 Fatty Acid-derived Mediators with Potent Anti-inflammatory Property
Using an unbiased lipidomics approach, Serhan’s group identified a series of novel anti-inflammatory lipid mediators from EPA and DHA. These include the E-series resolvins (RvE1 and RvE2) derived from EPA, as well as the D-series resolvins (RvD1-D6), the protectins (NPD1/PD1/PDX), and maresin (MaR1) derived from DHA (Fig. 2). These mediators constitute novel families of omega-3 PUFA-derived metabolites with potent anti-inflammatory, tissue-protective, and resolution-stimulating functions.
Fig. 2.
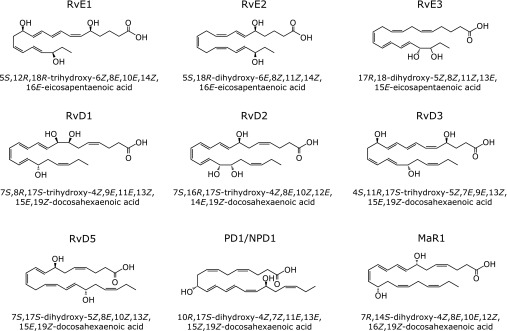
Structures of omega-3 PUFA-derived mediators.
In a murine model of asthma induced by ovalbumin (OVA), RvE1 inhibited airway inflammatory cell recruitment, ameliorated airway hyperresponsiveness and decreased production of the proinflammatory cytokines.(8,9) In pain research, RvE1 or RvD1 reduces inflammatory pain behaviors evoked by intraplantar formalin, carrageenan or complete Freund’s adjuvant (CFA).(10) In mouse burn models, systemically administered RvD2 effectively prevented thrombosis of the deep dermal vascular network and subsequent dermal necrosis.(11) In a colitis model, RvE1 protected against the development of 2,4,6-trinitrobenzene sulfonic acid-induced colitis.(12) In a sepsis model initiated by cecal ligation and puncture, RvD2 decreased both local and systemic bacterial burden, excessive cytokine production and neutrophil recruitment, while increasing peritoneal mononuclear cells and macrophage phagocytosis.(13) In self-resolving Escherichia coli infections, RvD1 and RvD5 each reduce bacterial titers and increased survival.(14)
In addition to mice, omega-3 fatty acid-derived mediators were also found in human plasma and serum following omega-3 PUFA supplementation.(15–17) These findings suggest that biologically relevant concentrations of these mediators can be achieved with omega-3 PUFA supplementation and could potentially contribute to the endogenous mechanisms by which omega-3 fatty acids exert their anti-inflammatory action.
LC-MS/MS-Based Lipidomics
A powerful method for the analysis of mono- and polyhydroxylated fatty acids is liquid chromatography-tandem mass spectrometry (LC-MS/MS) with electrospray ionization (ESI) (Fig. 3A). ESI is a soft ionization technology used to form either positive or negative ions without derivatization and decomposition. In case of fatty acid-derived mediators, ESI results in the removal of a proton to form [M-H]− carboxylate ions. The triple quadrupole mass spectrometer is capable of operating a MS/MS method called multiple reaction monitoring (MRM). A specified precursor ion is selected according to its mass-to-charge ratio in the first quadrupole mass filter, and is fragmented into product ions in the second chamber by collision-induced dissociation (CID). Then the third quadrupole mass filter is locked on its specified product ion. This MRM mode leads to further improvement of the detection and quantification limits when combined with high-resolution LC separations. We developed a comprehensive LC-ESI-MS/MS method applicable to the simultaneous detection and quantification of >300 PUFA metabolites including PGs, LTs, LXs, resolvins, protectins, and other AA-, EPA-, DHA-derived metabolites and their precursors (Fig. 3B).(18)
Fig. 3.
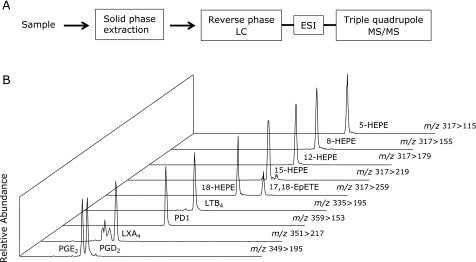
LC-MS/MS-based lipidomics. (A) Flow chart depicting the system of LC-ESI-MS/MS-based lipidomics. After solid phase extraction, samples are separated by HPLC, and fatty acid metabolites are detected and quantified by MRM using triple quadrupole MS/MS. (B) Representative MRM chromatograms of fatty acid metabolites are depicted.
Resolvin E3: a Novel Eosinophil-derived Lipid Mediator with Anti-inflammatory Property
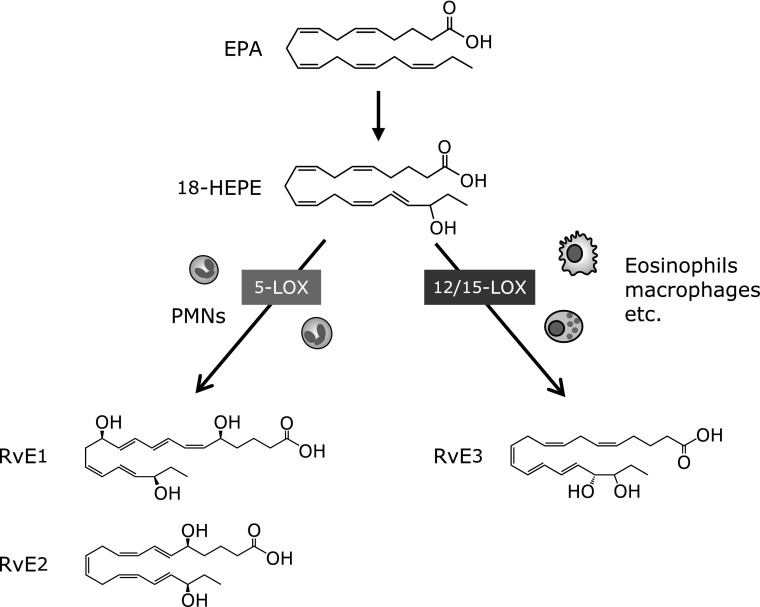
RvE1 and RvE2 are biosynthesized by human polymorphonuclear leukocytes (PMNs) via the 5-LOX pathway from a common precursor 18-HEPE.(15,19,20) 18-HEPE formation in vivo is related to dietary intake of EPA,(15) and a recent study demonstrated two parallel stereospecific pathways, 18R- and 18S-, in the biosynthesis of E-series Rvs both in human sera and murine exudates.(16) Analyses by unbiased target lipidomics using LC-MS/MS recently showed that eosinophils converted 18-HEPE into novel 8,18-dihydroxy-EPE (8,18-diHEPE), 11,18-diHEPE, 12,18-diHEPE, and 17,18-diHEPE.(21) Among those, 17,18-diHEPE, termed RvE3, displayed potent anti-inflammatory activity by blocking PMN infiltration in acute peritonitis, as do RvE1 and RvE2. However, unlike RvE1 and E2, both of which are biosynthesized by PMNs via the 5-LOX pathway, RvE3 is biosynthesized via the 12/15-LOX pathway, which is highly expressed in eosinophils, tissue resident macrophages, and airway epithelial cells (Fig. 4). RvE3 inhibited neutrophil chemotaxis in vitro at low nanomolar concentrations. Moreover, a recent study showed that the administration of RvE3 to LPS-exposed pregnant mice lowered the incidence of preterm birth.(22)
Fig. 4.
Biosynthesis of E-series resolvins. EPA-derived 18-HEPE is converted via the sequential actions of LOX, which leads to formation of E-series Rvs. 5-LOX expressed in PMNs converts 18-HEPE into RvE1 and RvE2. 18-HEPE is also converted via the 12/15-LOX pathway into potent anti-inflammatory mediator RvE3 (17,18-diHEPE).
RvE3 exists as four potential diastereomers resulting from different combinations of R/S configurations of C17 and C18. Recently, we established the total organic syntheses of four different stereoisomers of RvE3: (17R,18R)-, (17S,18S)-, (17S,18R)- and (17R,18S)-dihydroxy-5Z,8Z,11Z,13E,15E-EPE.(23) Based on matching of physical and biological properties, RvE3 was assigned the complete structure 17R,18R/S-dihydroxy-5Z,8Z,11Z,13E,15E-EPE.(24) These natural isomers (i.e., 17R,18S- and 17R,18R-diHEPE) prepared by total organic synthesis displayed a potent anti-inflammatory action by limiting neutrophil infiltrations both in vitro and in vivo. The unnatural stereoisomers (i.e., 17S,18S- and 17S,18R-diHEPE) were much less active compared with the natural isomers, demonstrating the stereoselective action of RvE3.
Novel Bioactive Products Formed via Omega-3 Epoxygenation of EPA
In addition to E-series Rvs which are formed via a common precursor 18-HEPE, we recently uncovered a novel EPA metabolic pathway via omega-3 epoxygenation, and found a novel bioactive metabolite 12-OH-17,18-EpETE with potent and stereoselective anti-inflammatory properties.(25) Intravenous administration of 12-OH-17,18-EpETE dose dependently blocked acute PMN infiltration in zymosan induced peritonitis. Also, 12-OH-17,18-EpETE at low nanomolar concentrations significantly reduced PMN migration speed toward the chemotactic gradient of LTB4. PMNs treated with 12-OH-17,18-EpETE exhibited less direct migration toward the chemotactic gradient of LTB4 and displayed multiple pseudopodia during migration. Interestingly, when compared with RvE3, both compounds reduced PMN migration speed toward the chemotactic gradient of LTB4, but only 12-OH-17,18-EpETE treatment exhibited less direct PMN migration.
In the body, 12-OH-17,18-EpETE is presumably biosynthesized through 12-hydro(per)oxidation of 17,18-EpETE or 17,18 epoxygenation of 12-HEPE. Also the epoxide moiety of 12-OH-17,18-EpETE can be rapidly hydrolyzed by epoxide hydrolase to form 12,17,18-triHETE. Biogenic 12-OH-17,18-EpETE exhibited potent anti-inflammatory activity, whereas structurally related 17,18-EpETE, 12-HEPE or 12,17,18-triHETE were essentially devoid of activity in zymosan-induced peritonitis. Also, the inhibitory effect of 12-OH-17,18-EpETE on LTB4-induced PMN polarization was dose dependent and displayed a pattern of structure–activity relationship to that of PMN inhibition in vivo.
The complete structures of two natural isomers were assigned as 12S-OH-17R,18S-EpETE and 12S-OH-17S,18R-EpETE, using chemically synthesized stereoisomers. These natural isomers displayed potent anti-inflammatory action, whereas the unnatural stereoisomers were essentially devoid of activity. The low nanomolar levels of compounds and the stereospecificity requirements for activity support the presence of a high-affinity receptor for 12-OH-17,18-EpETE on PMNs.
Perspectives
The formation of endogenous autacoids derived from omega-3 PUFA may explain in part the well-known, essential roles of the omega-3 PUFA in health and disease. E-series Rvs are biosynthesized from a common precursor 18-HEPE, and 12-OH-17,18-EpETE is formed from 17,18-EpETE. These oxidation reactions target omega-3 double bond of EPA, which distinguish it from omega-6 arachidonic acid. Based on these findings, now we propose that omega-3 oxidation may be an important structural feature for omega-3 PUFAs’ anti-inflammatory actions (Fig. 5). EPA is converted to 18-HEPE by aspirin-acetylated COX-2 or cytochrome P450 monooxygenase.(19,26) Also, several CYPs, including the CYP1A, CYP2C, and CYP2J subfamily members, can preferentially introduce a cis-epoxide at an omega-3 double bond of EPA to form 17,18-EpETE.(27–29) Cells expressing these enzymes are likely to be involved in regulating inflammatory responses via local production of anti-inflammatory metabolites.
Fig. 5.
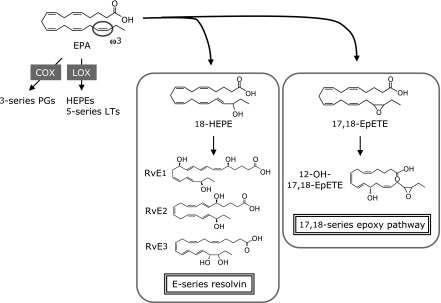
EPA metabolome in the body. EPA is converted by COX or LOX, which leads to the formation of 3-series PGs or 5-series LTs. In addition, recent studies uncovered novel EPA metabolic pathway via omega-3 hydroxylation and epoxygenation, and found potent anti-inflammatory metabolites such as E-series Rvs and 12-OH-17,18-EpETE. These metabolic pathways may represent an endogenous mechanism acting as an anti-inflammatory cascade after dietary intake of EPA.
Mediator lipidomics concerns the simultaneous and quantitative analysis of bioactive lipid mediators in biological systems. This technology could potentially identify the metabolic fingerprint of a disease for clinical diagnosis and treatment. Moreover, the recently identified endogenous anti-inflammatory mediators could lead to the development of novel therapeutics for diseases when non-resolving inflammation is suspected as a key component of pathogenesis.
References
- 1.De Caterina R, Endres S, Kristensen SD, Schmidt EB, editors. n-3 Fatty Acids and Vascular Disease. London: Springer-Verlag; 1993. [Google Scholar]
- 2.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 3.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 4.Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Eessent Fatty Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 7.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki H, Hisada T, Ishizuka T, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang N, Fredman G, Bäckhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 18.Arita M. Mediator lipidomics in acute inflammation and resolution. J Biochem. 2012;152:313–319. doi: 10.1093/jb/mvs092. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal anti-inflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjonahen E, Oh SF, Siegelman J, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Isobe Y, Arita M, Matsueda S, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287:10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita A, Kawana K, Tomio K, et al. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci Rep. 2013;3:3113. doi: 10.1038/srep03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urabe D, Todoroki H, Masuda K, Inoue M. Total syntheses of four possible stereoisomers of resolvin E3. Tetrahedron. 2012;68:3210–3219. [Google Scholar]
- 24.Isobe Y, Arita M, Iwamoto R, et al. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J Biochem. 2013;153:355–360. doi: 10.1093/jb/mvs151. [DOI] [PubMed] [Google Scholar]
- 25.Kubota T, Arita M, Isobe Y, et al. Eicosapentaenoic acid is converted via ω-3 epoxygenation to the anti-inflammatory metabolite 12-hydroxy-17,18-epoxyeicosatetraenoic acid. FASEB J. 2014;28:586–593. doi: 10.1096/fj.13-236224. [DOI] [PubMed] [Google Scholar]
- 26.Arita M, Clish CB, Serhan CN. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem Biophys Res Commun. 2005;338:149–157. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz D, Kisselev P, Ericksen SS, et al. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Lucas D, Goulitquer S, Marienhagen J, et al. Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lipid Res. 2010;51:1125–1133. doi: 10.1194/jlr.M003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold C, Markovic M, Blossey K, et al. Arachidonic acid-metabolizing cytochrome p450 enzymes are targets of ω-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]