Abstract
The relationships between the serum mineral concentrations and the endoscopic findings of esophageal varices have been poorly investigated. In this study, we investigated hepatitis virus-positive patients who had undergone a liver biopsy (n = 576) and 75 patients with compensated cirrhosis in order to evaluate the association of the zinc value with the severity of liver fibrosis and esophageal varices. The mean zinc values decreased with the progression of fibrosis (METAVIR score; F0–1: 71.3 ± 11.3, F2: 68.9 ± 11.7, F3: 66.3 ± 11.8, F4: 63.9 ± 15.0). In the hepatitis virus-related compensated cirrhosis, the mean zinc value decreased with the severity of varices (patients without varices: 66.3 ± 12.6, patients with low-risk varices: 62.5 ± 13.7, patients with high-risk varices: 55.6 ± 13.0). The zinc value was significantly lower in patients with varices than in those without varices (59.3 ± 13.6 vs 66.3 ± 12.6, p<0.05). The zinc value was also significantly lower in the patients with a high risk of bleeding than in those with a low risk (55.6 ± 13.0 vs 64.6 ± 13.1, p<0.01). These findings suggest that the zinc value is not only an indicator of an abnormal metal metabolism, but is also a simple parameter associated with hepatitis virus-related various conditions, including the degree of liver fibrosis and the severity of esophageal varices in compensated cirrhosis.
Keywords: liver cirrhosis, zinc, esophageal varices, biomarker, metabolism
Introduction
Variceal hemorrhage due to portal hypertension is one of the severe complications observed in patients with liver cirrhosis. Despite the recent progress in its treatment, variceal hemorrhage still carries a mortality rate of up to 20% within six weeks of the bleeding episode.(1–3) Although esophagogastroduodenoscopy (EGD) is the gold standard method to determine whether a cirrhotic patient should undergo a prophylactic treatment for variceal hemorrhage,(4,5) EGD is an expensive and uncomfortable burden for patients.
Recently, several noninvasive biomarkers of liver fibrosis have been examined to predict the presence and bleeding risk of varices in cirrhotic patients,(6–8) based on the significant relationship between the progression of liver fibrosis and portal hypertension. However, few clinical parameters have been shown to be associated with the presence of treatment-requiring high-risk varices in patients with well-compensated cirrhosis (Child–Pugh class A status), although noninvasive screening tests would be particularly useful for these asymptomatic cirrhotic patients. Furthermore, the relationships between the parameters of metallic metabolism and the endoscopic findings of esophageal varices have been poorly investigated.
Zinc is required for the activation of approximately 300 different metallo-enzymes and metal-activated enzymes in vivo, and is therefore considered to be one of the most important trace elements.(9–12) Zinc deficiency reduces various important vital functions, including protein synthesis, immunological reactions, skeletal growth and maturation, gonadal development, pregnancy and appetite.(13) The serum and hepatic zinc concentrations are decreased in patients with chronic liver diseases (CLD), and zinc depletion has been suggested to accelerate liver fibrosis.(14–16)
In the present study, we investigated the relationship between the histological grading of liver fibrosis and the blood zinc concentration in patients with hepatitis virus infection (hepatitis B virus: HBV or hepatitis C virus: HCV)-related CLD. We further analyzed the relationships of the zinc values with the endoscopic findings of esophageal varices in the compensated cirrhotic patients (Child–Pugh class A status) with hepatitis virus infection.
Materials and Methods
Patients and methods
In order to examine the relationship between the zinc concentration and the progression of liver fibrosis in CLD patients, we studied 576 patients with hepatitis virus infection who had undergone a percutaneous liver biopsy from January 2008 to July 2012. The chronic HBV infection was diagnosed based on a positive HBsAg status for at least six months. HCV infection was also diagnosed by the detection of HCV antibodies and HCV-RNA in the serum. Liver biopsy examinations were performed using the standard techniques. All liver samples were evaluated by well-trained pathologists at our institute with regard to the fibrosis stage and activity grade. Fibrosis was staged on a scale of F0–F4 (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; F4, liver cirrhosis) according to the METAVIR scoring system.(17) The histological evaluation of the biopsy tissues was also routinely performed in our department. All authors participated in the conferences about the histological evaluation, and the final results were confirmed by two authors (H.E and H.I) who received training for histological studies.
EGD was routinely performed in CLD outpatients at our institution according to the standard techniques. Esophageal varices detected by EGD were graded as I–IV according to the Paquet grading system,(18) and the presence of red signs on the varices was also evaluated. Patients with large varices (grade III–IV) or small varices with red signs were categorized to have treatment-requiring (high-risk) varices. All hepatitis virus-related compensated (Child–Pugh class A) cirrhotic patients admitted to our department for the treatment of esophageal varices from January 2008 to July 2011 were included in the present study as the “high-risk varices group”. Liver cirrhosis as the cause of portal hypertension was diagnosed by histological criteria and/or by the clinical (laboratory, endoscopic and/or ultrasonographic) findings.
In order to assess the zinc values in the compensated (Child–Pugh class A) cirrhotic patients without high-risk varices, we enrolled consecutive HBV or HCV-positive CLD patients who had been diagnosed with cirrhosis (F4 stage) by liver biopsy, but who were confirmed not to have treatment-requiring risky varices by the EGD examination within two months of the liver biopsy. The patients whose endoscopic findings were difficult to evaluate properly because of the past history of endoscopic treatment or the absence of EGD within two months of liver biopsy were excluded. The cirrhotic patients who did not have high-risk varices were categorized into two groups; patients without detectable varices were classified as the “no varices group” and patients with small varices without red signs were classified as the “low-risk varices group”.
In the present study, the values of two biomarkers of liver fibrosis (the APRI, the AST-to-platelet count ratio index and FIB-4) were calculated. The APRI and FIB-4 values were determined according to the formulas reported by Wai et al.(19) and Vallet-Pichard et al.(20) respectively: APRI = 100 × (AST level/upper limit of normal)/platelets [109/L] and FIB-4 = Age [years] × AST [U/L]/(platelets [109/L] × (ALT [U/L])1/2), in which the age of the patient is the age at the time of liver biopsy. In addition, we included the data of the branched-chain amino acids to tyrosine ratio (BTR) that is an indicator of amino acid imbalance, since we previously reported that the BTR value was associated with the progression of liver fibrosis and development of esophageal varices.(21)
All blood samples were obtained on the day of liver biopsy or endoscopic treatment for esophageal varices, and patients without a complete data set on the day of the liver biopsy or the endoscopic treatment were excluded from the study. Patients with the following conditions were also excluded from the study: the presence of other liver diseases, using immunosuppressive therapy, HIV virus co-infection. The study conformed to the ethical guidelines of the declaration of Helsinki, and written informed consent regarding the liver biopsy and use of clinical data was obtained from all patients on admission. This study was approved by the ethics committee of the institutional review board.
Statistical analysis
In the present study, we attempted to clarify whether the zinc value was associated with the progression of liver fibrosis in hepatitis virus-related CLD. The data for the comparisons among the groups “F0–1 vs F2 vs F3 vs F4” were evaluated by a non-repeated measurements ANOVA, and the statistical significance was further investigated with the Bonferroni correction. We also investigated whether the zinc values differed among the three groups (“no varices group”, “low-risk varices group” and “high-risk varices group”). The data for the comparisons among the groups were analyzed by a non-repeated measurements ANOVA with a subsequent Bonferroni correction. We also investigated whether the zinc concentration differed between the groups with or without varices, and the differences in the baseline characteristics of the two groups were also compared. In addition, we evaluated whether the zinc values were different between the groups with and without high-risk (treatment-requiring) varices. Quantitative variables with a normal distribution were expressed as the mean values ± SD, and those with an abnormal distribution were expressed as the median values (range). The statistical analysis between two groups was performed using Student’s t test or the Mann-Whitney U test, as appropriate. Statistical analysis was performed with the JMP 9 (SAS Institute Inc., Cary, NC).
Results
The zinc values decrease with the progression of liver fibrosis in patients with hepatitis virus-related CLD
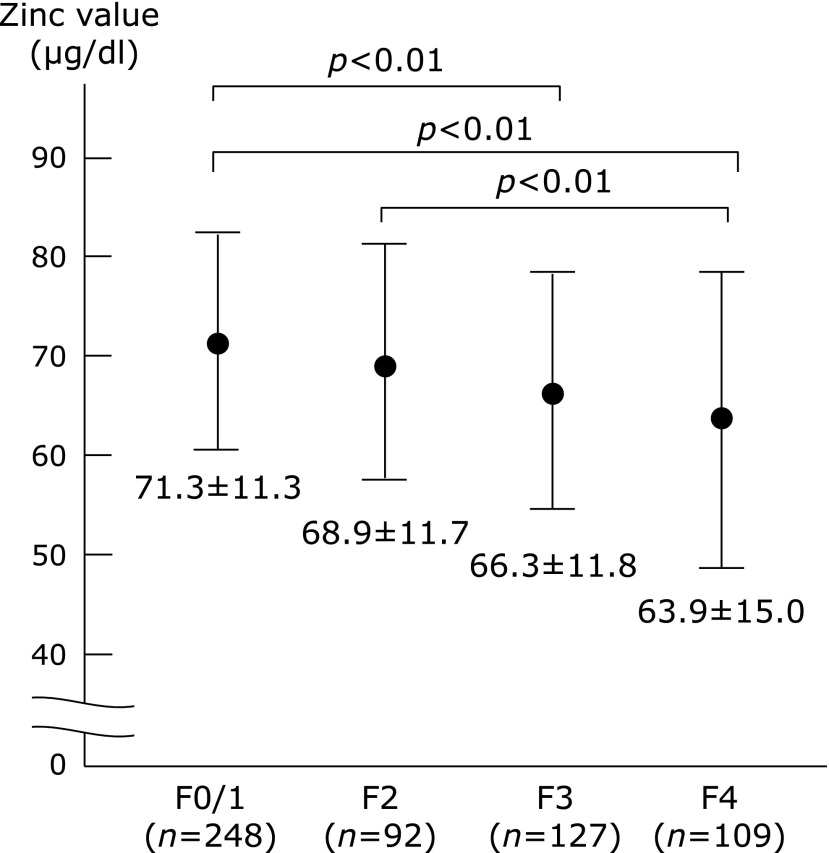
A total of 576 patients with HBV or HCV-related CLD were included in this study to investigate the association between the zinc value and the histological progression of liver fibrosis, based on the criteria described in the “Patients and methods” section. The characteristics of the patients are summarized in Table 1. The population included 265 (46%) males and 311 (54%) females, and the age of patients ranged from 23 to 83 years old (median 61 years old). The METAVIR liver fibrosis staging (17) showed that 328 (57%) patients had significant fibrosis (F2–F4), 236 (41%) patients had severe fibrosis (F3–F4) and 109 (19%) had cirrhosis (F4). The zinc values in the HBV or HCV-positive CLD patients decreased with the progression of liver fibrosis (Fig. 1).
Table 1.
Characteristics of the HBV or HCV-positive patients who received liver biopsy
| Age (years) | 61 (23–83) |
| Gender (Male/Female) | 265/311 |
| Etiology (HBV/HCV/HBV + HCV) | 121/454/1 |
| AST (IU/L) | 35 (10–756) |
| ALT (IU/L) | 36 (7–1,079) |
| γ-GTP (IU/L) | 29 (5–869) |
| ALP (IU/L) | 214 (96–774) |
| Total bilirubin (mg/dl) | 0.8 (0.1–2.3) |
| Albumin (g/dl) | 3.93 ± 0.37 |
| Prothrombin time (%) | 90.7 ± 12.1 |
| Hemoglobin (g/dl) | 13.5 ± 1.8 |
| Platelet (×103/mm3) | 162 ± 71 |
| Fibrosis stage: F0–1/F2/F3/F4 | 248/92/127/109 |
Quantitative variables were expressed as the mean values ± SD or median (range).
Fig. 1.
The values of zinc in relation to the METAVIR histological grading for liver fibrosis in patients with hepatitis virus (HBV or HCV)-related disease. The zinc value decreased as the fibrosis progressed. There was a significant difference between the F0/1 vs F4 and F3 groups. There was also a significant difference between the F2 vs F4 groups.
Characteristics of the hepatitis virus-related compensated cirrhotic patients and the clinical data
Since several biomarkers of liver fibrosis were shown to be related to the presence and/or bleeding risk of esophageal varices,(6–8) we next investigated whether the values of zinc were associated with the severity of esophageal varices in patients with hepatitis virus-related compensated cirrhosis (Fig. 2). Of the HBV or HCV-positive CLD patients in our department, 49 patients were found to have a high risk of variceal bleeding because they had large varices (grade III–IV) or small varices with red signs. In these patients with hepatitis virus-associated cirrhosis, the Child–Pugh classification was grade A in 19 patients, grade B in 28 patients and grade C in two patients. The 19 patients with Child–Pugh class A were first categorized as the hepatitis virus-related compensated cirrhosis with a high risk of variceal hemorrhage (“high-risk varices group”).
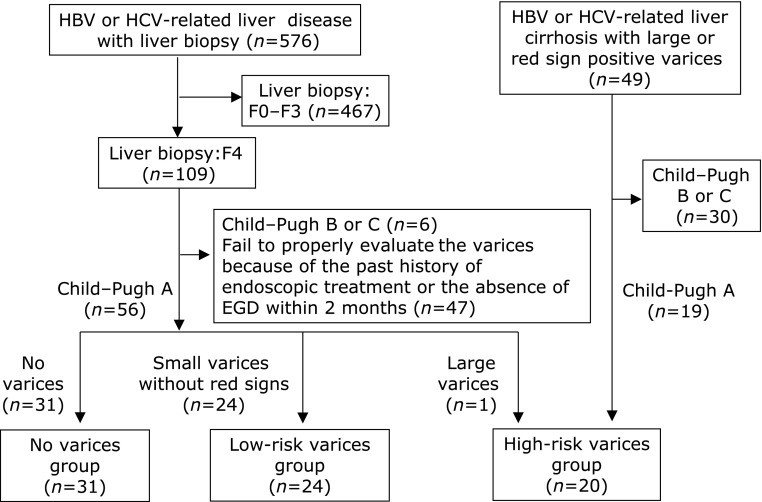
Fig. 2.
The algorithm used for the classification of the hepatitis virus (HBV or HCV)-positive patients with compensated (Child–Pugh class A) cirrhosis. Of the CLD patients in our department, 49 HBV or HCV-positive patients were found to have a high risk of variceal bleeding because they had large varices (grade III–IV) or small varices with red signs. In these hepatitis virus-related cirrhotic patients, the 19 patients with Child–Pugh class A were enrolled as the hepatitis virus-related compensated cirrhotic patients with a high risk of variceal hemorrhage (“high-risk varices group”). Of the 109 patients with a METAVIR score of F4 (shown in Fig. 1), we excluded the patients with the following conditions: Child–Pugh B status (n = 6), without proper evaluation of varices due to a past history of endoscopic treatment or the absence of EGD within two months (n = 47). The remaining 56 patients were enrolled in the present study, and 55 patients were categorized as having compensated cirrhosis with a low risk of variceal bleeding; 31 patients were diagnosed with liver cirrhosis without detectable varices (“no varices group”) and the remaining 24 patients had small varices without red signs (“low-risk varices group”). One patient with large varices was categorized as a Child–Pugh A patient with high-risk varices, so a total of 20 patients were finally enrolled as the “high-risk varices group”.
Next, in order to determine the characteristics of the compensated cirrhotic patients with a low risk of variceal bleeding, we examined the 109 patients who were categorized as compensated cirrhotic patients (109 patients with a METAVIR score of F4, as shown in Fig. 1). Among the 109 patients, six patients were excluded because of advanced liver cirrhosis. In addition, 47 patients whose variceal conditions were difficult to properly evaluate because of their past history of endoscopic treatment for varices or the absence of EGD within two months, were also excluded. Of the remaining 56 patients, 55 patients were confirmed to have a low risk of variceal bleeding; 31 patients were diagnosed with liver cirrhosis without detectable varices (“no varices group”) and the 24 patients had small varices without red signs (“low-risk varices group”). One patient who had large varices (grade III) was classified as a patient with a high risk of variceal bleeding; thus, a total of 20 patients were finally enrolled as the “high-risk varices group” (Fig. 2).
The characteristics of all 75 compensated cirrhotic patients (31 patients in the “no varices group”, 24 patients in the “low-risk varices group” and 20 patients in the “high-risk varices group”) are shown in Table 2. The population consisted of 42 (56%) males and 33 (44%) females, and the age of patients ranged from 23 to 82 years old (median 64 years old).
Table 2.
Characteristics of the total 75 patients with hepatitis virus (HBV or HCV)-positive compensated cirrhosis
| Age (years) | 64 (23–82) |
| Gender (Male/Female) | 42/33 |
| Etiology (HBV/HCV) | 10/65 |
| AST (IU/L) | 47 (19–328) |
| ALT (IU/L) | 38 (10–315) |
| γ-GTP (IU/L) | 41 (12–259) |
| ALP (IU/L) | 269 (127–681) |
| Total bilirubin (mg/dl) | 0.8 (0.3–2.3) |
| Albumin (g/dl) | 3.70 ± 0.36 |
| Hemoglobin (g/dl) | 12.3 ± 2.0 |
| Platelet (×103/mm3) | 118 ± 68 |
| Prothrombin time (%) | 83.1 ± 10.5 |
| Severity of varices (no/low-risk/high-risk) | 31/24/20 |
Quantitative variables were expressed as the mean values ± SD or median (range).
The zinc value is associated with the severity of varices in patients with hepatitis virus-related compensated cirrhosis
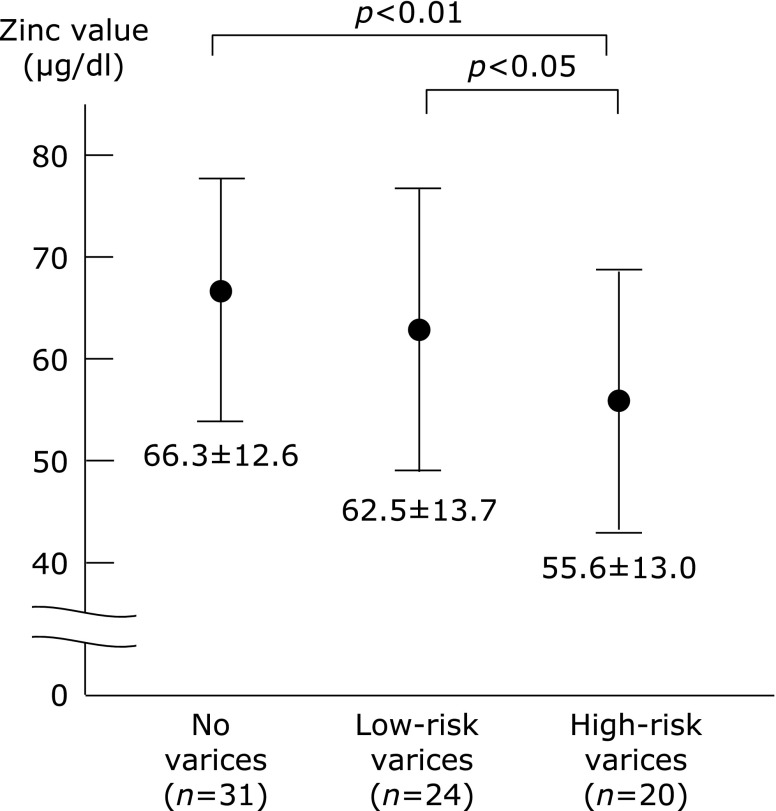
As shown in Fig. 3, the zinc concentrations were decreased as the bleeding risk increased, suggesting that a low zinc value was correlated with the severity of the endoscopic findings of varices in the compensated cirrhotic patients (Child–Pugh class A status) with hepatitis virus infection.
Fig. 3.
The zinc values in patients with hepatitis virus (HBV or HCV)-related compensated (Child–Pugh class A) cirrhosis. The serum zinc values were decreased as the bleeding risk of esophageal varices increased. There was a significant difference between the “no varices group” vs the “low-risk varices group”, and the “low-risk varices group” vs the “high-risk varices group.
We also examined the differences in the zinc values between the patients with varices and patients without varices. Comparing the 44 patients with varices (the “high-risk varices group” and the “low-risk varices group”) and 31 patients without varices (the “no varices group”), the zinc value was significantly lower in patients with varices than that in patients without varices, suggesting that a decreased zinc value was associated with the presence of varices in patients with hepatitis virus-related compensated cirrhosis (Fig. 3 and Table 3). The zinc value was found to be significantly lower in the presence of varices, while the ALP value was higher and the AST, ALT, albumin, prothrombin time (%) and hemoglobin values were lower. BTR, which is an indicator of amino acid imbalance, was significantly lower in patients with varices. However, the values of two well-known fibrosis-related markers, APRI and FIB-4 indices, were not significantly different between the two groups (Table 3).
Table 3.
Characteristics of the hepatitis virus associated compensated cirrhotic patients with or without varices
| Varices detected by EGD |
|||
|---|---|---|---|
| Absent (n = 31) | Present (n = 44) | p value | |
| Age (years) | 63 (23–76) | 64 (31–82) | ns |
| Gender (Male/Female) | 13/18 | 29/15 | ns |
| Etiology (HBV/HCV) | 3/28 | 7/37 | ns |
| AST (IU/L) | 56 (24–328) | 41 (19–128) | <0.01 |
| ALT (IU/L) | 53 (12–315) | 31 (10–136) | <0.01 |
| γ-GTP (IU/L) | 39 (14–259) | 41 (12–242) | ns |
| ALP (IU/L) | 232 (133–494) | 281.5 (127–681) | <0.05 |
| Total bilirubin (mg/dl) | 0.7 (0.3–1.8) | 0.8 (0.3–2.3) | ns |
| Albumin (g/dl) | 3.82 ± 0.29 | 3.61 ± 0.39 | <0.05 |
| Hemoglobin (g/dl) | 13.1 ± 1.9 | 11.8 ± 1.9 | <0.01 |
| Platelet (×103/mm3) | 123 ± 45 | 114 ± 80 | ns |
| Prothrombin time (%) | 86.9 ± 10.2 | 80.4 ± 10.0 | <0.05 |
| Zn (µg/dl) | 66.3 ± 12.6 | 59.4 ± 13.7 | <0.05 |
| APRI | 1.78 (0.44–8.42) | 1.34 (0.25–5.88) | ns |
| FIB-4 | 1.40 (0.26–2.90) | 1.59 (0.30–4.19) | ns |
| BTR | 5.25 ± 1.29 | 4.56 ± 1.26 | < 0.05 |
Quantitative variables were expressed as the mean values ± SD or median (range). ns: not significant, APRI: AST-to-platelet ratio index, BTR: branched-chain amino acids to tyrosine ratio.
When we compared the 20 patients in the “high-risk varices group” with the 55 patients in the “no varices group” or the “low-risk varices group”, the zinc value was significantly lower in the patients in the “high-risk varices group” (Fig. 3 and Table 4), suggesting that a low zinc value is related to an increased risk of variceal hemorrhage. We also found a significantly lower ALT, hemoglobin and BTR level, as well as a lower percentage of female patients, in the “high-risk varices group” (Table 4). The values of APRI and FIB-4 indices, were not found to be related to the presence of high-risk varices. Furthermore, among all of the blood parameters examined, the zinc value showed the smallest p value (p = 0.009<0.01) regarding the comparison between the two groups with or without a high risk of varices.
Table 4.
Characteristics of the hepatitis virus associated compensated cirrhotic patients with or without high-risk varices
| High risk varices detected by EGD |
|||
|---|---|---|---|
| Absent (n = 55) | Present (n = 20) | p value | |
| Age (years) | 64 (23–78) | 63.5 (31–82) | ns |
| Gender (Male/Female) | 24/31 | 2/18 | <0.01 |
| Etiology (HBV/HCV) | 5/50 | 5/15 | ns |
| AST (IU/L) | 50 (19–328) | 44 (21–81) | ns |
| ALT (IU/L) | 48 (10–315) | 31 (15–86) | <0.05 |
| γ-GTP (IU/L) | 47 (14–259) | 34.5 (12–159) | ns |
| ALP (IU/L) | 262 (127–681) | 281 (191–462) | ns |
| Total bilirubin (mg/dl) | 0.7 (0.3–2.0) | 0.8 (0.6–2.3) | ns |
| Albumin (g/dl) | 3.74 ± 0.34 | 3.61 ± 0.40 | ns |
| Hemoglobin (g/dl) | 12.7 ± 1.8 | 11.3 ± 2.2 | <0.05 |
| Platelet (×103/mm3) | 121 ± 63 | 110 ± 80 | ns |
| Prothrombin time (%) | 83.5 ± 11.2 | 82.1 ± 8.7 | ns |
| Zn (µg/dl) | 64.6 ± 13.1 | 55.6 ± 13.0 | <0.01 |
| APRI | 1.42 (0.30–8.42) | 1.46 (0.25–3.92) | ns |
| FIB-4 | 1.47 (0.26–4.19) | 1.59 (0.30–3.90) | ns |
| BTR | 5.01 ± 1.30 | 4.43 ± 1.24 | <0.05 |
Quantitative variables were expressed as the mean values ± SD or median (range). ns: not significant, APRI: AST-to-platelet ratio index, BTR: branched-chain amino acids to tyrosine ratio.
Discussion
Despite the importance of EGD examination to evaluate the risk of variceal hemorrhage,(4,5,22,23) many patients are unable or unwilling to undergo the uncomfortable examination. In particular, patients with a well-maintained liver function and Child–Pugh class A status, who do not have any apparent clinical symptoms, are often reluctant to undergo the endoscopic study. However, esophageal varices are present in about 40% of patients with compensated cirrhosis and in 60% of those with decompensated disease and ascites,(24,25) and it is therefore important to identify a non-invasive biomarker related to the presence and the bleeding risk of varices even in compensated (asymptomatic) cirrhotic patients.
Although several biomarkers of liver fibrosis have been proposed to predict the presence of varices, few studies have investigated the relationships between the endoscopic variceal findings and the fibrosis-related markers in compensated cirrhotic patients with well-maintained liver function (Child–Pugh class A status). In particular, there have been few studies regarding the association between the metabolic parameters and the endoscopic findings of varices in compensated cirrhotic patients, although a recent study showed the relationship between insulin-resistance and the development of esophageal varices.(26)
We previously investigated the ratio of two glycated proteins (glycated albumin, GA and glycated hemoglobin, HbA1c) proteins in patients with CLD, and reported that this ratio (the GA/HbA1c ratio) was increased with the progression of liver fibrosis in patients with HCV-related CLD and non-alcoholic steatohepatitis.(27,28) We also reported that the GA/HbA1c ratio was associated with the severity of the esophageal varices in HCV-related cirrhotic patients.(29) Furthermore, we recently focused on the amino acid imbalance in CLD, and found the BTR to be decreased with the development and progression of varices.(21)
A low serum zinc concentration is a well-known metabolic disorder in patients with CLD, and previous studies reported the relationship between the progression of liver disease and the decreased zinc values in CLD patients. In patients with CLD, the blood zinc concentrations were lower in patients with liver cirrhosis or hepatocellular carcinoma than in patients with chronic hepatitis,(14–16,30,31) leading us to hypothesize that the reduced blood zinc value could be associated with the formation and progression of varices. Interestingly, supplementation with zinc has been shown to improve the prognosis of cirrhotic patients, as well as the cirrhosis-related symptoms.(11,12,16,32) In patients receiving oral supplementation with zinc, maintenance of the serum zinc concentration at more than 80 µg/dl was the most important factor associated with cancer-free survival.(32)
In the present study, we showed that CLD-patients with hepatitis virus infection already have a low zinc concentration before the progression to cirrhosis (Fig. 1). We also showed that the value of zinc was decreased in line with the endoscopic severity of varices in patients with hepatitis virus-related compensated cirrhosis. These results were consistent with the previous reports regarding the relationship between a low serum zinc value and the progression of CLD from chronic hepatitis to liver cirrhosis. Our findings suggest that the zinc value is not only a parameter indicating an abnormal metal metabolism, but is also a simple parameter associated with various clinical conditions in hepatitis virus-related CLD patients, such as the histological stage of liver fibrosis and the severity of varices in the compensated cirrhotic patients. However, it remains unknown whether the findings in this study can be applied to patients with other etiologies, patients with less advanced fibrosis (F1–F3) and patients with decompensated cirrhosis.
There are several eminent markers of advanced liver fibrosis such as a platelet count, APRI and FIB-4 index. However, these parameters were not significantly different between the groups with or without (high-risk) varices (Table 3 and 4). Unlike that observed in HCV-positive patients, noninvasive biomarkers are sometimes reported to not sufficiently evaluate the degree of liver fibrosis in HBV-positive patients,(33,34) and therefore the data obtained from HBV-positive patients may be at least partly responsible for our results. In addition, a recent cross-sectional, longitudinal cohort study concluded that the platelet count is not a sufficient non-invasive marker for varices.(6,35) However, it is difficult to fully clarify the reasons, and a future study with a larger number of patients should be important, because the small number of enrolled patients may also affect the results.
It would be interesting if we could determine cut-off values of the zinc concentration available as an alternative to EGD examinations. We performed the receiver operating characteristic (ROC) analyses in order to determine the optimal cut-off values based on the following criteria: “with or without varices” and “with or without high-risk varices”. Although we obtained possible cut-off values (zinc values of 59.0 µg/dl and 56.0 µg/dl, respectively), it was not easy to identify the cut-off values of the zinc that had a sufficient diagnostic performance, perhaps because the relatively small number of patients were enrolled in the present study. Therefore, our results do not directly indicate that the zinc value alone can substitute for EGD examinations, and further investigations in a larger number of patients are important.
In summary, we have investigated patients with hepatitis virus-related CLD and shown that the zinc value is associated with the histological progression of liver fibrosis and the severity of esophageal varices in compensated cirrhosis. The liver plays an important role in various aspects of metabolism; however, there have been only a few studies, including our previous reports that investigated the correlations between metabolic parameters and the presence of varices or the bleeding risk of varices. Future studies will be needed to examine the correlations of metabolic parameters with the presence of varices or the bleeding risk of varices in a larger number of patients.
Acknowledgments
This study was supported by a Grant-in-Aid for Health and Labor Sciences Research from the Ministry of Health, Labour, and Welfare of Japan to S.N.
Abbreviations
- CLD
chronic liver disease
- EGD
esophagogastroduodenoscopy
- HBV
hepatitis B virus
- HCV
hepatitis C virus
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G, De Franchis R, Cooperative Study Group Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C, Thabut D, Kamath PS, Shah VH. Oesophageal varices in cirrhotic patients: from variceal screening to primary prophylaxis of the first oesophageal variceal bleeding. Liver Int. 2010;31:108–119. doi: 10.1111/j.1478-3231.2010.02351.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Lim JK, Members of Veterans Affairs Hepatitis C Resource Center Program Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802–1829. doi: 10.1038/ajg.2009.191. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Baveno V Faculty Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Sebastiani G, Tempesta D, Fattovich G, et al. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale study. J Hepatol. 2010;53:630–638. doi: 10.1016/j.jhep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R. Non-invasive (and minimally invasive) diagnosis of oesophageal varices. J Hepatol. 2008;49:520–527. doi: 10.1016/j.jhep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Thabut D, Moreau R, Lebrec D. Noninvasive assessment of portal hypertension in patients with cirrhosis. Hepatology. 2011;53:683–694. doi: 10.1002/hep.24129. [DOI] [PubMed] [Google Scholar]
- 9.McClain CJ, Kasarskis EJ, Jr, Allen JJ. Functional consequences of zinc deficiency. Prog Food Nutr Sci. 1985;9:185–226. [PubMed] [Google Scholar]
- 10.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130 (5S Suppl):1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka S, Matsumura H, Nakamura H, et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr. 2009;45:292–303. doi: 10.3164/jcbn.08-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himoto T, Hosomi N, Nakai S, et al. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand J Gastroenterol. 2007;42:1078–1087. doi: 10.1080/00365520701272409. [DOI] [PubMed] [Google Scholar]
- 13.Umeta M, West CE, Haidar J, Deurenberg P, Hautvast JG. Zinc supplementation and stunted infants in Ethiopia: a randomised controlled trial. Lancet. 2000;355:2021–2026. doi: 10.1016/S0140-6736(00)02348-5. [DOI] [PubMed] [Google Scholar]
- 14.Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605–1609. doi: 10.1002/hep.1840080622. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa T. Can zinc enhance response interferon therapy for patients with HCV-related liver disease? World J Gastroenterol. 2012;18:3196–3200. doi: 10.3748/wjg.v18.i25.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Saito H, Higashimoto M, Hibi T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: A pilot study. Hepatol Res. 2007;37:405–409. doi: 10.1111/j.1872-034X.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- 17.The French METAVIR Cooperative Study Group Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 18.Paquet KJ. Prophylactic endoscopic sclerosing treatment of esophageal wall in varices—a prospective controlled trial. Endoscopy. 1982;14:4–5. doi: 10.1055/s-2007-1021560. [DOI] [PubMed] [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 20.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto H, Sakai Y, Aizawa N, et al. Association of amino acid imbalance with the severity of liver fibrosis and esophageal varices. Ann Hepatol. 2013;12:471–478. [PubMed] [Google Scholar]
- 22.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 23.Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut. 2000;46 (Suppl III):1–15. doi: 10.1136/gut.46.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schepis F, Cammà C, Niceforo D, et al. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33:333–338. doi: 10.1053/jhep.2001.21410. [DOI] [PubMed] [Google Scholar]
- 25.D'Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol. 1997;11:243–256. doi: 10.1016/s0950-3528(97)90038-5. [DOI] [PubMed] [Google Scholar]
- 26.Eslam M, Ampuero J, Jover M, et al. Predicting portal hypertension and variceal bleeding using non-invasive measurements of metabolic variables. Ann Hepatol. 2013;12:588–598. [PubMed] [Google Scholar]
- 27.Aizawa N, Enomoto H, Imanishi H, et al. Elevation of the glycated albumin to glycated hemoglobin ratio during the progression of hepatitis C virus-related liver fibrosis. World J Hepatol. 2012;4:11–17. doi: 10.4254/wjh.v4.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bando Y, Kanehara H, Aoki K, et al. The glycated albumin to glycated haemoglobin ratio increases along with fibrosis stage in non-alcoholic steatohepatitis. Ann Clin Biochem. 2012;49 (Pt 4):387–390. doi: 10.1258/acb.2012.011139. [DOI] [PubMed] [Google Scholar]
- 29.Sakai Y, Enomoto H, Aizawa N, et al. Relationship between elevation of glycated albumin to glycated hemoglobin ratio in patients with a high bleeding risk of esophageal varices. Hepatogastroenterology. 2012;59:2280–2284. doi: 10.5754/hge12064. [DOI] [PubMed] [Google Scholar]
- 30.Riggio O, Merli M, Capocaccia L, et al. Zinc supplementation reduce blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology. 1992;16:785–789. doi: 10.1002/hep.1840160326. [DOI] [PubMed] [Google Scholar]
- 31.Ebara M, Fukuda H, Hatano R, et al. Metal contents in the liver of patients with chronic liver disease caused by hepatitis C virus. Reference to hepatocellular carcinoma. Oncology. 2003;65:323–330. doi: 10.1159/000074645. [DOI] [PubMed] [Google Scholar]
- 32.Katayama K, Sakakibara M, Imanaka K, et al. Effect of zinc supplementation in patients with type C liver cirrhosis. Open J Gastroenterol. 2011;1:28–34. [Google Scholar]
- 33.Sebastiani G, Castera L, Halfon P, et al. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther. 2011;34:1202–1216. doi: 10.1111/j.1365-2036.2011.04861.x. [DOI] [PubMed] [Google Scholar]
- 34.Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012;12:14. doi: 10.1186/1471-230X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qamar AA, Grace ND, Groszmann RJ, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47:153–159. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]