Abstract
With the aim of developing effective anti-inflammatory drugs, we have been investigating the biochemical effects of shikonin of “Shikon” roots, which is a naphthoquinone with anti-inflammatory and antioxidative properties. Shikonin scavenged reactive oxygen species like hydroxyl radical, superoxide anion (O2•−) and singlet oxygen in previous studies, but its reactivity with reactive oxygen species is not completely understood, and comparison with standard antioxidants is lacking. This study aimed elucidation of the reactivity of shikonin with nitric oxide radical and reactive oxygen species such as alkyl-oxy radical and O2•−. By using electron paramagnetic resonance spectrometry, shikonin was found unable of reacting with nitric oxide radical in a competition assay with oxyhemoglobin. However, shikonin scavenged alkyl-oxy radical from 2,2'-azobis(2-aminopropane) dihydrochloride with oxygen radical absorbance capacity, ORAC of 0.25 relative to Trolox, and showed a strong O2•−-scavenging ability (42-fold of Trolox; estimated reaction rate constant: 1.7 × 105 M−1s−1) in electron paramagnetic resonance assays with CYPMPO as spin trap. Concerning another source of O2•−, the phagocyte NADPH oxidase (Nox2), shikonin inhibited the Nox2 activity by impairing catalysis when added before enzyme activation (IC50: 1.1 µM; NADPH oxidation assay). However, shikonin did not affect the preactivated Nox2 activity, although having potential to scavenge produced O2•−. In conclusion, shikonin scavenged O2•− and alkyl-oxy radical, but not nitric oxide radical.
Keywords: Trolox, superoxide, naphthoquinone, nitric oxide, ORAC
Introduction
With the goal of developing effective drugs for inflammatory diseases, we have been investigating the mechanisms of action and molecular targets of shikonin, which is an active substance of the roots of Lithospermum erythrorhizon (Japanese name: “Shikon”), an herbal medicine used for burns and injuries since ancient times. The chemical structure of shikonin consists of one naphthazarin ring (5,8-dihydroxy-1,4-naphthoquinone) with a side chain (1-hydroxy-4-methyl-3-pentenyl) at its position 2. Shikonin takes the R configuration and its naturally existing S enantiomer is called alkannin. The pharmacological effects of shikonin consist especially of anti-inflammatory effects in vitro and in vivo.(1–3)
The mechanisms of the anti-inflammatory activity of shikonin and its derivatives involve direct inhibition of pro-inflammatory enzymes, and influence on the expression of key molecules in the inflammation response. Such molecules included kinases involved in cellular pro-inflammatory responses like spleen tyrosine kinase (Syk, that is crucial for degranulation response in basophils),(4) and mitogen-activated kinases (MAPKs) such as MAP kinase kinase (MEK), extracellular signal-regulated kinases (ERK), and p38-MAPK.(5) The inhibition of the MAPKs that promote expression of inflammatory cytokines and mediators might be involved in the shikonin-caused suppression of tumor necrosis factor (TNF)-α release,(6) as well as in the decreased nitric oxide (NO) production by lipopolysaccharide-stimulated RAW 264.7 macrophages.(5) Previously shown decreased expression of TNF-α and inducible nitric oxide synthase in RAW 264.7 macrophages and other cells(7–9) could result from inhibition of above MAPKs which are involved in the transcriptional activation of inflammatory responses.
Shikonin also targets enzymatic activity of all isoforms of nitric oxide synthase (NOS).(5) This suggested that the suppression of the endothelium-dependent relaxation of rat thoracic aorta in response to acetylcholine by shikonin might occur due to inhibition of endothelial NOS. At that time, we wondered whether in addition to endothelial NOS inhibition, direct reaction of shikonin with NO could be also involved in the inhibition of vascular response, but to the best of our knowledge, this was unknown.
Concerning the radical scavenging activity of shikonin against reactive oxygen species (ROS), ROS such as superoxide anion (O2•−), hydroxyl radical, singlet oxygen, and tert-butylperoxyl radical were scavenged (Table 1).(10–12) However, its radical scavenging activity is not completely elucidated, and comparison with standard antioxidants is lacking. In addition, conflicting results about its O2•−-scavenging ability remain unexplained: although shikonin reacted with O2•− generated by the hypoxanthine/xanthine oxidase systems (electron paramagnetic resonance, EPR assays),(10,12) one derivative, acetylshikonin, was unable to quench O2•− generated by the activated NADPH oxidase 2 system (Nox2 system) isolated from stimulated polymorphonuclear leukocytes (cytochrome c reduction assay).(13) Nevertheless, shikonin is reported to inhibit the Nox2 activity (i.e., O2•− generation)(13) if added previous to enzyme activation in a cell-free reconstitution assay. In the reconstitution of Nox2 activity in the cell-free assay, the membrane component (Nox2, a.k.a. flavocytochrome b558) and cytosolic components of the Nox2 system are combined in the presence of an amphiphile such as a fatty acid or detergent.(14) The amphiphile leads to assembly of membrane and cytosolic components then forming activated Nox2 complex.(14–16) In this case, the inhibition by shikonin seemed to be directly on the Nox2 enzyme catalysis and not through scavenging of O2•−.
Table 1.
Review of the ROS-scavenging activity of shikonin
| ROS | IC50 (µM) | Assay | Reference |
|---|---|---|---|
| O2•− | 7.2 | EPR† | Sekine et al. (ref. 10) |
| 17 | EPR† | Gao et al. (ref. 12) | |
| •OH | 40§ | EPR† | Sekine et al. (ref. 11) |
| 108 | EPR† | ref. 12 | |
| BuOO• | 27 | EPR‡ | ref. 12 |
| 113 | EPR† | ref. 12 |
The sources of superoxide (O2•−), hydroxyl radical (•OH), singlet oxygen (1O2) and tert-butyl peroxyl radical (BuOO•) were respectively, hypoxanthine/xanthine oxidase system, Fenton reaction, hematoporphyrin irradiated with UVB, and tert-butyl hydroperoxide reacting with methemoglobin and diethylenetriamine-N,N,N',N'',N''-pentaacetic acid. †spin trap: 5,5-dimethyl-1-pyrroline-N-oxide; ‡spin trap: 2,2,6,6-tetramethyl-4-piperidone hydrochloride; §determined for shikonin and alkannin in mixture (82:18).
Since reactive NO species, ROS, and inflammation are associated with the pathogenesis of many diseases, a therapeutic compound with both antioxidant and anti-inflammatory effects like shikonin might be promising in their prevention and treatment. In this study, we examined the possibility of direct reaction between shikonin and NO radical and addressed its ROS-scavenging ability using sensitive EPR spectrometry including Trolox as a standard antioxidant. The effect of shikonin on the O2•−-producing phagocyte Nox2 system was also revisited. The results are discussed aiming a better understanding of the ROS-scavenging properties of shikonin.
Material and Methods
Materials
Shikonin and alkannin were purchased from Wako Pure Chem. Ind. Ltd. (Osaka, Japan) and stocked frozen (−20°C) in small aliquots as 30 mM solutions in dimethylsulfoxide (DMSO; Wako). Myristic acid, sodium salt (MA), 2,2'-azobis(2-aminopropane) dihydrochloride (AAPH), riboflavin, Trolox, sodium diethyldithiocarbamate (DETC), and ferrous sulfate were also from Wako. Cytochrome c (type VI from horse heart), β-NADPH, superoxide dismutase (SOD), and oxyhemoglobin (Oxy-Hb) were from Sigma. Spin trapping reagent 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline-N-oxide (CYPMPO) was from Radical Research (Tokyo, Japan). 3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine (NOC-7), EDTA2Na, and n-heptyl-β-thioglucoside were from Dojindo Laboratories (Kumamoto, Japan). All the reactions were performed under aerobic conditions, and the solutions were not deaerated.
NO radical generation
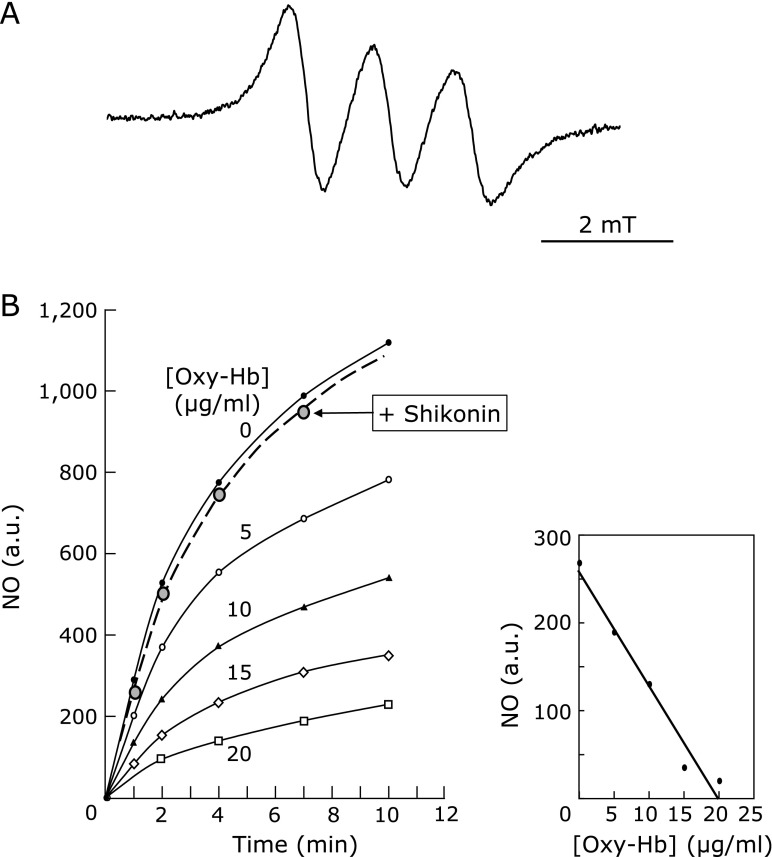
NO radical was generated by NOC-7 (final concentration in the assay, 100 µM), which is a spontaneous NO-releasing agent.(17) The reaction mixture consisted of 50 mM DETC dissolved in an emulsion prepared with INTRAFAT injection 20% (Takeda Pharmaceutical Co. Ltd.; Osaka, Japan) in phosphate buffer, pH 7.0, and supplemented with FeSO4. Addition of NOC-7 did not change the pH of the reaction. The NO radical was stably trapped as a ternary complex, (DETC)2-Fe2+-NO, and then detected by EPR spectrometry at 25°C, being signal intensity plotted against time to derive initial rates of NO production. Then, increasing amounts of oxyhemoglobin (Oxy-Hb: 0–20 µg/ml) were added to capture NO radical, which caused a linear decrease in signal intensity (Fig. 1). The reactivity of shikonin or alkannin with NO was tested competitively with Oxy-Hb, by addition to this system. Solvent DMSO showed no effects in this reaction.
Fig. 1.
Shikonin reactivity with NO. The reaction of shikonin with NO was tested in a competition assay with oxyhemoglobin (Oxy-Hb) and monitored by EPR. The reaction consisted of 100 µM NOC-7 in 50 mM DETC in INTRAFAT emulsion, pH 7.0, supplemented with FeSO4, to which shikonin was added (see Materials and Methods). (A) a representative EPR spectrum of the (DETC)2-Fe2+-NO complex. (B) On the left, changes of signal intensity of the (DETC)2-Fe2+-NO complex by Oxy-Hb but not by shikonin. Oxy-Hb concentrations of 0, 5, 10, 15, and 20 µg/ml are shown by black circles, white circles, black triangles, white rhombus, and white squares, respectively. The assay in the presence of shikonin (0.5 mM) is shown by the dashed line on grey circles. On the right, the NO-reacting activity of Oxy-Hb is shown as a function of concentration. Results are representative of duplicate experiments.
Alkyl-oxy radical generation
The alkyl-oxy radical (RO•) adduct, where R represents the H2N(HN)C-C(CH3)2-group, was generated by incubation of 100 mM AAPH dissolved in 2:8 (v/v) mixture of acetonitrile and 100 mM sodium phosphate buffer, pH 7.4, supplemented with 10 mM CYPMPO. The alkyl-oxy radical was produced by illumination of the mixture as previously described.(18) Tested compounds were added to the reaction mixture (60-µl volume) at the concentrations indicated in the legends, except for Trolox, which was assayed with acetonitrile omitted. EPR spectra were recorded after preincubation of reaction mixtures for 5 min at 37°C. Data analysis and calculation of oxygen radical absorbance capacity (ORAC) against AAPH-derived radical were done as described,(18) by plotting I0/I – 1 against [AOx]0/[ST]0, where I0 and I are the EPR peak heights in the presence of spin trap alone (ST, CYPMPO) and spin trap plus antioxidant (AOx), respectively, and [AOx]0 and [ST]0 are respectively the initial concentrations of the antioxidant and the spin trap. The plot results in a line with slope of kAOx/kST, where kAOx and kST are the reaction rate constants for the radical reaction with antioxidant and spin trap, respectively.(18) When the scavenging activity was expressed relative to Trolox, the results were presented as means ± propagated error to reflect the combined uncertainties from both the antioxidant and Trolox in the calculations.
O2•− generation
O2•− was generated by illumination of riboflavin, as follows. In this reaction, light photoreduced riboflavin reduces oxygen to O2•−. An illuminator (catalog number RUVF-203SR, Radical Research) equipped with a 200-W Xe arc lamp (emitting visible light in the range of 400 to 700 nm; San-ei Electronics, Osaka, Japan) linked by an optical fiber was used to irradiate light to the following reaction components: 1 µM riboflavin, 5 mM EDTA2Na and 10 mM CYPMPO in a 2:8 (v/v) mixture of acetonitrile and 100 mM sodium phosphate buffer, pH 7.4. Tested compounds (Trolox, shikonin, or alkannin) were added to the reaction mixture (volume, 60 µl) and irradiated for 3 min at a fixed intensity of 100 lux after transfer into an EPR cell for spectrum recording. Due to the sensitivity of shikonins to light, illumination intensity was lowered but at a level to keep sufficient S/N ratios in EPR data collection. The stability of shikonin towards light was previously reported to be in the order of few hours (t1/2 = 2 h at exposure to 5,000 lux in aqueous buffer with ethanol),(19) and the restricted 3-min irradiation at lower intensity in our experiments had no influence on both the naphthoquinones. Data analyses were performed as described above for alkyl-oxy radical.
Electron paramagnetic resonance spectrometry (EPR)
EPR spectra of (DETC)2-Fe2+-NO complex were measured at 25°C. NO was expressed as arbitrary units by evaluation of the peak heights from characteristic low-field EPR peaks, in the absence or presence of Oxy-Hb, respectively. EPR spectra of (DETC)2-Fe2+-NO complex were recorded on RE-1X X-band (JEOL, Tokyo, Japan). The spectrometer settings used for recording were as follows: field modulation width, 0.1 mT; microwave power, 10 mW; field scan rate, 336.5 ± 5 mT/3 min; modulation frequency, 100 kHz; time constant, 0.3 s. For EPR detections of the alkyl-oxy radical and O2•−, an RE-1X X-band (JEOL) and an RRX-1XS (Radical Research) EPR spectrometers were used, respectively. Typical spectrometer settings were as follows: field modulation width, 0.1 mT; microwave power, 6 mW; field scan rate, 336.2 ± 7.5 mT/2 min; frequency, 100 kHz; time constant, 0.1 s.
Neutrophil isolation and subcellular fractionation
Resting neutrophils were obtained from porcine peripheral blood as described previously,(20) starting from 2 L of blood. Membranes (105 × g pellet) and cytosol (supernatant fraction) were obtained from centrifugation of postnuclear fraction after cell break by sonication.(21) Membranes were washed in 1 M NaCl and repelleted before solubilization with 1% (w/w) n-heptyl-β-thioglucoside in 10 mM Pipes, pH 7.3, 100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, supplemented with protease inhibitors and 20% (v/v) glycerol.(21,22) In experiments using preactivated Nox2 system, the membrane fraction from stimulated neutrophils was used. Briefly, neutrophils were stimulated by treatment with MA (100 nmoles/107 cells for 10 s) followed by reaction stop with cold Ca+2-free Krebs Ringer phosphate buffer and washing before cell fractionation as above. The flavocytochrome b558 heme content in resting and stimulated membrane fractions was quantified by difference spectra upon reduction with sodium dithionite.(23)
Reconstitution of O2•− generation in cell-free assay
NADPH oxidase activity (referred as Nox2 activity throughout) was assayed in a cell-free reconstitution assay using solubilized resting neutrophil membranes plus cytosol, and an optimal amount of MA as a stimulant.(23) Usually, enzyme activation was performed in two steps: first, a preincubation step of 5 min in a volume of 0.1 ml activation buffer (65 mM sodium phosphate buffer, pH 6.8, with 1.2 mM MgCl2, 1 mM EGTA, and 2 mM sodium azide) which contained membranes (1 pmol Nox2/flavocytochrome b558), cytosol (150 µg protein), 2.5 µM GTP-γ-S, and 34.4 µM MA (final concentrations), then followed by dilution to 0.8 ml with activation buffer. Activity was evaluated by either SOD-sensitive cytochrome c reduction (SOD: 200 U/ml; cytochrome c: 30 µM, ε550–540 nm = 19.1 mM−1cm−1) or NADPH oxidation (125 µM; ε340 nm = 6.2 mM−1cm−1), in assays done, respectively, in plastic cuvettes and in black, narrow quartz cuvettes (1-cm light path). The effects of shikonin or alkannin were tested by addition of either of them 3 min before MA.
Determination of Nox2 enzyme activity of stimulated membranes
Membranes from MA-stimulated neutrophils that contain preassembled Nox2 complex were obtained as described above (’Neutrophil isolation and subcellular fractionation’ section). The effect of shikonin on the O2•−-generating activity of stimulated membranes (2 pmol heme) was assayed after preincubation with shikonin at room temperature for 5 min (cytochrome c reduction) or 2 min before addition of NADPH (NADPH oxidation). Assay volumes and concentrations of cytochrome c and NADPH were the same as in the cell-free assay. Activities were presented as mol O2•− or NADPH/s/mol heme.
Protein determination
The protein content of membrane and cytosol fractions was determined by Lowry method, using bovine serum albumin as a standard.
Results
ROS-scavenging activity of shikonin
Shikonin does not scavenge NO
The reactivity of shikonin as a scavenger of NO was investigated by testing its ability to decrease the EPR signal of the spin adduct (DETC)2-Fe2+-NO (Fig. 1A). The addition of increasing amounts of Oxy-Hb decreases signal intensity of the (DETC)2-Fe2+-NO adduct in a dose-dependent manner, showing 50% inhibition (IC50) at 10 µg Oxy-Hb/ml under the conditions assayed herein (Fig. 1B). However, addition of shikonin or alkannin to this assay had no influence on the signal intensity even at a high final concentration of 0.5 mM (Fig. 1B). It can be concluded that neither shikonin nor alkannin scavenges NO or interacts with the (DETC)2-Fe2+-NO spin adduct.
Shikonin decreases alkyl-oxy radical EPR signal
Next, the scavenging effect of shikonin on AAPH-derived alkyl-oxy radical was examined and compared with that of Trolox in a competition assay with CYPMPO. Shikonin caused lowering of the characteristic peak heights of the alkyl-oxy radical adduct, and the plot of I0/I – 1 as a function of [antioxidant]/[CYPMPO](18) resulted in a linear relationship crossing the origin (data not shown), of which slope corresponded to the ratio kAOx/kCYPMPO of 58 ± 6 (Table 2; mean ± SD, n = 5). The ratios for alkannin and Trolox were 46 ± 3 (n = 5) and 237 ± 23 (n = 5), respectively. The ORAC values relative to Trolox, calculated as previously(18) from the slopes, were 0.25 and 0.20 for shikonin and alkannin, respectively (Table 2). Although lower than Trolox, the scavenging effect of shikonin was comparable to that reported for N-acetylcysteine.(18)
Table 2.
Alkyl-oxy radical-scavenging activity of shikonin
| ORAC† | ||
|---|---|---|
| kAOx/kCYPMPO | relative to Trolox | |
| Shikonin | 58 ± 6 | 0.25 ± 0.03 |
| Alkannin | 46 ± 3 | 0.20 ± 0.02 |
| N-acetylcysteine | 0.40‡ | |
The scavenging of alkyl-oxy radical derived from AAPH was determined by EPR in a competition assay with CYPMPO, as described in Materials and Methods. Shikonin, alkannin or Trolox was assayed at concentrations of 50 and 100 µM. The kAOx/kCYPMPO values are means ± SD (n = 5). †ORAC: Oxygen Radical Absorbance Capacity, the scavenging activity relative to Trolox is expressed as means ± propagated error (n = 5), to reflect both the standard deviations of the antioxidant and Trolox in the calculations: i.e., division of kAOx/kCYPMPO of shikonin or alkannin by that of Trolox kAOx/kCYPMPO = 237 ± 23; means ± SD, n = 5). ‡From Kohri et al. (ref. 18).
Shikonin reacts with O2•− in the EPR assay
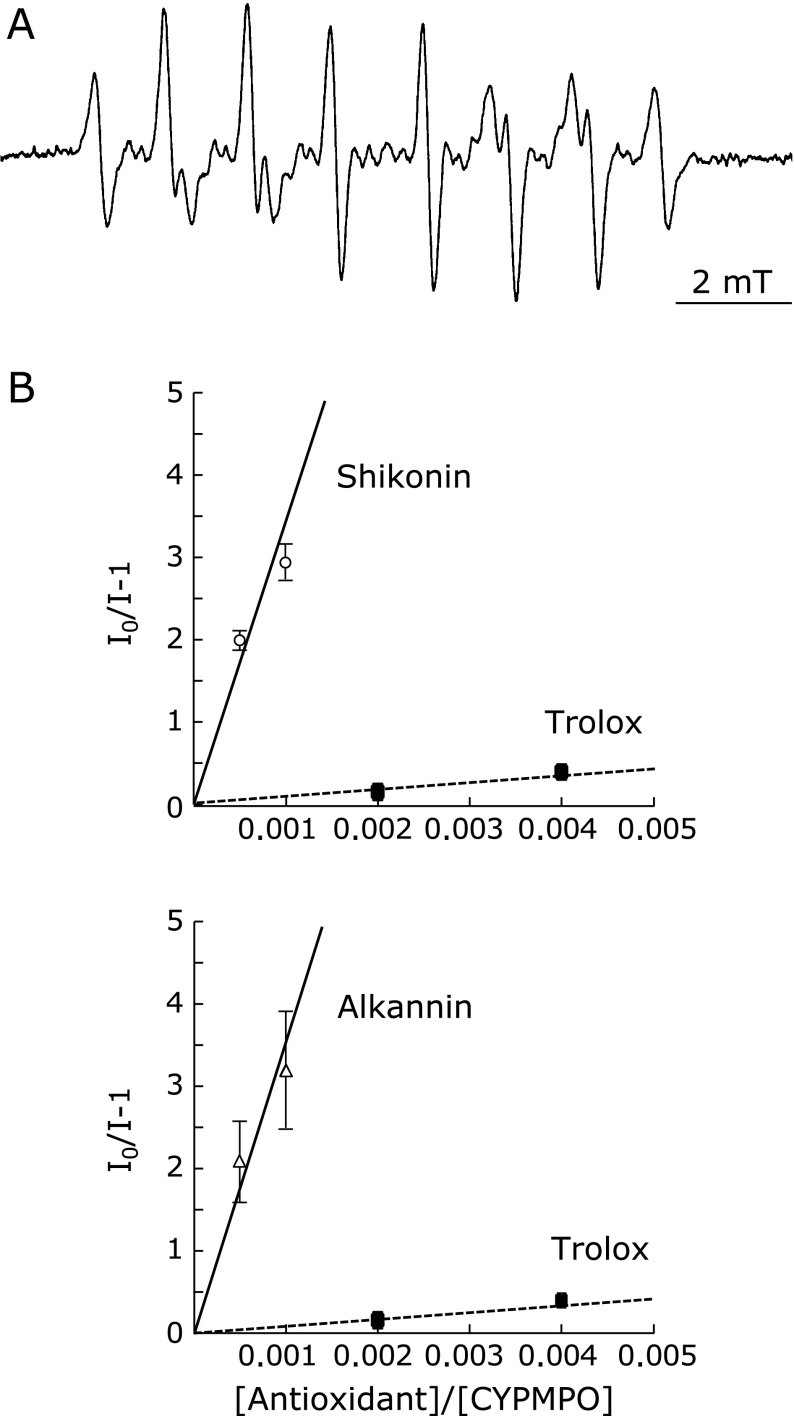
Next, the O2•−-scavenging effect of shikonin was tested in a competition assay with CYPMPO. Addition of shikonin to the reaction decreased the EPR signal of the CYPMPO-O2•− adduct (Fig. 2A), leading to lowering of the characteristic peak heights in a dose-dependent manner (data not shown). Signals from the reduced form of shikonin did not overlap with those of the CYPMPO-O2•− adduct. Similarly to the reaction with the alkyl-oxy radical, the plot of I0/I – 1 against [AOx]0/[CYPMPO]0 for reaction with O2•− gave a zero-crossing line (Fig. 2B), with slope corresponding to the ratio kAOx/kCYPMPO. The plots of shikonin or alkannin gave significantly higher slopes relative to Trolox (Table 3, Fig. 2B): 3,460 ± 530, and 3,550 ± 480 (means ± SD, n = 5), respectively. The slope kAOx/kCYPMPO for Trolox was 83 ± 12 (n = 5). Then, the O2•−-scavenging activity relative to Trolox (that is, when Trolox O2•−-scavenging activity equals 1) was 42.0 ± 8.8 and 43.0 ± 8.5 (means ± propagated error; n = 5), respectively, for shikonin and alkannin (Table 3). From the above slope mean value and by using the rate constant for the reaction of O2•− with CYPMPO (k = 48 M−1s−1),(24) the second-order rate constant of the reaction of O2•− with shikonin could be estimated by calculation as 1.7 × 105 M−1s−1.
Fig. 2.
Shikonin reactivity with O2•−. The reaction of shikonin with O2•− was tested by EPR in a competition assay with CYPMPO (see Materials and Methods). (A) a representative EPR spectrum of the CYPMPO-O2•− adduct. (B) Shikonin or alkannin was used at concentrations of 5 and 10 µM, and Trolox, at 20 and 40 µM. Resulted signal decreases are shown as a plot of I0/I – 1 against [antioxidant]0/[CYPMPO]0. The slopes of the lines represent kAOx/kCYPMPO for shikonin (upper panel), alkannin (lower panel), and Trolox (squares, both panels) as a standard. I0: EPR peak height in the presence of spin trap alone; I: EPR signal height in the presence of spin trap plus antioxidant. Plotted values are means ± SD (n = 5).
Table 3.
O2•−-scavenging activity of shikonin
| O2•−-scavenging activity† | ||
|---|---|---|
| kAOx/kCYPMPO | relative to Trolox | |
| Shikonin | 3,460 ± 530 | 42.0 ± 8.8‡ |
| Alkannin | 3,550 ± 480 | 43.0 ± 8.5‡ |
†The scavenging of O2•− generated by illumination of riboflavin was determined by EPR in a competition assay with CYPMPO, as described in Materials and Methods. Assay conditions are the same as described in Fig. 2. The kAOx/kCYPMPO of Trolox was 83 ± 12 (n = 5). kAOx/kCYPMPO values are means ± SD (n = 5). ‡Scavenging activities relative to Trolox are expressed as means ± propagated error, to reflect both the standard deviations of the antioxidant and Trolox.
Effects of shikonin on the O2•−-generating enzyme, Nox2 system
Shikonin does not affect preactivated Nox2 activity
The reaction of shikonin with Nox2-derived O2•− was examined by testing the effects of shikonin on the O2•−-generating activity of solubilized membranes from stimulated neutrophils, which contain preassembled Nox2 complex. In the SOD-inhibitable cytochrome c reduction assay, shikonin showed no significant effects: the rates relative to the vehicle control (as 100%, specific activity: 2.63 ± 0.22 mol O2•−/s/mol heme; mean ± SD; n = 3) were 107.5%, 106.1%, 100.2%, and 74.9%, respectively, for final concentrations of 0.1, 1.0, 10, and 100 µM shikonin preincubated with membranes (2 pmol heme/assay) for 5-min before addition of NADPH (see Methods). This was a quite lower scavenging activity than that expected from the results of EPR assay, which indicated that few µM shikonin scavenged higher level of O2•−.
An explanation for this disagreement could be that shikonin loses to cytochrome c in the competition for trapping O2•−. Indeed, faster rate constants were reported for the reaction of O2•− with cytochrome c, in the range of 4.0 × 105 to 1.1 × 106 M−1s−1,(25–28) than that for the reaction of shikonin with O2•− estimated herein. Thus the inability of shikonin to scavenge O2•− in the presence of cytochrome c might be because O2•− is trapped faster by cytochrome c.
The no influence of a shikonin derivative, acetylshikonin, on the preactivated Nox2 enzyme on basis of cytochrome c reduction,(13) could have been influenced by the faster reaction of O2•− with cytochrome c, as described above. To examine the effects of shikonin on preactivated Nox2 enzyme without inclusion of cytochrome c, the activity was checked by NADPH oxidation using solubilized membranes from stimulated neutrophils. The oxidation of NADPH reflects the electron flow from NADPH to oxygen via FAD and hemes of the Nox2 enzyme redox center, Nox2 (i.e., flavocytochrome b558). Rates of NADPH oxidation expressed in mol NADPH/s/mol heme, at the shikonin concentrations shown within parentheses, were: 5.2 ± 1.4 (0 µM), 5.3 ± 0.2 (5 µM), and 5.6 ± 1.4 (30 µM) (mean ± SD; n = 3). Shikonin showed no effects on the NADPH oxidation rates even at a relatively high concentration of 30 µM.
These results suggested that shikonin does not influence preactivated Nox2 enzyme: under aerobic conditions, electrons flow from NADPH to oxygen and generate O2•− as indicated by the continuous NADPH oxidation, and if cytochrome c was added at this step, a complete SOD-sensitive cytochrome c reduction could be observed, proving that the final product is O2•−. It can be concluded that shikonin does not affect catalysis of the activated Nox2 enzyme. Although not directly evidenced by the assay method, in agreement with the reactivity of shikonin with O2•− observed in the EPR experiment (Table 3), shikonin would be scavenging O2•− from activated Nox2 in the absence of cytochrome c.
Shikonin inhibits Nox2 enzyme previous to enzyme activation
On the other hand, according to Kawakami et al.,(13) acetylshikonin inhibited Nox2 activity in the cell-free reconstitution assay if added previous to enzyme activation. We revisited the question to assure whether the effect is related or not with reaction with O2•−.
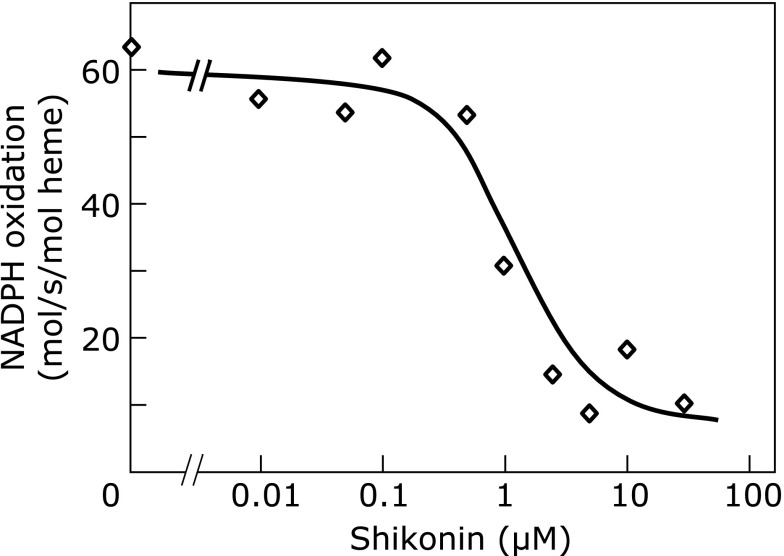
We examined the effects of shikonin on the Nox2 activity in the cell-free assay using detergent solubilized membranes and cytosol from resting neutrophils. Shikonin was added to membranes and cytosol fractions 3 min previous to activation of Nox2 enzyme with MA. After activation for 5 min, the enzyme activity was monitored by NADPH oxidation. Shikonin higher than 0.1 µM gradually inhibited Nox2 activity (IC50: 1.1 µM; Fig. 3). The suppression of NADPH oxidation indicated that electron flow was damaged, suggesting that shikonin affected the Nox2 enzyme catalysis. When cytochrome c was included in the assay as an indicator of O2•− generation, a similar inhibition curve as that shown in Fig. 3, with an IC50 of 1.5 µM for shikonin and 2.2 µM for alkannin was observed (data not shown). Because O2•−, if formed in the assay, reacts faster with cytochrome c than with shikonin for the reasons described above (Shikonin does not affect preactivated Nox2 activity), the inhibition of cytochrome c reduction meant inhibition of Nox2 activity. Therefore, regarding Nox2 enzyme before its activation, the inhibition by shikonin is direct on the enzyme activity but not because of reaction with O2•−.
Fig. 3.
Effects of shikonin on the cell-free reconstitution of Nox2 enzyme. Detergent-solubilized membranes (1 pmol heme-equivalent) and cytosol (150 µg protein) were incubated in the absence or presence of shikonin for 3 min in 0.1 mL of activation buffer (see Materials and Methods), before a 5-min stimulation with 27.5 nmol of myristic acid. The mixture was then transferred to a black cuvette preset in the spectrophotometer containing NADPH (final 125 µM) in activation buffer (final assay volume: 0.8 mL). Decrease in the absorbance at 340 nm indicates NADPH oxidation. Values are means of assays done at least in duplicate, of a representative experiment out of two.
Discussion
In this study, we aimed a better understanding of the effects of shikonin as an antioxidant. Firstly, its scavenging ability for NO and ROS like O2•− and alkyl-oxy radical was investigated in EPR assays. For scavenging activity of O2•− and alkyl-oxy radical, the evaluation was done using competition with CYPMPO and comparison with Trolox as a standard antioxidant. Secondly, its effects on phagocyte Nox2 activity before and after enzyme activation were examined.
Because the previously observed inhibition of the acetylcholine-induced relaxation of precontracted aortic vascular smooth muscle suggested existence of targets in addition to endothelial NOS,(5) it was investigated whether shikonin could directly react with NO radical. This was not the case, since shikonin did not compete with Oxy-Hb for NO (Fig. 1B). These results suggested that together with endothelial NOS,(5) other targets could be involved in its effects on the aortic vascular endothelium and smooth muscle and these remain to be fully elucidated.
Analyses of the ability of shikonin to scavenge alkyl-oxy radicals with Trolox as a reference (Table 2) showed ORAC values comparable to genistein (an isoflavone) and N-acetylcysteine in similar EPR assay conditions.(18) The present antioxidant activity of shikonin and alkannin relative to alkyl-oxy radicals in Trolox equivalents showed a close value as that of previously reported in scavenging of AAPH-derived radical in the crocin bleaching assay (ORAC: 0.34).(29)
Shikonin showed strong reactivity with O2•− as shown by its ability to suppress CYPMPO-O2•− adduct peaks (data not shown). Its reactivity with O2•− in comparison with Trolox in the spin trapping assay with CYPMPO was 42 times higher (Fig. 2, Table 3). The rate constant estimated in our assay for shikonin (1.7 × 105 M−1s−1; Results, Shikonin reacts with O2•− in the EPR assay) was an order lower than that reported previously under different assay conditions (1.4 × 106 M−1s−1).(10) Our results indicated that shikonin is a stronger scavenger of O2•− than Trolox. In order to compare the O2•−-scavenging effect with those of previous reports which evaluated it on basis of IC50 in the assays, the IC50 of shikonin in our EPR assay was calculated according to Mitsuta et al.(30) using the followings: [CYPMPO] = 10 mM, the estimated rate constant for O2•− reaction with shikonin (1.7 × 105 M−1s−1), and that with CYPMPO from Kamibayashi et al. (48 M−1s−1).(24) The result was 2.9 µM, which is lower than those reported previously for shikonin (Table 1).(10,12) The herein estimated IC50 for O2•−-scavenging ability was comparable to those of l-DOPA, caffeic acid and dopamine (1.2–2.1 µM) assayed in competition with CYPMPO, which is the group of strong antioxidants that places second after epigallocatechins and gallic acid (0.35–0.62 µM).(31 and refs. therein)
Shikonin, however, could not inhibit O2•− generation from preactivated Nox2 enzyme from stimulated neutrophils in the SOD-sensitive cytochrome c reduction assay (Results, Shikonin does not affect preactivated Nox2 activity), similar to a previous study with acetylshikonin.(13) An explanation for this observation could be a masking effect due to the lower reaction rate of shikonin with O2•− than that of cytochrome c, as predicted from the respective rate constants. Herein, it was assured that the preactivated Nox2 activity is intact since NADPH oxidation remained unchanged (Results, same section as above). Electrons flow through the redox carriers in flavocytochrome b558 in the following order according to midpoint redox potentials: NADPH/NADP+, FAD/FADH2, and hemes. Finally, oxygen is reduced to O2•−.(15 and refs. therein) Since under the assay conditions, NADPH is mostly consumed for O2•−-generation (because, as described in Results, the rate of NADPH oxidation fitted with that of SOD-sensitive cytochrome c reduction according to the reaction NADPH + 2 O2 → NADP+ + H+ + 2 O2•−),(32) the product O2•− is reacting with shikonin. It can be assumed that in the aerobic conditions of the assay containing around 3-fold Km of NADPH (reported Km for Nox2 in solubilized membranes: 45 µM),(33) the turnover of the enzyme would be constant and depending only on the amount of active enzyme complex, so that NADPH oxidation would remain constant during the assay. Although NADPH oxidation is not direct evidence for reaction of O2•− with shikonin, the O2•−-scavenging activity evidenced in the EPR assay (Fig. 2, Table 3) suggests that shikonin is free to react with Nox2-derived O2•− in the absence of other competitors like cytochrome c.
The inhibition of the Nox2 activity appeared when shikonin was added before enzyme activation (IC50, 1.1 µM in the cell-free assay; Fig. 3: NADPH oxidation assay), but it was not because of scavenging of O2•−. The inhibition was on enzyme catalysis, as shown by impaired NADPH oxidation, and O2•− was not produced (the reduction of cytochrome c was also inhibited with an IC50 of 1.5 µM; Results, Shikonin inhibits Nox2 enzyme previous to enzyme activation). Prevention of catalysis could involve direct targeting of redox centers or other catalytically relevant sites of the Nox2 enzyme complex, and/or impairment of assembly of components of Nox2 system. Since shikonin is known to react with thiols,(34) targeting of sensitive cysteines in the Nox2 system, whose existence was suggested by thiol-modifying inhibitors like phenylarsine oxide(35–37) and gliotoxin,(38,39) might be involved. Previous studies also suggested failure in the assembly of Nox2 system in acetylshikonin-treated neutrophils due to an impaired translocation of cytosolic p47phox to the membranes.(40) Whatever the mechanisms of the inhibition, the fact that shikonin inhibits respiratory burst of neutrophils before activation could have implications in its effects in vivo, in modulation of inflammation-related events.
A finding against reactivity of shikonin with O2•− was that acetylshikonin was unable to scavenge O2•− produced by the dihydroxyfumaric acid autoxidation as detected by nitroblue tetrazolium reduction.(40) In this assay, no substantial kinetic competition would be expected from nitroblue tetrazolium having a slower rate constant for the reaction with O2•− (5 × 104 M−1s−1)(41) than that estimated for the reaction with shikonin. The reason for the inability of shikonin to prevent this reaction needs further elucidation.
Some studies have analyzed the radical scavenging activity of shikonin theoretically on basis of molecular density functional theory, which allows calculation of parameters such as ionization potential (indicator of electron-donating ability) and polarity index (log P, i.e., partition coefficient of the compound in n-octane/water as an index of hydrophilicity and lipophilicity).(29,42,43) These chemical properties would be influenced by the reaction system, such as the presence of other agents able to impose thermodynamic and kinetic barriers for shikonin to react with ROS.
In conclusion, shikonin was shown to react with alkyl-oxy radical and O2•− anion but not with NO. Given that oxidative stress and inflammation are linked each other in the pathology of many diseases, the fact of shikonin being a powerful scavenger of O2•−, with anti-inflammatory effects as well, might expand its future therapeutic applications.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (#21590106) from the MEXT to H. T.-O.
Abbreviations
- AAPH
2,2'-azobis(2-aminopropane) dihydrochloride
- CYPMPO
5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline-N-oxide
- DETC
sodium diethyldithiocarbamate
- DMSO
dimethylsulfoxide
- EPR
electron paramagnetic resonance
- MA
myristic acid, sodium salt
- NO
nitric oxide
- NOC-7
3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine
- NOS
nitric oxide synthase
- Nox2
NADPH oxidase 2
- O2•−
superoxide anion
- ORAC
oxygen radical absorbance capacity
- Oxy-Hb
oxyhemoglobin
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin and related naphthazarin natural products. Angew Chem Int Ed. 1999;38:270–301. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 3.Papageorgiou VP, Assimopoulou AN, Ballis AC. Alkannins and shikonins: a new class of wound healing agents. Curr Med Chem. 2008;15:3248–3267. doi: 10.2174/092986708786848532. [DOI] [PubMed] [Google Scholar]
- 4.Takano-Ohmuro H, Yoshida LS, Yuda Y, Morioka K, Kitani S. Shikonin inhibits IgE-mediated histamine release by human basophils and Syk kinase activity. Inflamm Res. 2008;57:484–488. doi: 10.1007/s00011-008-8067-9. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida LS, Kawada T, Irie K, et al. Shikonin directly inhibits nitric oxide synthases: possible targets that affect thoracic aorta relaxation response and nitric oxide release from RAW 264.7 macrophages. J Pharmacol Sci. 2010;112:343–351. doi: 10.1254/jphs.09340fp. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida LS, Kawada T, Irie K, et al. New targets of shikonin that impair RAW 264.7 macrophages and vascular smooth muscle responses. J Pharmacol Sci. 2010;112 (Suppl. 1):149. doi: 10.1254/jphs.09340fp. [DOI] [PubMed] [Google Scholar]
- 7.Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor α promoter in vivo. J Biol Chem. 2004;279:5877–5885. doi: 10.1074/jbc.M309185200. [DOI] [PubMed] [Google Scholar]
- 8.Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH, Kang JJ. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-κB signaling. J Ethnopharmacol. 2008;120:264–271. doi: 10.1016/j.jep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Nam KN, Son MS, Park JH, Lee EH. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-κB: neuroprotective implications. Neuropharmacology. 2008;55:819–825. doi: 10.1016/j.neuropharm.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Sekine T, Masumizu T, Maitani Y, Nagai T. Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. Intl J Pharm. 1998;174:133–139. [Google Scholar]
- 11.Sekine T, Masumizu T, Maitani Y, Takayama K, Kohno M, Nagai T. Effect of shikonin and alkannin on hydroxyl radical generation system concerned with iron ion. Yakugaku Zasshi. 1998;118:609–615. doi: 10.1248/yakushi1947.118.12_609. [DOI] [PubMed] [Google Scholar]
- 12.Gao D, Kakuma M, Oka S, Sugino K, Sakurai H. Reaction of β-alkannin (shikonin) with reactive oxygen species: detection of β-alkannin free radicals. Bioorg Med Chem. 2000;8:2561–2569. doi: 10.1016/s0968-0896(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami N, Koyama Y, Tanaka J, Ohara A, Hayakawa T, Fujimoto S. Inhibitory effect of acetylshikonin on the activation of NADPH oxidase in polymorphonuclear leukocytes in both whole cell and cell-free systems. Biol Pharm Bull. 1996;19:1266–1270. doi: 10.1248/bpb.19.1266. [DOI] [PubMed] [Google Scholar]
- 14.Molshanski-Mor S, Mizrahi A, Ugolev Y, Dahan I, Berdichevsky Y, Pick E. Cell-free assays: the reductionist approach to the study of NADPH oxidase assembly, or “all you wanted to know about cell-free assays but did not dare to ask”. Methods Mol Biol. 2007;412:385–428. doi: 10.1007/978-1-59745-467-4_25. [DOI] [PubMed] [Google Scholar]
- 15.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 17.Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J Org Chem. 1993;58:1472–1476. [Google Scholar]
- 18.Kohri S, Fujii H, Oowada S, et al. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant’s scavenging capacity against AAPH-derived free radicals. Anal Biochem. 2009;386:167–171. doi: 10.1016/j.ab.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Cho MH, Paik YS, Hahn TR. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J Agr Food Chem. 1999;47:4117–4120. doi: 10.1021/jf9902853. [DOI] [PubMed] [Google Scholar]
- 20.Kakinuma K, Kaneda M, Chiba T, Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem. 1986;261:9426–9432. [PubMed] [Google Scholar]
- 21.Fujii H, Kakinuma K. Electron transfer reactions in the NADPH oxidase system of neutrophils-involvement of an NADPH-cytochrome c reductase in the oxidase system. Biochim Biophys Acta. 1991;1095:201–209. doi: 10.1016/0167-4889(91)90100-c. [DOI] [PubMed] [Google Scholar]
- 22.Miki T, Yoshida LS, Kakinuma K. Reconstitution of superoxide-forming NADPH oxidase activity with cytochrome b558 purified from porcine neutrophils. Requirement of a membrane-bound flavin enzyme for reconstitution of activity. J Biol Chem. 1992;267:18695–18701. [PubMed] [Google Scholar]
- 23.Yoshida LS, Saruta F, Yoshikawa K, Tatsuzawa O, Tsunawaki S. Mutation at histidine 338 of gp91phox depletes FAD and affects expression of cytochrome b558 of the human NADPH oxidase. J Biol Chem. 1998;273:27879–27886. doi: 10.1074/jbc.273.43.27879. [DOI] [PubMed] [Google Scholar]
- 24.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Rad Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 25.Koppenol WH, van Buuren KJ, Butler J, Braams R. The kinetics of the reduction of cytochrome c by the superoxide anion radical. Biochim Biophys Acta. 1976;449:157–168. doi: 10.1016/0005-2728(76)90130-4. [DOI] [PubMed] [Google Scholar]
- 26.Seki H, Ilan YA, Ilan Y, Stein G. Reactions of the ferri-ferrocytochrome-c system with superoxide/oxygen and CO2−/CO2 studied by fast pulse radiolysis. Biochim Biophys Acta. 1976;440:573–586. doi: 10.1016/0005-2728(76)90043-8. [DOI] [PubMed] [Google Scholar]
- 27.McCord JM, Crapo KJ, Fridovich I. Superoxide dismutase assays: a review of methodology. In: Michelson AM, McCord JM, Friedovich I, editors. Superoxide and Superoxide Dismutases. New York: Academic Press; 1977. pp. 11–17. [Google Scholar]
- 28.Butler J, Hoey BM. The one-electron reduction potential of several substrates can be related to their reduction rates by cytochrome P-450 reductase. Biochim Biophys Acta. 1993;1161:73–78. doi: 10.1016/0167-4838(93)90198-z. [DOI] [PubMed] [Google Scholar]
- 29.Ordoudi SA, Tsermentseli SK, Nenadis N, Assimopoulou AN, Tsimidou MZ, Papageorgiou VP. Structure-radical scavenging activity relationship of alkannin/shikonin derivatives. Food Chem. 2011;124:171–176. [Google Scholar]
- 30.Mitsuta K, Mizuta Y, Kohno M, Hiramatsu M, Mori A. The application of ESR-spin-trapping technique to the evaluation of SOD-like activity of biological substances. Bull Chem Soc Jpn. 1990;63:187–191. [Google Scholar]
- 31.Nakajima A, Sakurai Y, Matsuda E, et al. Ability of water-soluble biosubstances to eliminate hydroxyl and superoxide radicals examined by spin-trapping ESR measurements: two-dimensional presentation of antioxidative ability. Biosci Biotechnol Biochem. 2013;77:324–331. doi: 10.1271/bbb.120749. [DOI] [PubMed] [Google Scholar]
- 32.Babior BM. Activation of the respiratory burst oxidase. Environ Health Perspect Dec. 1994;102 (Suppl 10):53–56. doi: 10.1289/ehp.94102s1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross AR, Parkinson JF, Jones OT. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984;223:337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao D, Hiromura M, Yasui H, Sakurai H. Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol Pharmaceut Bull. 2002;25:827–832. doi: 10.1248/bpb.25.827. [DOI] [PubMed] [Google Scholar]
- 35.Le Cabec V, Maridonneau-Parini I. Complete and reversible inhibition of NADPH oxidase in human neutrophils by phenylarsine oxide at a step distal to membrane translocation of the enzyme subunits. J Biol Chem. 1995;270:2067–2073. doi: 10.1074/jbc.270.5.2067. [DOI] [PubMed] [Google Scholar]
- 36.Kutsumi H, Kawai K, Johnston RB, Jr, Rokutan K. Evidence for participation of vicinal dithiols in the activation sequence of the respiratory burst of human neutrophils. Blood. 1995;85:2559–2569. [PubMed] [Google Scholar]
- 37.Doussiere J, Poinas A, Blais C, Vignais PV. Phenylarsine oxide as an inhibitor of the activation of the neutrophil NADPH oxidase - identification of the beta subunit of the flavocytochrome b component of the NADPH oxidase as a target site for phenylarsine oxide by photoaffinity labeling and photoinactivation. Eur J Biochem. 1998;251:649–658. doi: 10.1046/j.1432-1327.1998.2510649.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida LS, Abe S, Tsunawaki S. Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem Biophys Res Commun. 2000;268:716–723. doi: 10.1006/bbrc.2000.2192. [DOI] [PubMed] [Google Scholar]
- 39.Nishida S, Yoshida LS, Shimoyama T, Nunoi H, Kobayashi T, Tsunawaki S. Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase. Infect Immun. 2005;73:235–244. doi: 10.1128/IAI.73.1.235-244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JP, Tsao LT, Raung SL, Hsu MF, Kuo SC. Investigation of the inhibition by acetylshikonin of the respiratory burst in rat neutrophils. Br J Pharmacol. 1997;121:409–416. doi: 10.1038/sj.bjp.0701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auclair C, Voisin E. Nitroblue tetrazolium reduction. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 123–132. [Google Scholar]
- 42.Wu HQ, Huang ZS, Bu XZ, et al. The molecular mechanisms involved in the cytotoxicity of alkannin derivatives. Eur J Med Chem. 2005;40:1341–1345. doi: 10.1016/j.ejmech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Taubert D, Breitenbach T, Lazar A, et al. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Rad Biol Med. 2003;35:1599–1607. doi: 10.1016/j.freeradbiomed.2003.09.005. [DOI] [PubMed] [Google Scholar]