Abstract
The song system of birds consists of several neural pathways. One of these, the anterior forebrain pathway, is necessary for the acquisition but not for the production of learned song in zebra finches. It has been shown that the anterior forebrain pathway sequentially connects the following nuclei: the high vocal center, area X of lobus parolfactorius, the medial portion of the dorsolateral thalamic nucleus, the lateral magnocellular nucleus of anterior neostriatum (IMAN), and the robust nucleus of the archistriatum (RA). We now show in zebra finches (Taeniopygia guttata) that IMAN cells that project to RA also project to area X, forming a feedback loop within the anterior forebrain pathway. The axonal endings of the IMAN projection into area X form cohesive and distinct domains. Small injections of tracer in subregions of area X backfill a spatially restricted subset of cells in IMAN, that, in turn, send projections to RA that are arranged in horizontal layers, which may correspond to the functional representation of vocal tract muscles demonstrated by others. We infer from our data that there is a myotopic representation throughout the anterior forebrain pathway. In addition, we suggest that the parcellation of area X into smaller domains by the projection from IMAN highlights a functional architecture within X, which might correspond to units of motor control, to the representation of acoustic features of song, or both.
Full text
PDF

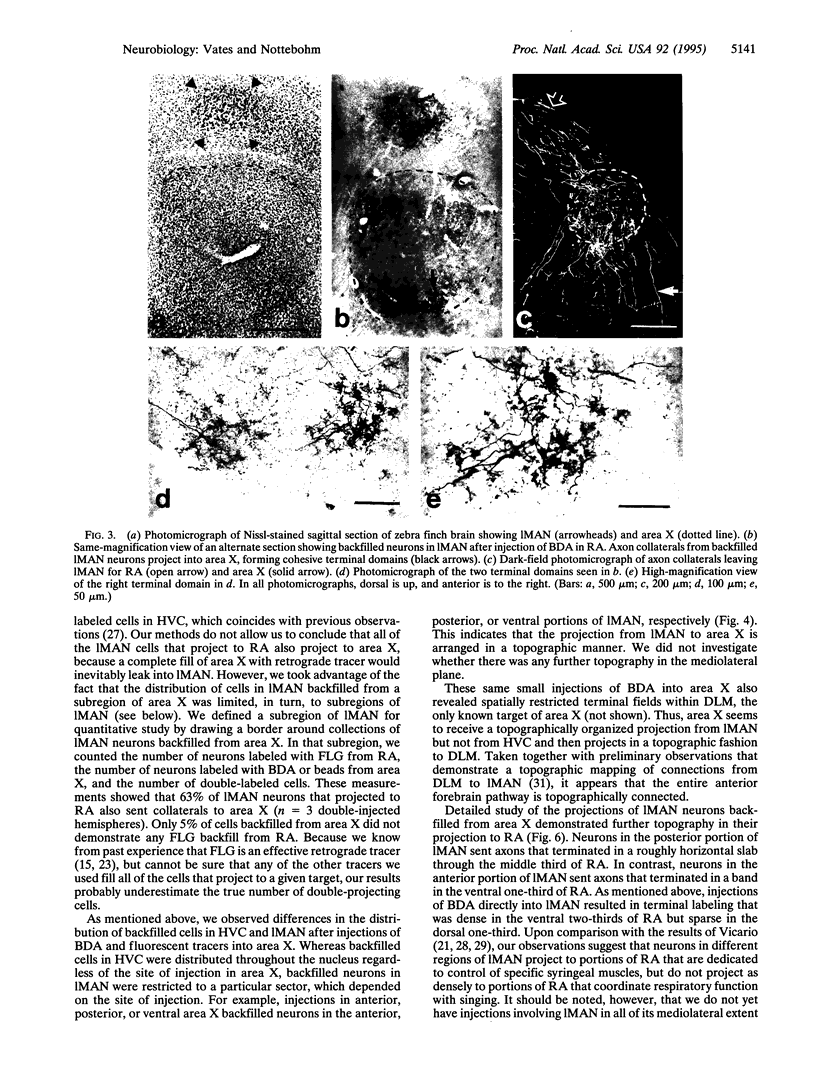


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Buylla A., Theelen M., Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Vicario D. S. Simple microcomputer system for mapping tissue sections with the light microscope. J Neurosci Methods. 1988 Sep;25(2):165–173. doi: 10.1016/0165-0270(88)90155-0. [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Halsema K. A., Brown S. A., Miesner E. A. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989 Jan 8;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Miesner E. A., Arnold A. P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984 May 25;224(4651):901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K., Arnold A. P. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: a quantitative electron microscopic study. J Neurosci. 1991 Jul;11(7):2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Gurney M. E. Auditory responses in the zebra finch's motor system for song. Brain Res. 1981 Sep 21;221(1):192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965 Dec;22(7):770–783. [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983 May;3(5):1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci. 1986 Jun;6(6):1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland J. S., Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. G., Nottebohm F. Role of a telencephalic nucleus in the delayed song learning of socially isolated zebra finches. J Neurobiol. 1993 Aug;24(8):1045–1064. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Kelley D. B., Paton J. A. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982 Jun 1;207(4):344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Okuhata S., Saito N. Synaptic connections of thalamo-cerebral vocal nuclei of the canary. Brain Res Bull. 1987 Jan;18(1):35–44. doi: 10.1016/0361-9230(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Paton J. A., O'Loughlin B. E., Nottebohm F. Cells born in adult canary forebrain are local interneurons. J Neurosci. 1985 Nov;5(11):3088–3093. doi: 10.1523/JNEUROSCI.05-11-03088.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C., Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991 Sep;11(9):2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued L., Kyriakidis K., Heimer L. In vivo anterograde and retrograde axonal transport of the fluorescent rhodamine-dextran-amine, Fluoro-Ruby, within the CNS. Brain Res. 1990 Aug 27;526(1):127–134. doi: 10.1016/0006-8993(90)90258-d. [DOI] [PubMed] [Google Scholar]
- Simpson H. B., Vicario D. S. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990 May;10(5):1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F., Nordeen E. J., Nordeen K. W. Characterization of neurons born and incorporated into a vocal control nucleus during avian song learning. Brain Res. 1993 Aug 27;620(2):335–338. doi: 10.1016/0006-8993(93)90176-n. [DOI] [PubMed] [Google Scholar]
- Sohrabji F., Nordeen E. J., Nordeen K. W. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990 Jan;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Stokes T. M., Leonard C. M., Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974 Aug 1;156(3):337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Veenman C. L., Reiner A., Honig M. G. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992 Mar;41(3):239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Vicario D. S. A new brain stem pathway for vocal control in the zebra finch song system. Neuroreport. 1993 Jul;4(7):983–986. doi: 10.1097/00001756-199307000-00037. [DOI] [PubMed] [Google Scholar]
- Vicario D. S. Motor mechanisms relevant to auditory-vocal interactions in songbirds. Brain Behav Evol. 1994;44(4-5):265–278. doi: 10.1159/000113581. [DOI] [PubMed] [Google Scholar]
- Vicario D. S. Organization of the zebra finch song control system: II. Functional organization of outputs from nucleus Robustus archistriatalis. J Comp Neurol. 1991 Jul 22;309(4):486–494. doi: 10.1002/cne.903090405. [DOI] [PubMed] [Google Scholar]
- Vicario D. S., Yohay K. H. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. J Neurobiol. 1993 Apr;24(4):488–505. doi: 10.1002/neu.480240407. [DOI] [PubMed] [Google Scholar]
- Vu E. T., Mazurek M. E., Kuo Y. C. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994 Nov;14(11 Pt 2):6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J. M. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol. 1993 Dec 8;338(2):225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]









