Abstract
Leishmania, a protozoan parasite, causes a wide range of human diseases ranging from the localized self-healing cutaneous lesions to fatal visceral leishmaniasis. Toxicity of traditional first line drugs and emergence of drug-resistant strains have worsened the situation. DNA topoisomerase II in kinetoplastid protozoan parasites are of immense interest as drug target because they take part in replication of unusual kinetoplast DNA network. In this study, we have taken target-based therapeutic approaches to combat leishmaniasis. Two isobenzofuranone compounds, viz., (1) 3,5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH3) and (2) (4-bromo)-3′-hydroxy-5′-(4-bromophenyl)-benzophenone(JVPH4) were synthesized chemically and characterized by NMR and mass spectrometry analysis. Activity of type II DNA topoisomerase of leishmania (LdTOPII) was monitored by decatenation assay and plasmid cleavage assay. The antiparasitic activity of these compounds was checked in experimental BALB/c mice model of visceral leishmaniasis. Isobenzofuranone derivatives exhibited potent antileishmanial effect on both antimony (Sb) sensitive and resistant parasites. Treatment with isobenzofuranone derivatives on promastigotes caused induction of reactive oxygen species (ROS)-mediated apoptosis like cell death in leishmania. Both the compounds inhibited the decatenation activity of LdTOPII but have no effect on bi-subunit topoisomerase IB. Treatment of LdTOPII with isobenzofuranone derivatives did not stabilize cleavage complex formation both in vitro and in vivo. Moreover, treatment with isobenzofuranone derivatives on Leishmania donovani-infected mice resulted in clearance of parasites in liver and spleen by induction of Th1 cytokines. Taken together, our data suggest that these compounds can be exploited as potential antileishmanial agents targeted to DNA topoisomerase II of the parasite.
Keywords: Antileishmanial agents, apoptosis, DNA topoisomerase II, isobenzofuranone, Leishmania donovani
Introduction
Leishmaniasis is one of the major fatal parasitic diseases with significant morbidity and mortality worldwide and is caused by protozoan parasites belonging to the genus Leishmania (Herwaldt 1999). Depending on the species variation, the disease can have a wide range of clinical manifestation and categorized into three different types, namely, cutaneous, mucocutaneous, and visceral leishmaniasis. Cutaneous leishmaniasis is the most common form and visceral leishmaniasis is the most severe form (Coler and Reed 2005). Kala-azar or visceral leishmaniasis caused by Leishmania donovani is a major problem in the developing countries (Topno et al. 2010). Most of the drugs used for the therapy of this disease are antimonial preparations such as sodium stibogluconate (pentostam) and meglumine antimonate (glucantime) and are known as first-line drugs. The second-line drugs like amphotericin B, miltefosine, and paromomycin although clinically used are all severely toxic and as a consequence lead to patient noncompliance and emergence of drug-resistant strains (Ashutosh and Goyal 2007; Chappuis et al. 2007). Presently, there is no available vaccine against leishmaniasis and therefore chemotherapy remains the major medical mode for managing the diseases (Kedzierski 2010).
DNA topoisomerases are long known as targets for antibacterial and anticancer therapy (Pommier et al. 2010). The enzymes participate in all kind of DNA metabolic processes. Based on the number of strands they cleave, these enzymes are classified as type I or type II DNA topoisomerase (Champoux 2001). DNA topoisomerase II in kinetoplastid protozoan parasites are of immense interest because they take part in replication of unusual kinetoplast DNA network (kDNA) inside mitochondria (Shapiro 1994; Das et al. 2008). Type II DNA topoisomerases have emerged as principal therapeutic targets, with a group of targeting agents having a broad spectrum of antiparasitic activity (Bakshi and Shapiro 2003). DNA topoisomerase II of leishmania consists of an N-terminal ATPase, a central DNA-binding, and an unconserved C-terminal domain (Sengupta et al. 2003, 2005a,b). The inhibitors of type II topoisomerase can be divided into two classes: topoisomerase II poisons and catalytic inhibitors or class II inhibitors (Nitiss 2009). Poisons stabilize the covalent enzyme–DNA complex and block rejoining of the DNA break. These include bacterial DNA gyrase poisons such as quinolone antibiotics and eukaryotic topoisomerase II poisons doxorubicin, amsacrine, etoposide, and teniposide (Pommier et al. 2010). Class II inhibitors interfere with DNA topoisomerase II during different stages of the catalytic cycle without trapping the covalent enzyme–DNA complexes (Larsen et al. 2003). All class II drugs mediate their action by either binding to the enzyme, which prevents them to sit on the substrate DNA, (e.g., merbarone and acetyl boswellic acids) (Fortune and Osheroff 1998; Syrovets et al. 2000) or by interacting with the ATPase domain and thus interfering with the ATPase activity of the enzyme that produces “closed clamps”, for example, bisdioxopiperazines ICRF-187 and ICRF-193 (Jensen et al. 2000). This class II inhibitors are ‘catalytic inhibitors’ and they exert their antiproliferative effects by depleting the essential enzymatic function (Larsen et al. 2003; Nitiss 2009).
Leishmania donovani infection causes strong immunosuppressive effect in the host (Awasthi et al. 2004) by downregulating the production of reactive oxygen species (ROS) and nitric oxide (NO) within the host macrophages (Lima-Junior et al. 2013). Leishmania infection impairs the production of host protective (Th1) cytokines, for example, interferon-γ (IFN-γ), interleukin-12, and interleukin-2 and induces the production of the disease-promoting (Th2) cytokines, for example, transforming growth factor β (TGF-β), interleukin-10, and interleukin-4 (Kemp et al. 1993). Consequently, a potential therapy for leishmaniasis would not only be limited to the antileishmanial property of the molecule but also be to modulate immune responses mediated by the parasite-infected immune cells (Walker et al. 1999).
Phthalides or 1(3H)-Isobenzofuranone are a prominent class of natural products that possess significant biological properties (Lin et al. 2005). Phthalides also serve as valuable synthetic intermediates. An aryl-substituted isobenzofuranone-1(3H)-one was found to be the inhibitors of the lymphocyte pore-forming protein perforin (Spicer et al. 2012). Antiplatelet activity of 3-butyl-6-bromo-1(3H)-isobenzofuranone was reported both in vitro and ex vivo in rat platelets aggregation (Ma et al. 2012). Several compounds in the group of 1(3H)-Isobenzofuranone exhibited anticancer activity (Ye et al. 1998; Karna et al. 2010). In a recent communication, it was reported that different derivatives of 2,4-dihydroxybenzophenone exhibited leishmanicidal activity against Leishmania amazonensis (MacielRezende et al. 2013).
In this study, we have shown that the chemically synthesized 3,5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH3) and (4-bromo)-3′-hydroxy-5′-(4-bromophenyl)-benzophenone (JVPH4) exhibit potent antileishmanial property. Both the compounds are cytotoxic to antimony-sensitive and also to antimony-resistant parasites. JVPH3 and JVPH4 inhibit LdTOPII activity by interfering the initial topoisomerase II–DNA binary complex formation. Isobenzofuranone derivatives mediated inhibition of topoisomerase II hampers the basic metabolic process in cell and ultimately lead to apoptosis like cell death of the parasite. Moreover, these compounds modulate host immune response by increasing the production of Th1 cytokines in experimental mouse model and reduced the parasite burden in liver and spleen of the infected animal. Our data demonstrate for the first time the novel antileishmanial property of isobenzofuranone derivatives that exert their action via targeting topoisomerase II in leishmania.
Materials and Methods
Chemicals
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Invitrogen Life Technologies. dimethyl sulfoxide (DMSO), pyruvate kinase, lactate dehydrogenase, camptothecin, and etoposide were purchased from Sigma chemicals (St. Louis, MO). Recombinant human topoisomerase IIα (hTOPIIα) was purchased from Topogen Inc. (Port Orange, FL). Recombinant LdTOPIB was prepared as described previously (Das et al. 2006). All drugs were dissolved in 100% DMSO at a concentration of 20 mmol/L and stored at −20°C.
Synthesis of 3,5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH-3) and (4-bromo)-3′-hydroxy-5′-(4-bromophenyl)-benzophenone (JVPH-4)
To a 50 mL flask fitted with magnetic stirrer were added activated I2 (45 mg, 20 mol%), K2CO3 (276 mg, 2 mmol/L), (E)-1,4-bis(4-chlorophenyl)but-2-ene-1,4-dione (234 mg, 1 mmol/L) or (E)-1,4-bis(4-bromophenyl)but-2-ene-1,4-dione (394 mg, 1 mmol/L) and methyl acetoacetate (128 mg, 1.1 mmol/L) in dry i-PrOH (10 mL). The reaction mixture was then stirred under reflux at 110°C for 0.5 h. After disappearance of the starting material (monitored by thin layer chromatography) the reaction mixture was allowed to cool at room temperature. Solvent was removed from the reaction mixture under reduced pressure in vacuum. The residue was then diluted with water (10 mL) followed by extraction with CHCl3 (3 × 25 mL). The organic layer was collected, washed with brine, and then dried over anhydrous Na2SO4. Removal of solvent resulted in a solid mass which was subjected to column chromatography over neutral alumina using petroleum ether and increasing proportion of chloroform as eluent. Petroleum ether: chloroform (70:30) eluent gave a solid which was recrystallized from chloroform–petroleum ether as a white solid to afford 3,5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH-3) (235 mg, 60%), whereas elution of the corresponding reaction mixture (E)-1,4-bis(4-bromophenyl)but-2-ene-1,4-dione with petroleum ether: chloroform (50:50) afforded a solid which was recrystallized to give a white solid (4-bromo)-3′-hydroxy-5′-(4-bromophenyl)-benzophenone (JVPH-4) (150 mg, 35%).
Parasite maintenance and cultures
Leishmania donovani, sodium antimony gluconate (SAG)-sensitive (SbS) MHOM/IN/1983/AG83 (AG83) and the one SAG-resistant (SbR) strain, GE1 were used. Amastigotes obtained from the spleens of infected hamsters were cultured at 22°C in Medium199 (M199) media to obtain promastigotes. Promastigotes were further grown in 10% (v/v) heat-inactivated fetal bovine serum (FBS) as described (Saha et al. 2013).
Measurement of cell viability
Leishmania donovani AG83 promastigotes and laboratory developed SAG-resistant (SbR) GE1 parasites (3.0 × 106 cells mL−1) were incubated with different concentrations of JVPH3 and JVPH4 for 12 h, following which the survival percentage was estimated by MTT assay. Parasites treated with 0.5% DMSO served as controls. MTT is reduced to purple formazan in the mitochondria of living cells. Formazan is then solubilized, and the concentration determined by optical density at 570 nm. Percentage of viable promastigotes in each treatment groups was determined with respect to untreated control cells (Roy et al. 2008).
Measurement of ROS
Promastigotes (2.0 × 106 cells mL−1) were treated with JVPH3 or JVPH4 for indicated time periods. Parasites treated with 0.5% DMSO served as controls. After different treatments, parasites were washed and loaded with a cell permeant dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for 1 h. Fluorometric measurements (λex = 510 nm and λem = 530 nm) were performed in triplicate, and the results were expressed as in the mean fluorescence intensity per 106 cells (Roy et al. 2008).
Measurement of reduced glutathione level
Leishmania donovani promastigotes (2.0 × 106 cells mL−1) were treated with JVPH3 or JVPH4 at different times. Parasites treated with 0.5% DMSO served as controls. The cells were then lysed by cell lysis buffer according to the manufacturer’s protocol (Apo Alert glutathione assay kit; Clontech, Mountain View, CA). Cell lysates were incubated with monochlorobimane for 3 h and glutathione levels were detected by fluorometer (λex = 395 nm and λem = 480 nm) (Sen et al. 2004). Spectrofluorometric data presented here are representative of three experiments.
Measurement of total fluorescent lipid peroxidation product
Promastigotes (2.0 × 106 cells mL−1) were treated with JVPH3 or JVPH4 for indicated time periods. Parasites treated with 0.5% DMSO served as controls. After different treatments, parasites were washed twice with 1X PBS and the pellet was resuspended in 2 mL of 15% SDS-PBS solution. The fluorescence intensities of the total fluorescent lipid peroxidation products were measured with excitation at 360 nm and emission at 430 nm and expressed as relative fluorescence units with respect to quinine sulfate (Sen et al. 2004). Spectrofluorometric data presented here are representative of three experiments.
Double staining with annexinV and PI
Externalization of phosphatidyl serine on the outer membrane of untreated and JVPH3- and JVPH4-treated promastigotes was measured by the binding of FITC-annexinV and PI using an annexinV staining kit (Invitrogen BioServices India Pvt. Ltd, Bangalore, India). Flow cytometry was carried out for treated and untreated parasites. The gating was done so that the FL-1 channel denotes the mean intensity of FITC-annexinV, whereas the FL-2 channel denotes the mean intensity of PI (Chowdhury et al. 2012).
DNA fragmentation assay
The extent of DNA fragmentation after drug treatments was estimated using the cell death detection ELISA kit (Roche Diagnostics Corporation, Indianapolis, IN). Promastigote samples (5.0 × 106 cells mL−1) were collected 0, 2, 4, and 6 h post treatment with these compounds and the histone-associated DNA fragments (mononucleosome and oligonucleosome) were detected using the manufacturer’s protocol (Chowdhury et al. 2012).
Purification of recombinant LdTOPII
Escherichia coli BL21 (DE3) pLysS cells harboring pET16bLdTOPII, (Sengupta et al. 2005b) were induced at 0.6 OD600 with 0.5 mmol/L isopropyl β-D-1-thiogalactopyranoside at 22°C for 4 h. The cells were harvested and resuspended in phosphate buffer (pH 7.8) containing 300 mmol/L NaCl, 200 μg mL−1 lysozyme, 0.1% Triton X-100, 0.25% Sarkosyl and 1 mmol/L phenylmethylsulfonyl fluoride. The lysates obtained after sonication on ice was cleared by centrifugation. The cleared lysate was passed through Ni-nitrilo triacetic acid (Ni-NTA) agarose column (Qiagen India Pvt. New Delhi, India). After washing with phosphate buffer (pH 7.8) containing 300 mmol/L NaCl and 30 mmol/L imidazole, elution was done using 250 mmol/L imidazole. For further purification, the fractions were pooled dialyzed and chromatographed on phosphocellulose column (P11 cellulose, Whatman) as described (Sengupta et al. 2005b).
Decatenation assay
Decatenation assays were performed in total volumes of 25 μL containing 25 mmol/L Tris-HCl pH 7.9, 10 mmol/L MgCl2, 0.1 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 mmol/L DTT, 50 mmol/L NaCl, 10% glycerol, 2 mmol/L adenosine triphosphate (ATP), 200 ng of kDNA from L. donovani strain UR6, and one unit of purified LdTOPII or hTOPIIα. One unit of enzyme activity is defined as the amount of enzyme needed for 50% decatenation of 200 ng kDNA networks into minicircles. The assays were carried at 30°C for 30 min and the reaction products were analyzed by electrophoresis on a 1% agarose gel, stained with ethidium bromide (EtBr) (0.5 μg mL−1), and photographed under UV illumination (Sengupta et al. 2003).
Plasmid cleavage reaction
Cleavage reaction was performed as described previously (Sengupta et al. 2005b). A 20 μL reaction mixture contained 200 ng negatively supercoiled pRYG DNA, 10 mmol/L Tris–HCl (pH 7.5), 100 mmol/L KCl, 0.1 mmol/L EDTA, 5 mmol/L MgCl2, 0.5 mmol/L DTT, 2 mmol/L ATP, 30 μg mL−1 bovine serum albumin (BSA), and 5 unit of enzymes. Reaction mixture was incubated at 37°C for 30 min and terminated by the addition of 0.5% SDS and 10 mmol/L EDTA. The mixture was further incubated with 100 μg /mL proteinase K at 37°C for 30 min and analyzed by electrophoresis on a 1% agarose gel. EtBr at a final concentration of 0.5 μg/mL was included in the gel to resolve the linear product (Form III) from the supercoiled molecule (Form I). Control assays always contained an amount of drug diluent (DMSO) equivalent to that present in drug containing reaction.
DNA unwinding assay
Unwinding assay was performed with 50 fmol of supercoiled and relaxed pBluscript (SK+) DNA in the presence or absence of different concentrations of JVPH3 and JVPH4 in a 50 μL reaction mixture as described (Pommier et al. 1987). Relaxed DNA was prepared by treatment of the supercoiled plasmid DNA with excess of topoisomerase I, followed by proteinase K digestion at 37°C, phenol/chloroform extraction, and ethanol precipitation. After incubation at 37°C for 15 min, reactions were terminated by the addition of prewarmed stop solution (5% SDS, 15% Ficoll and 0.25% Bromophenol Blue) and electrophoresed on to 1% agarose gel. The DNA band was stained with 0.5 μg mL−1 of EtBr and visualized by UV light.
ATPase assay
ATPase measurements were carried out by the pyruvate kinase/lactate dehydrogenase assay described previously (Sengupta et al. 2005a). Reaction mixture contained 0.1 mmol/L NADH, 2 mmol/L phosphoenolpyruvate, 2 mmol/L ATP, three units of pyruvate kinase, and four units of lactate dehydrogenise. Reactions were initiated by mixing the reaction mixture and ATP, both preequilibrated to 30°C. Decrease in NADH concentration was monitored by measuring the absorbance at 340 nm in a UV–visible spectrophotometer.
Electrophoretic mobility shift assay
The labeling of 36-mer oligonucleotide 1(5′ATGAAATCTAACAATGCGCTCATCGT CATCCTCGGC3′) containing high-affinity topoisomerase II binding site was done using polynucleotide kinase (Roche Biochemicals). The labeled oligonucleotide 1 was annealed to oligonucleotide 2 (3′TACTTTAGATTGTTACGCGAGTAGCAGTAGGACCG5′) in a buffer containing 40 mmol/L Tris–HCl (pH 7.5), 20 mmol/L MgCl2, and 50 mmol/L NaCl at 70°C for 1 h and allowed to cool down slowly to room temperature. Briefly, the reaction was done in a 20 μL binding buffer containing 50 mmol/L Tris– HCl (pH 7.5), 1 mmol/L DTT, 4 mmol/L MgCl2, 50 mmol/L KCl, and 15 μg mL−1 BSA and 5 pmol of 36 bp duplex oligonucleotide and LdTOPII enzyme and varying concentrations of JVPH3 and JVPH4. Reaction mixtures were incubated at 4°C for 30 min and electrophoresed through 7% nondenaturing polyacrylamide gel and autoradiographed (Sengupta et al. 2005b).
Immunoband depletion assay
Promastigotes (2.0 × 107) were cultured for 12 h at 22°C with or without drugs. Nuclear fractions were isolated as previously described (Sen et al. 2004). The nuclear fractions after lysis with 1% SDS were subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were electrophoretically transferred on to nitrocellulose membranes. Immunoblotting of immobilized proteins was carried out using a rabbit antibody raised against ATPase domain (43 kDa) of L. donovani topoisomerase II (Sengupta et al. 2005a).
Bioethics
BALB/c mice, originally obtained from Jackson Laboratories, Bar Harbor, ME and reared in the Institute Animal facilities, were used for experimental purposes with prior approval of the Animal Ethics Committee. The studies and animal handling were approved by IICB Animal Ethical Committee (Registration no. 147/1999, CPCSEA), registered with Committee for the purpose of Control and Supervision on Experiments on Animals (CPCSEA), Govt. of India.
Infection of mice and treatment regimen
For experimental visceral infections, female BALB/c mice (4–6 week old and 20–25 g each) were injected via intracardiac route with 2X107 hamster spleen-transformed L. donovani promastigotes. Three weeks post infection, JVPH3 and JVPH4 were administered to infected animals via intraperitoneal routes at 5 and 10 mg kg−1 body weight separately twice a week for a period of 3 weeks (Chowdhury et al. 2012). Visceral infection was determined by Giemsa-stained impression smears of spleen and liver from 6-week infected mice and reported as Leishman Donovan Units (LDU), calculated as the number of parasites per 1000 nucleated cells × organ weight (in mg) (Stauber 1956).
Estimation of cytokine levels by enzyme-linked immunosorbent assay
Splenocytes were isolated from different groups of BALB/c mice by mechanical disruption of spleen and lysis of red blood corpuscles by 0.14 mol/L Tris-buffered NH4Cl. Cells were plated in triplicate at 5.0 × 105 cells mL−1 in 96-well plates (BD Biosciences, San Diego, CA) and allowed to proliferate for next 48 h at 37°C in 5% CO2 incubator in presence or absence of 25 μg mL−1 soluble leishmanial antigen (SLA) (Mukherjee et al. 2013). Cytokines production by SLA pulsed splenocytes from different mice groups was determined by ELISA kit (BD Biosciences) as per manufacturer’s instruction.
Statistical analysis
Data are provided as means ± SEM or means ± SD, of the number of independent experiments. Data were tested for significance using the paired Student’s t-test. Differences were considered statistically significant when P < 0.05. Statistical analysis and graphical representation were performed with GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA).
Results
Synthesis and spectral analysis of isobenzofuranone derivatives
Two isobenzofuranone derivatives, namely, 3, 5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH-3) and (4-bromo)-3′-hydroxy-5′-(4-bromophenyl) benzophenone (JVPH-4) were synthesized chemically as described in “Materials and Methods” section. The chemical structure of JVPH3 and JVPH4 is presented in Figure 1A and B, respectively.
Figure 1.
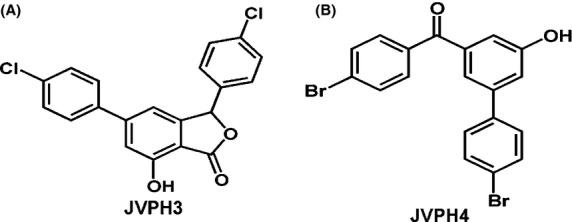
Chemical structure of isobenzofuranone derivatives. 3,5-bis(4-chlorophenyl)-7-hydroxyisobenzofuran-1(3H)-one (JVPH3) (A) and (4-bromo)-3′-hydroxy-5′-(4-bromophenyl)-benzophenone (B).
Spectral data of JVPH3 were given below.
IR νmax (KBr, cm−1): 3210, 1728, 8211H NMR (CDCl3, 600 MHz): δ 7.79 (s, 1H), 7.55-7.57 (m, 4H), 7.40 (dd, J = 6.6, 1.8 Hz, 2H), 7.20 (dd, J = 6.6, 1.8 Hz, 2H), 7.15 (s, 1H), 6.40 (s, 1H); 13C NMR (CDCl3, 150 MHz): δ 171.4, 156.4, 150.1, 149.9, 138.3, 134.8, 132.3, 132.2, 129.0 (2C), 128.8 (4C), 123.8, 123.4, 114.8, 112.8, 109.8, 83.1; EI MS m/z: 392.86 [M+Na]+; Anal. Calcd. For C20H12Cl2O3: C, 64.71; H, 3.26; Found: C, 64.62; H, 3.22.
Spectral data of JVPH4 were given below
IR νmax (KBr, cm−1): 3249, 1635, 8221H NMR (CDCl3, 600 MHz): δ 7.78 (d, J = 8.4, 2H), 7.49–7.43 (m, 6H), 7.40 (d, J = 8.4, 2H), 7.26 (s, 1H); 13C NMR (CDCl3, 150 MHz): δ 195.1, 156.3, 141.9, 139.3, 139.2, 138.1, 135.5, 134.2, 132.1, 131.8, 131.5, 129.1 (2C), 128.8, 128.4 (2C), 121.1, 118.2,115.5; EI MS m/z: 432.05 [M]+; Anal. Calcd. For C19H12Br2O2: C, 52.81; H, 2.80; Found: 52.59, 2.72.
Isobenzofuranone derivatives exhibited cytotoxic activity on L. donovani by induction of apoptosis like cell death
To determine the cytotoxic effect of isobenzofuranone derivatives (JVPH3 and JVPH4) on the growth of leishmania, the L. donovani AG83 promastigotes and on laboratory grown antimony-resistant (SbR) GE1 parasite were exposed to five different concentrations of JVPH3 and JVPH4 for 12 h, after which the cell viability was determined by the MTT assay as described under “Materials and Methods” (Fig. 2A and B). Cells treated with 0.2% DMSO served as control. IC50 values of the compounds on AG83 and GE1 promastigotes are given in Table 1. We checked the morphological changes in JVPH3- and JVPH4-treated parasite by microscopic study and no attributable phenotypic changes were observed (data not shown). Antileishmanial effect of both JVPH3 and JVPH4 was further checked on amastigote form of AG83 and GE1 parasite (Fig. S1A and B).
Figure 2.
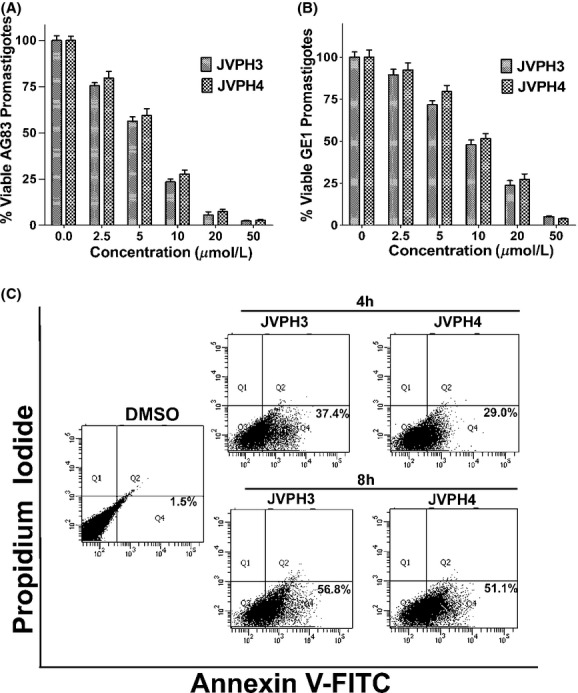
Cytotoxic effect of isobenzofuranone derivatives on Leishmania donovani and cell death. Percentage of viable AG83 and GE1 promastigotes after treatment with different concentrations of JVPH3 and JVPH4 were plotted in (A) and (B), respectively. The results are shown as mean ± SD of three independent experiments. Flow cytometric analysis of L. donovani promastigote death through PCD/necrotic processes using annexin V-FITC and PI in FL-1 versus FL-2 channels. The cells in the bottom right quadrant in each of the panels indicated apoptosis (C).
Table 1.
IC50 value (μmol/L) of isobenzofuranone compounds on antimony sensitive and resistant promastigotes
| Compounds | Parasite name | IC50 (μmol/L) |
|---|---|---|
| JVPH3 | AG83 (SbS) | 5.40 ± 0.0014 |
| GE1(SbR) | 9.81 ± 0.0017 | |
| JVPH4 | AG83 (SbS) | 6.01 ± 0.0011 |
| GE1(SbR) | 11.54 ± 0.0012 |
The results shown are the means of three independent experiments and represent mean ± SD from three independent experiments.
Next, we checked that whether this cytotoxic effect of isobenzofuranones was due to the occurrence of cell death in leishmania. Promastigotes were treated with 10 μmol/L of each JVPH3 and JVPH4 for 4 and 8 h, and then the annexinV-positive cell population was determined by flow cytometry (Fig. 2C). Flow cytometry analysis revealed that JVPH3 treatment caused 37.4% and 56.8% annexinV-positive parasite for 4 and 8 h treatment, respectively. Similarly JVPH4 treatment for 4 h caused 29% annexinV-positive and for 8 h caused 51.1% annexinV-positive parasites, respectively.
To understand whether JVPH3 and JVPH4 induce ROS inside cells, promastigotes were exposed to 10 μmol/L JVPH3 and JVPH4 for different time points. The time course experiment suggested that both the compounds induce ROS inside the parasite (Fig. S2A). Pretreatment of the parasite with N-acetyl cysteine (NAC), an antioxidant followed by treatment with JVPH3 did not show any induction of ROS in the experimental condition. We have also monitored other downstream events of ROS generation inside the cells such as peroxidation of membrane lipid molecules and reduction of endogenous antioxidant such as reduced glutathione level inside the cell after drug treatment. Treatment of the parasite with JVPH3 and JVPH4 increased the level of lipid peroxidation in a time-dependent way, (Fig. S2B) on the other hand, level of reduced glutathione decreased drastically with increasing time of incubation increased (Fig. S2C).
We also checked the level of DNA fragmentation in JVPH3- and JVPH4-treated parasite. For these purposes, promastigotes were exposed to 20 μmol/L of JVPH3 or JVPH4 for different time points. The extent of DNA fragmentation in treated parasites was estimated using an enzyme-linked immunosorbent assay (ELISA)-based assay and time-dependent increase of DNA fragmentations were observed in both JVPH3- and JVPH4-treated parasites (Fig. S2D).
Isobenzofuranone derivatives inhibit the activity of LdTOPII but not LdTOPIB
We have checked the inhibitory effect of isobenzofuranone derivatives on the activity of LdTOPII. Decatenation assay was performed with the purified fraction of recombinant DNA topoisomerase (LdTOPII) and the release of minicircle DNA from kDNA network was checked by agarose gel electrophoresis (Fig. 3A). LdTOPII was incubated with different concentrations of JVPH3 and JVPH4 along with the substrate kDNA. Dose–response study revealed that both JVPH3 and JVPH4 completely inhibited decatenation activity of LdTOPII at 25μmol/L concentration. We also checked the inhibitory effect of isobenzofuranone derivatives on the activity of hTOPIIα. Both JVPH3 and JVPH4 inhibited decatenation activity of hTOPIIα in much more higher concentration (200 μmol/L) as compared to LdTOPII (Fig. S3).
Figure 3.
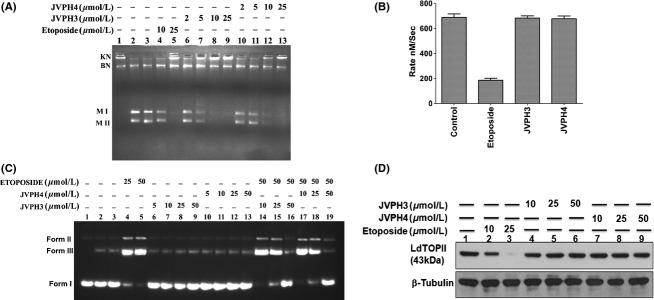
Inhibitory activity of isobenzofuranone derivatives on LdTOPII. Purified LdTOPII was incubated with 200 ng kDNA in presence of different concentrations of JVPH3 and JVPH4. Lane 1, kDNA only; lane 2, kDNA treated with LdTOPII; lane 3, same as lane 2 but in presence of DMSO. KN and BN indicate kDNA network and broken network, respectively. M II and M I indicate the released minicircles super coiled and nicked form (A). ATPase activity of LdTOPII was measured by the pyruvate kinase/lactate dehydrogenase assay. Rate of LdTOPII-mediated ATP hydrolysis in presence of (50 μmol/L) etoposide and 50 μmol/L of JVPH3/JVPH4 were measured (B). The results are shown as mean ± SD of three independent experiments. Inhibition of etoposide-induced cleavage complex formation by isobenzofuranone derivatives was analyzed by cleavage reaction and agarose gel electrophoresis. Lane 1, negatively supercoiled pRYG DNA; lane 2, pRYG DNA with LdTOPII alone; lane 3 same as lane 2 but in presence of proteinase k treatment (C). In vivo LdTOPII-mediated cleavage complex stabilization by isobenzofuranone derivates was analyzed by immunoband depletion assay. Equal amounts of protein from different treatments were subjected to SDS-PAGE followed by immunoblotting with antibody raised against ATPase domain of LdTOPII. β-tubulin served as loading control (D).
To check the effect of JVPH3 and JVPH4 on the ATPase activity of LdTOPII, ATPase activity was measured by the pyruvate kinase/lactate dehydrogenase assay as described in “Materials and Methods” section. It was observed that both JVPH3 and JVPH4 did not interfere with LdTOPII-mediated ATP hydrolysis (Fig. 3B). Etoposide inhibits the rate of enzyme-catalyzed ATP hydrolysis used as positive control in the experiments.
To check the effect of JVPH3 and JVPH4 on LdTOPIB activity, plasmid DNA relaxation assay was performed. None of these compounds exhibited any inhibitory effect on LdTOPIB (Fig. S4). To check whether JVPH3 and JVPH4 intercalate into DNA, DNA unwinding assay was performed with supercoiled and relaxed plasmid DNA as described in “Materials and Methods” section. Supercoiled and relaxed plasmid DNAs were separately treated with LdTOPIB in the presence of different concentrations of JVPH3 and JVPH4 (Fig. S5A and B). A net negative supercoiling of the relaxed substrate DNA was introduced in the presence of strong intercalative agent EtBr (positive control), but JVPH3 and JVPH4 had no effect on the topological state of the DNA up to 200μmol/L concentration.
Isobenzofuranone derivatives inhibit cleavable complex formation by abrogating topoisomerase–DNA interaction
To explore the mechanism of inhibition of LdTOPII, electrophoretic mobility shift assays were performed to study the effect of JVPH3 and JVPH4 on the initial topoisomerase II–DNA binary complex formation. 32P labeled 36 bp duplex linear DNA containing high-affinity binding site of topoisomerase II was used as substrate (Sengupta et al. 2005b). LdTOPII binds with this DNA substrate and caused the mobility shift of the labeled DNA. When LdTOPII was preincubated with increasing concentrations of JVPH3 and JVPH4, prior to the addition of 36 bp duplex DNA, no shift of mobility was observed. These results suggest that JVPH3 and JVPH4 prevented the primary interaction between LdTOPII and the substrate DNA (Fig. S6).
Since the binding of TOPII and DNA precedes the enzyme–DNA cleavable complex formation, LdTOPII-mediated DNA cleavage was assayed with different concentrations of JVPH3 and JVPH4. For this purpose, plasmid cleavage reaction was performed under equilibrium condition by reacting LdTOPII with pRYG DNA. Etoposide was used as positive control which stabilizes the cleavage complex formation. As shown in Figure 3C, both 25 and 50 μM etoposide convert closed circular DNA (Form I) to linear DNA (Form III) by stabilization of the “cleavable complex” (lanes 4 and 5). Lane 2 shows the formation of nicked circular DNA (Form II) and linear DNA (Form III) as a result of cleavage of pRYG DNA with LdTOPII alone. This serves as the background cleavage of LdTOPII. When the cleavage assay was performed with increasing concentrations of these compounds with LdTOPII, no remarkable linear products (lanes 6–9 for JVPH3, lanes 10–13 for JVPH4) were observed, whereas etoposide at 25 and 50 μmol/L stabilized the cleavable complex. Moreover, when topoisomerase II was preincubated with 10, 25, and 50 μmol/L concentrations of these derivatives before the addition of 50 μmol/L etoposide (lanes 14–16 for JVPH3 and lanes 17–19 for JVPH4), the etoposide-mediated cleavage was inhibited drastically with increasing concentrations of these compounds and was completely inhibited at 50 μmol/L concentration of each derivative. These results suggest that JVPH3 and JVPH4 derivatives inhibit the binding of enzyme to substrate DNA and thus inhibit cleavable complex formation. To understand whether such in vitro observations were comparable in vivo JVPH3- and JVPH4-treated L. donovani AG83 promastigotes were assayed for immunoband depletion experiments as described in “Materials and Methods”. If topoisomerase II can form a covalent complex with genomic DNA inside the cells, then topoisomerase II–DNA cleavable complex cannot enter into the gel. It was observed that etoposide treatment for 6 h on promastigotes caused the depletion of the immunoband of topoisomerase II. On the other hand, when the promastigotes were treated with increasing concentrations of JVPH3 and JVPH4, no remarkable depletion of the respective bands was observed (Fig. 3D).
Treatment with isobenzofuranone derivatives reduced splenic and liver parasite burden and polarized Th1 immune response
To understand whether these compounds can be exploited as antileishmanial agents, the compounds were administered in an experimental visceral leishmaniasis mouse model as described in “Materials and Methods” section. Treatment with JVPH3 and JVPH4 (5 mg kg−1 day−1) caused ∼71.30% and ∼58.80% reduction of liver parasite burden (Fig. 4A) and ∼69.50% and ∼58.40% reduction of splenic parasitic burden (Fig. 4B), respectively, compared to the parasitic load in infected animal liver and spleen tissues. The effect was more pronounced with 10 mg kg−1 day−1 of JVPH3 and JVPH4 administration, as there was huge reduction of hepatic and splenetic parasite burden compared to infected control tissues. Treatment of both the compounds induced ROS and NO in splenocytes compared to drug untreated infected control (Fig. S7). For further investigation of antileishmanial mechanism of JVPH3 and JVPH4, we measured Th1 and Th2 cytokines level by ELISA analysis. Both JVPH3 and JVPH4 caused the increased production of Th1 cytokine (IL-12 and TNF-α) and reduced Th2 cytokine (IL-10) production compared to drug untreated infected mice. The results demonstrated in Figure 4C suggest that treatment of JVPH3 in two different doses (5 and 10 mg kg−1 day−1) caused the induction of IL-12 level of ∼ 4.50 and ∼5.68 fold and of TNF-α level of ∼11.40 and ∼16.02-fold, respectively, compared to drug untreated infected mice. Similarly, JVPH4 in two different doses (5 and 10 mg kg−1 day−1) caused the induction of IL-12 level of ∼ 4.21 and ∼5.32-fold and TNF-α level of ∼10.70 and ∼15.17-fold, respectively, compared to drug untreated infected mice. However, Th2 cytokine IL-10 decreased after JVPH3 and JVPH4 treatment, compared to infected controls. Treatment of JVPH3 in two different doses (5 and 10 mg kg−1 day−1) caused the reduction of IL-10 level of ∼2.48 and ∼7.90-fold, respectively, compared to drug untreated infected mice (Fig. 4D). Similarly, JVPH4 in two different doses (5 and 10 mg kg−1 day−1) caused the reduction of IL-10 level of ∼2.24 and ∼8.42-fold compared to drug untreated infected mice. Collectively, these data suggest that isobenzofuranone derivatives confer immunobalance in L. donovani infected mice by switching to Th1 response.
Figure 4.
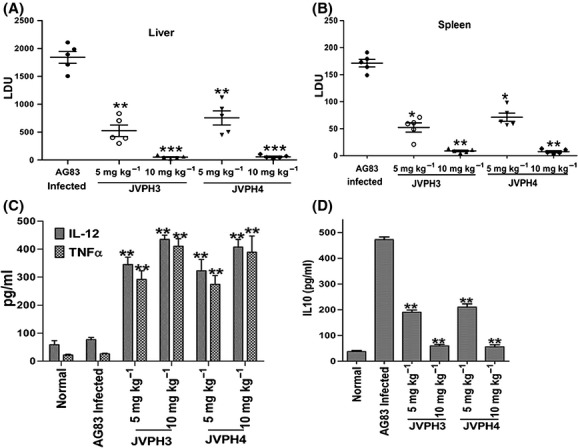
In vivo antileishmanial effects of isobenzofuranone derivatives on Leishmania donovani infected BALB/c mouse model of visceral leishmaniasis. Animals were sacrificed and liver (A) and splenic (B) parasite load was determined by stamp-smear method and expressed as LDU for all groups. Untreated, infected mice were used as controls. Data are presented as mean ± SEM (n = 5 animal per group). Splenocytes isolated from different group of mice were plated aseptically and incubated with 25 μg mL−1 SLA for 48 h. IL-12 and TNF-α in supernatants of splenocyte cultures were assayed by ELISA (C). IL-10 in supernatants of splenocyte cultures were assayed by ELISA (D). Values represent the mean ± SD. (3–5 mice/group) *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t-test as compared to different JVPH3 and JVPH4 treatment groups with infection control). ns indicates that differences are not significant.
Discussion
Because of the emergence of drug unresponsive strains of leishmania and toxic side effects of available drugs, it is necessary to identify newer targets and also to develop newer therapeutic agents to combat this infection. In this study, we have identified two novel isobenzofuranone derivatives, namely, JVPH3 and JVPH4 that exhibited potent antileishmanial effect on both antimony sensitive and resistant parasites via targeting DNA topoisomerase II. This study also illustrates the molecular mechanism of inhibition of LdTOPII and the mechanism of cell death. Earlier studies suggested several natural products and synthetic molecules that exhibited antileishmanial effect by targeting bi-subunit topoisomerase IB of L. donovani (Ray et al. 1998; Sen et al. 2004; Das et al. 2008; Roy et al. 2008). Flavonoids such as quercetin, luteolin, and baicalein have been shown to possess antileishmanial property by targeting topoisomerase IB and inducing DNA damage in leishmania (Das et al. 2006, 2008; BoseDasgupta et al. 2008). The pentacyclic triterpenoid, dihydrobetulinic acid (DHBA) also manifests antileishmanial activity by catalytically inhibiting the bi-subunit topoisomerase IB (Chowdhury et al. 2003, 2011). Very few compounds have recently been identified that exhibit antileishmanial effect by targeting leishmanial topoisomerase II (Mittra et al. 2000). The compounds JVPH3 and JVPH4 inhibited the decatenation activity of LdTOPII but have no inhibitory effect on bi-subunit topoisomerase IB activity. A topoisomerase reaction has three general mechanistic steps: binding of the enzyme to the substrate DNA, strand breakage and subsequent strand passage through the break and strand religation (Stewart et al. 1998; Koster et al. 2005). It is evident from EMSA analysis that both JVPH3 and JVPH4 inhibit the enzyme DNA complex formation and suggest that these compounds act on DNA-binding step. Since the binary complex formation precedes the enzyme–DNA cleavable complex formation, it was expected that isobenzofuranone would not stabilize the cleavable complex. Unlike etoposide, a known eukaryotic DNA topoisomerase II class I inhibitor that stabilizes cleavage complex, JVPH3 and JVPH4 do not form any cleavage complex in vitro, suggesting that these compounds act as catalytic inhibitors. Interestingly, when topoisomerase II was incubated with JVPH3 and JVPH4 prior to treatment with etoposide, the compounds abrogated the cleavage complex formation. The plausible explanation of this finding might be that the isobenzofuranone derivatives interfere with the DNA-binding activity of the enzymes, thus it cannot bind with the DNA and became unable to stabilize cleavage complex formation. This mechanism was verified by in vivo immunoband depletion assay.
DNA topoisomerase II exists both in the nucleus and in the mitochondria of leishmania (Shapiro 1994). The mtDNA or kDNA network of leishmania contains two types of DNA molecules; ‘maxicircles and minicircles’ (Simpson 1986). TOPII plays a vital role in kDNA metabolism (Shapiro 1994). Inhibition of LdTOPII by isobenzofuranone derivatives may interfere mitochondrial function. Increased population of annexinV-positive cells and DNA fragmentation in isobenzofuranones treated parasites indicate that isobenzofuranones promoted the apoptosis like cell death in leishmania. JVPH3 and JVPH4 induced ROS inside the parasite, cause the depletion of endogenous antioxidant level like reduced glutathione, and increase the production of lipid peroxidation in leishmania. Isobenzofuranone derivatives do not cause DNA fragmentation by the stabilization of LdTOPII–cleavage complex, rather it may occur due to ROS-mediated oxidative DNA lesions and frequent DNA modifications.
The antileishmanial activity of isobenzofuranone derivatives was validated in L. donovani-infected BALB/c mice. At 10 mg kg−1 body weight, there was almost complete clearance of parasites from the liver and spleen of the infected mice. Healing in visceral leishmaniasis is well associated with the development of strong cell-mediated immunological responses, such as T-cell response and NO production, which help in activating macrophages to kill the intracellular parasites (Kemp et al. 1993; Diefenbach et al. 1999). Both ROS and reactive nitrogen intermediates in activated macrophages play important role in parasite killing. Increased level of ROS and NO in JVPH3/JVPH4-treated mouse splenocytes strongly supports that these isobenzofuranone derivatives reduced the parasite burden in infected mice via activation of macrophages. An effective leishmanicidal response against L. donovani infection is dependent on the balance between Th1 and Th2 cytokines (Kemp et al. 1993). Therapy with isobenzofuranone derivatives mounts polarized Th1 responses with enhanced IL-12, TNF-α and NO production and reduced Th2-associated cytokine IL-10, production in all four groups of mice compared with the infected control groups. Collectively, it is evident that isobenzofuranones treatment on infected mice shifted the balance from Th2 to Th1 response.
In conclusion, our results documented that the novel isobenzofuranone derivatives exhibited potent antileishmanial effect on both antimony sensitive and resistant parasites targeting type II DNA topoisomerase and polarizing Th1 response during the healing of leishmaniasis in mouse model. As the target-based chemotherapy remains the only choice for the treatment of leishmaniasis, isobenzofuranone derivatives are the promising candidates for the development of antileishmanial drugs.
Acknowledgments
We thank S. Roy, the Director of our Institute, for his interest in this work. We also acknowledge Kshudiram Naskar for help during mice handling. The work is supported by Department of Biotechnology (DBT), Government of India (BT/PR4456/MED/29/355/2012) and Indo-Brazil project (DST/INT/Brazil/RPO-01/2009/2) of Department of Science and Technology (DST) and the chemistry part was supported by Council of Scientific and Industrial Research (CSIR), New Delhi in the form of Network Project (CSC 0108).
Glossary
- ATP
adenosine triphosphate
- DHBA
dihydrobetulinic acid
- BSA
bovine serum albumin
- EtBr
ethidium bromide
- FBS
fetal bovine serum
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- hTOPIIα
human topoisomerase IIα
- kDNA
kinetoplast DNA
- LDU
Leishman Donovan Units
- M199
Medium199
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAC
N-acetyl cysteine
- Ni-NTA
Ni-nitrilo triacetic acid
- NO
nitric oxide
- PCD
programmed cell death
- ROS
reactive oxygen species
- SAG
sodium antimony gluconate
- SLA
soluble leishmanial antigen
- TGF-β
transforming growth factor-β
Disclosures
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Clearance of internalized SbS (AG83) and SbR (GE1) Leishmania donovani parasite from in vitro infected mouse macrophage in culture. Macrophages (Mϕ) were isolated from BALB/c mice 36–48 h post injection (intraperitoneal) with 2% (w/v) hydrolyzed starch by peritoneal lavage with ice-cold phosphate-buffered saline and infected with early-passaged. L donovani AG83 promastigotes and laboratory developed SbR (GE1) parasites in vitro. Infected macrophages, after subsequent washing, were incubated with different concentrations of JVPH3 and JVPH4 and left for another 24-h period. Macrophages were fixed, and intracellular amastigotes were counted by Giemsa staining. The number of internalized AG83 and GE1 amastigotes within each infected macrophages was counted under bright field microscope in (A) and (B), respectively. The results are shown mean ± SD of three independent experiments.
Figure S2. Isobenzofuranone derivatives induced the generation of ROS and fragmentation of genomic DNA in Leishmania donovani. ROS generation for the promastigotes treated with JVPH3/JVPH4 for different times was measured by flurometric analysis of H2DCFDA at 530 nm (A). The level of fluorescent products of lipid peroxidation was measured at 430 nm (B) and the intracellular GSH level was measured at 480 nm (C). The percentage of DNA fragmentation after treatment with JVPH3 and JVPH4 was plotted against time of incubation (D). The results are shown mean ± SD of three independent experiments.
Figure S3. Effect of isobenzofuranone derivatives on hTOPIIα activity. hLdTOPIIα was incubated with 200 ng kDNA in presence of different concentrations of JVPH3 and JVPH4. Lane 1, kDNA only; lane 2, kDNA treated with LdTOPII; lane 3, same as lane 2 but in presence of DMSO. KN indicates kDNA network. M II and M I indicate the released minicircles super coiled and nicked form.
Figure S4. Effect of isobenzofuranone derivatives on LdTOPIB activity. The type I DNA topoisomerase was assayed by decreased mobility of the relaxed isomers of supercoiled pBS (SK+) [pBluescript (SK+)] DNA in agarose gel. Different concentrations (2, 5, 10, 20, and 50 μmol/L) of JVPH3 and JVPH4 were incubated with LdTOPIB followed by electrophoresis in 1% agarose gel. Camptothecin (CPT), a known topoisomerase I inhibitor, was used as positive control. The gels were stained EtBr (0.5 μg mL−1) and photographed under UV illumination. Lane 1, pBS (SK+) DNA; lane 2, pBS (SK+) DNA with LdTOPIB; lane 3, same as lane 2 but in presence of 2% DMSO; lane 4, same as lane 2, but the enzyme was incubated with 25 μmol/L CPT. NM, nicked monomer; RM, relaxed monomer; SM, supercoiled monomer.
Figure S5. Effect of isobenzofuranone derivatives on DNA unwinding. Unwinding assay was performed with supercoiled and relaxed plasmid DNA. Negatively supercoiled pBS (SK+) DNA was treated with LdTOPIB in presence of different concentrations of JVPH3 and JVPH4 (A). Relaxed pBS(SK+) DNA was treated with LdTOPIB in presence of different concentrations of JVPH3 and JVPH4 (B). EtBr (0.1 and 1 μg mL−1) is used as positive control in the experiments. Lane 1, pBS (SK+) DNA; lane 2, pBS(SK+) DNA with LdTOPIB; lanes 3–4, same as lane 2 but in presence of 0.1 and 1 μg mL−1 EtBr; lanes 5–9, same as lane 2, but in presence of increasing concentration of JVPH3. Lanes 10–14, same as lane 2, but in presence of increasing concentration of JVPH4. NM, nicked monomer; RM, relaxed monomer; SM, supercoiled monomer.
Figure S6. Effect of isobenzofuranone derivatives on LdTOPII–DNA binary complex formation. Effect of isobenzofuranone derivatives on DNA-binding activity of LdTOPII proteins was analyzed by EMSA analysis. Lane 1, labeled 36-mer duplex oligonucleotide; lane 2, 36-mer oligonucleotide incubated with of LdTOPII; lanes 3 and 4 same as lane 2, but incubated in presence of JVPH3 (10 and 25 μmol/L); and lanes 5 and 6 same as lane 2 but incubated in presence of JVPH4 (10 and 25 μmol/L).
Figure S7. In vivo generation of ROS and NO from infected and isobenzofuranone derivatives treated BALB/c mice. Splenocytes (2.0 × 106) from different experimental mice groups were pulsed with SLA for 48 h in 5% CO2 incubator at 37°C. Level of ROS in splenocytes was measured by H2DCFDA probe (A) and NO content in the culture supernatant was measured by Griess reagent (B). Data are presented as mean ± SEM (n = 5 animal per group). *P < 0.05, **P < 0.01, and ***P < 0.005 (Student’s t-test), as compared to different JVPH3 and JVPH4 treatment groups with infection control.
References
- Ashutosh SS, Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol. 2007;56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J Med Res. 2004;119:238–258. [PubMed] [Google Scholar]
- Bakshi RP, Shapiro TA. DNA topoisomerases as targets for antiprotozoal therapy. Mini Rev Med Chem. 2003;3:597–608. doi: 10.2174/1389557033487863. [DOI] [PubMed] [Google Scholar]
- BoseDasgupta S, Das BB, Sengupta S, Ganguly A, Roy A, Dey S, et al. The caspase-independent algorithm of programmed cell death in Leishmania induced by baicalein: the role of LdEndoG, LdFEN-1 and LdTatD as a DNA ‘degradesome’. Cell Death Differ. 2008;15:1629–1640. doi: 10.1038/cdd.2008.85. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Chowdhury AR, Mandal S, Goswami A, Ghosh M, Mandal L, Chakraborty D, et al. Dihydrobetulinic acid induces apoptosis in Leishmania donovani by targeting DNA topoisomerase I and II: implications in antileishmanial therapy. Mol Med. 2003;9:26–36. [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Mukherjee T, Sengupta S, Chowdhury SR, Mukhopadhyay S, Majumder HK. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Mol Pharmacol. 2011;80:694–703. doi: 10.1124/mol.111.072785. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Mukherjee T, Mukhopadhyay R, Mukherjee B, Sengupta S, Chattopadhyay S, et al. The lignan niranthin poisons Leishmania donovani topoisomerase IB and favours a Th1 immune response in mice. EMBO Mol Med. 2012;4:1126–1143. doi: 10.1002/emmm.201201316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Das BB, Sen N, Roy A, Dasgupta SB, Ganguly A, Mohanta BC, et al. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res. 2006;34:1121–1132. doi: 10.1093/nar/gkj502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BB, Ganguly A, Majumder HK. DNA topoisomerases of Leishmania: the potential targets for anti-leishmanial therapy. Adv Exp Med Biol. 2008;625:103–115. doi: 10.1007/978-0-387-77570-8_9. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Schindler H, Rollinghoff M, Yokoyama WM, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;284:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Merbarone Inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J Biol Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Jensen LH, Nitiss KC, Rose A, Dong J, Zhou J, Hu T, et al. A novel mechanism of cell killing by anti-topoisomerase II bisdioxopiperazines. J Biol Chem. 2000;275:2137–2146. doi: 10.1074/jbc.275.3.2137. [DOI] [PubMed] [Google Scholar]
- Karna P, Zughaier S, Pannu V, Simmons R, Narayan S, Aneja R. Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent. J Biol Chem. 2010;285:18737–18748. doi: 10.1074/jbc.M109.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski L. Leishmaniasis vaccine: where are we today? J Glob Infect Dis. 2010;2:177–185. doi: 10.4103/0974-777X.62881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M, Kurtzhals JA, Bendtzen K, Poulsen LK, Hansen MB, Koech DK, et al. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993;61:1069–1073. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature. 2005;434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther. 2003;99:167–181. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, et al. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- Lin G, Chan SS-K, Chung H-S, Li S-L. Chemistry and biological activities of naturally occurring phthalides. In: Atta-ur-Rahaman, editor. Studies in natural products chemistry. Vol. 32. Amsterdam, Netherlands: Elsevier; 2005. pp. 611–669. Part L. [Google Scholar]
- Ma F, Gao Y, Qiao H, Hu X, Chang J. Antiplatelet activity of 3-butyl-6-bromo-1(3H)-isobenzofuranone on rat platelet aggregation. J Thromb Thrombolysis. 2012;33:64–73. doi: 10.1007/s11239-011-0647-9. [DOI] [PubMed] [Google Scholar]
- MacielRezende CM, de Almeida L, Costa ÉD, Pires FR, Alves KF, Viegas C, Jr, et al. Synthesis and biological evaluation against Leishmania amazonensis of a series of alkyl-substituted benzophenones. Bioorg Med Chem. 2013;21:3114–3119. doi: 10.1016/j.bmc.2013.03.045. [DOI] [PubMed] [Google Scholar]
- Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S, et al. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med. 2000;6:527–541. [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Mukhopadhyay R, Bannerjee B, Chowdhury S, Mukherjee S, Naskar K, et al. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc Natl Acad Sci USA. 2013;110:582. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Covey JM, Kerrigan D, Markovits J, Pham R. DNA unwinding and inhibition of mouse leukemia L1210 DNA topoisomerase I by intercalators. Nucleic Acids Res. 1987;15:6713–6731. doi: 10.1093/nar/15.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hazra B, Mittra B, Das A, Majumder HK. Diospyrin, a bisnapthaquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol. 1998;54:994–999. doi: 10.1124/mol.54.6.994. [DOI] [PubMed] [Google Scholar]
- Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, Jaisankar P, et al. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol. 2008;74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- Saha S, Mukherjee T, Chowdhury S, Mishra A, Chowdhury SR, Jaisankar P, et al. The lignan glycosides lyoniside and saracoside poison the unusual type IB topoisomerase of Leishmania donovani and kill the parasite both in vitro and in vivo. Biochem Pharmacol. 2013;86:1673–1687. doi: 10.1016/j.bcp.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, et al. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 2004;11:924–936. doi: 10.1038/sj.cdd.4401435. [DOI] [PubMed] [Google Scholar]
- Sengupta T, Mukherjee M, Mandal C, Das A, Majumder HK. Functional dissection of the C-terminal domain of type II DNA topoisomerase from the kinetoplastid hemoflagellate Leishmania donovani. Nucleic Acids Res. 2003;31:5305–5316. doi: 10.1093/nar/gkg727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sengupta T, Mukherjee M, Das A, Mandal C, Das R, Mukherjee T, et al. Characterization of the ATPase activity of topoisomerase II from Leishmania donovani and identification of residues conferring resistance to etoposide. Biochem J. 2005a;390:419–426. doi: 10.1042/BJ20042128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta T, Mukherjee M, Das R, Das A, Majumder HK. Characterization of the DNA-binding domain and identification of the active site residue in the ‘Gyr A’ half of Leishmania donovani topoisomerase II. Nucleic Acids Res. 2005b;33:2364–2373. doi: 10.1093/nar/gki527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro TA. Mitochondrial topoisomerase II activity is essential for kinetoplast DNA minicircle segregation. Mol Cell Biol. 1994;14:3660–3667. doi: 10.1128/mcb.14.6.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Spicer JA, Huttunen KM, Miller CK, Denny WA, Ciccone A, Browne KA, et al. Inhibition of the pore-forming protein perforin by a series of aryl-substituted isobenzofuran-1(3H)-ones. Bioorg Med Chem. 2012;20:1319–1336. doi: 10.1016/j.bmc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Stauber AL. Resistance to the Khartoum strain of Leishmania donovani. Rice Inst Pam. 1956;45:80–96. [Google Scholar]
- Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ. A model for the mechanism of human topoisomerase I. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Buchele B, Gedig E, Slupsky JR, Simmet T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIalpha. Mol Pharmacol. 2000;58:71–81. doi: 10.1124/mol.58.1.71. [DOI] [PubMed] [Google Scholar]
- Topno RK, Das VN, Ranjan A, Pandey K, Singh D, Kumar N, et al. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in Bihar, India. Am J Trop Med Hyg. 2010;83:502–506. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, et al. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-γ-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clearance of internalized SbS (AG83) and SbR (GE1) Leishmania donovani parasite from in vitro infected mouse macrophage in culture. Macrophages (Mϕ) were isolated from BALB/c mice 36–48 h post injection (intraperitoneal) with 2% (w/v) hydrolyzed starch by peritoneal lavage with ice-cold phosphate-buffered saline and infected with early-passaged. L donovani AG83 promastigotes and laboratory developed SbR (GE1) parasites in vitro. Infected macrophages, after subsequent washing, were incubated with different concentrations of JVPH3 and JVPH4 and left for another 24-h period. Macrophages were fixed, and intracellular amastigotes were counted by Giemsa staining. The number of internalized AG83 and GE1 amastigotes within each infected macrophages was counted under bright field microscope in (A) and (B), respectively. The results are shown mean ± SD of three independent experiments.
Figure S2. Isobenzofuranone derivatives induced the generation of ROS and fragmentation of genomic DNA in Leishmania donovani. ROS generation for the promastigotes treated with JVPH3/JVPH4 for different times was measured by flurometric analysis of H2DCFDA at 530 nm (A). The level of fluorescent products of lipid peroxidation was measured at 430 nm (B) and the intracellular GSH level was measured at 480 nm (C). The percentage of DNA fragmentation after treatment with JVPH3 and JVPH4 was plotted against time of incubation (D). The results are shown mean ± SD of three independent experiments.
Figure S3. Effect of isobenzofuranone derivatives on hTOPIIα activity. hLdTOPIIα was incubated with 200 ng kDNA in presence of different concentrations of JVPH3 and JVPH4. Lane 1, kDNA only; lane 2, kDNA treated with LdTOPII; lane 3, same as lane 2 but in presence of DMSO. KN indicates kDNA network. M II and M I indicate the released minicircles super coiled and nicked form.
Figure S4. Effect of isobenzofuranone derivatives on LdTOPIB activity. The type I DNA topoisomerase was assayed by decreased mobility of the relaxed isomers of supercoiled pBS (SK+) [pBluescript (SK+)] DNA in agarose gel. Different concentrations (2, 5, 10, 20, and 50 μmol/L) of JVPH3 and JVPH4 were incubated with LdTOPIB followed by electrophoresis in 1% agarose gel. Camptothecin (CPT), a known topoisomerase I inhibitor, was used as positive control. The gels were stained EtBr (0.5 μg mL−1) and photographed under UV illumination. Lane 1, pBS (SK+) DNA; lane 2, pBS (SK+) DNA with LdTOPIB; lane 3, same as lane 2 but in presence of 2% DMSO; lane 4, same as lane 2, but the enzyme was incubated with 25 μmol/L CPT. NM, nicked monomer; RM, relaxed monomer; SM, supercoiled monomer.
Figure S5. Effect of isobenzofuranone derivatives on DNA unwinding. Unwinding assay was performed with supercoiled and relaxed plasmid DNA. Negatively supercoiled pBS (SK+) DNA was treated with LdTOPIB in presence of different concentrations of JVPH3 and JVPH4 (A). Relaxed pBS(SK+) DNA was treated with LdTOPIB in presence of different concentrations of JVPH3 and JVPH4 (B). EtBr (0.1 and 1 μg mL−1) is used as positive control in the experiments. Lane 1, pBS (SK+) DNA; lane 2, pBS(SK+) DNA with LdTOPIB; lanes 3–4, same as lane 2 but in presence of 0.1 and 1 μg mL−1 EtBr; lanes 5–9, same as lane 2, but in presence of increasing concentration of JVPH3. Lanes 10–14, same as lane 2, but in presence of increasing concentration of JVPH4. NM, nicked monomer; RM, relaxed monomer; SM, supercoiled monomer.
Figure S6. Effect of isobenzofuranone derivatives on LdTOPII–DNA binary complex formation. Effect of isobenzofuranone derivatives on DNA-binding activity of LdTOPII proteins was analyzed by EMSA analysis. Lane 1, labeled 36-mer duplex oligonucleotide; lane 2, 36-mer oligonucleotide incubated with of LdTOPII; lanes 3 and 4 same as lane 2, but incubated in presence of JVPH3 (10 and 25 μmol/L); and lanes 5 and 6 same as lane 2 but incubated in presence of JVPH4 (10 and 25 μmol/L).
Figure S7. In vivo generation of ROS and NO from infected and isobenzofuranone derivatives treated BALB/c mice. Splenocytes (2.0 × 106) from different experimental mice groups were pulsed with SLA for 48 h in 5% CO2 incubator at 37°C. Level of ROS in splenocytes was measured by H2DCFDA probe (A) and NO content in the culture supernatant was measured by Griess reagent (B). Data are presented as mean ± SEM (n = 5 animal per group). *P < 0.05, **P < 0.01, and ***P < 0.005 (Student’s t-test), as compared to different JVPH3 and JVPH4 treatment groups with infection control.


