Abstract
Influenza viruses collected from regions of Asia, Africa and Oceania between 2009 and 2012 were tested for their susceptibility to two new neuraminidase inhibitors, peramivir and laninamivir. All viruses tested had normal laninamivir inhibition. However, 3·2% (19/599) of A(H1N1)pdm09 viruses had highly reduced peramivir inhibition (due to H275Y NA mutation) and <1% (6/1238) of influenza B viruses had reduced or highly reduced peramivir inhibition, with single occurrence of variants containing I221T, A245T, K360E, A395E, D432G and a combined G145R+Y142H mutation. These data demonstrate that despite an increase in H275Y variants in 2011, there was no marked change in the frequency of peramivir- or laninamivir-resistant variants following the market release of the drugs in Japan in 2010.
Keywords: Antiviral susceptibility, laninamivir, neuraminidase inhibitors, peramivir
Introduction
Currently, the neuraminidase inhibitors (NAIs) are the only class of antivirals that are effective against circulating influenza A and B viruses due to widespread resistance against the older adamantane class of drugs.1 Two NAIs, orally administered oseltamivir (Tamiflu®) and inhaled zanamivir (Relenza®), have been licensed in many countries since 1999. In 2010, two new NAIs, peramivir (Rapiacta®) and laninamivir (Inavir®), were licensed in Japan.2 Peramivir has also been licensed in South Korea3 and most recently in China, as a response to concerns of A(H7N9) avian influenza.4 Peramivir is delivered intravenously and therefore is well suited to treating severely ill patients who may be unable to use oral or inhaled formulations.5 Laninamivir octanoate, a prodrug of laninamivir, is administered by inhalation (in a powdered form) and is quickly converted into its active form in the lungs.6 However, compared with oseltamivir and zanamivir, which are administered twice daily for 5 days, laninamivir is long-acting, requiring only a single administration for retention of the drug for at least five days.7 During 2011–2012, laninamivir was the largest selling NA inhibitor in Japan.8 As with any new antiviral agents that are released to the market, it is important to monitor circulating influenza strains for the development of resistance to both peramivir and laninamivir.9–11 This is particularly pertinent given the widespread resistance to the NAI oseltamivir that occurred in seasonal A(H1N1) viruses in 2008.12,13 Here, we evaluate the peramivir and laninamivir susceptibility of influenza A and B viruses circulating in parts of Asia, Africa and Oceania between 2009 and 2012, a time frame that spans pre- and post-market launch of these drugs in Japan and South Korea.
Sample selection and classification criteria
Approximately 2500 influenza A and 1238 influenza B viruses collected from 19 countries and territories in Asia, Africa and Oceania via the WHO Global Influenza Surveillance and Response System (GISRS) between 2009 and 2012, were screened for peramivir and laninamivir susceptibility. Viruses were received from the following countries and territories: Australia (2492), Brunei (5), Cambodia (164), Fiji (23), Hong Kong (4), Kenya (8), Macau (SAR, China) (101), Malaysia (44), New Caledonia (9), New Zealand (457), Papua New Guinea (10), Philippines (120), Singapore (196), Solomon Islands (1), South Africa (17), South Korea (10), Sri Lanka (27), Taiwan (6) and Thailand (92). Peramivir susceptibility and laninamivir susceptibility were assessed using a functional fluorescence-based NA inhibition assay as described by Hurt et al.14 Sequence analysis was conducted using standard methods.
In line with the recommendations implemented by the WHO GISRS Expert Committee for Antiviral Resistance in Influenza, virus susceptibility was classified based on fold differences compared with the respective NA inhibitor median IC50 of the matching subtype/type.15
Peramivir susceptibility
The peramivir susceptibility of 2548 influenza A and 1238 influenza B viruses was tested, of which 96·8% (580/599) of A(H1N1)pdm09 and 99·4% (1231/1238) of influenza B viruses demonstrated normal peramivir inhibition, while all A(H3N2) isolates (n = 1949) exhibited normal peramivir inhibition. The mean (±standard deviation) peramivir IC50 of the influenza B viruses with normal inhibition was 0·74 ± 0·33 nm, four-fold higher than the mean IC50 of the influenza A(H1N1)pdm09 or A(H3N2) viruses (Table 1). In addition, there was no significant difference in the median peramivir IC50s of B Victoria compared with B Yamagata lineage viruses exhibiting normal inhibition.
Table 1.
Overall median and mean peramivir and laninamivir IC50 of influenza viruses with normal inhibition*
| NA inhibitors | A(H1N1)pdm09 IC50 (nm) | A(H3N2) IC50 (nm) | B IC50 (nm) |
|---|---|---|---|
| Peramivir | |||
| No. tested, n | 580 | 1949 | 1231 |
| Median (p25, p75) | 0·13 (0·10, 0·24) | 0·20 (0·15, 0·27) | 0·68 (0·50, 0·90) |
| Mean ± SD | 0·17 ± 0·10 | 0·18 ± 0·08 | 0·74 ± 0·33 |
| Laninamivir | |||
| No. tested, n | 511 | 1950 | 1238 |
| Median (p25, p75) | 0·22 (0·19, 0·30) | 0·60 (0·45, 0·80) | 2·37 (1·92, 3·00) |
| Mean ± SD | 0·27 ± 0·05 | 0·62 ± 0·05 | 3·26 ± 0·26 |
To determine the overall median and mean peramivir and laninamivir IC50 of influenza viruses with normal inhibition, the median for all viruses tested in 2009, 2010, 2011 and 2012 was initially calculated. Viruses with IC50 10-fold or more (or five-fold or more for influenza B) above the median were then removed from the data set. The final median and mean IC50 of normal inhibition viruses were then recalculate and are displayed in Table 1.
Nineteen A(H1N1)pdm09 viruses (19/599, 3·2%) had highly reduced peramivir inhibition (Figure 1), with a mean IC50 value of 31·3 ± 10·3 nm, 241-fold above the median peramivir IC50 of A(H1N1)pdm09 viruses with normal inhibition. Genetic analysis of these viruses revealed that they all contained the H275Y NA substitution (N1 numbering, codon 274 in N2 numbering), a mutation known to confer highly reduced oseltamivir inhibition.12 Forty-two per cent (8/19) of the H275Y variants detected were from a cluster of cases in Australia in 2011,16 but additional strains were also detected in other regions of Australia (2009; 2011; 2012, n = 5) and from countries such as Thailand (2010, n = 1), Singapore (2010, n = 3), Brunei (2011, n = 1) and Philippines (2011, n = 1) where peramivir and laninamivir are not licensed for use.
Figure 1.
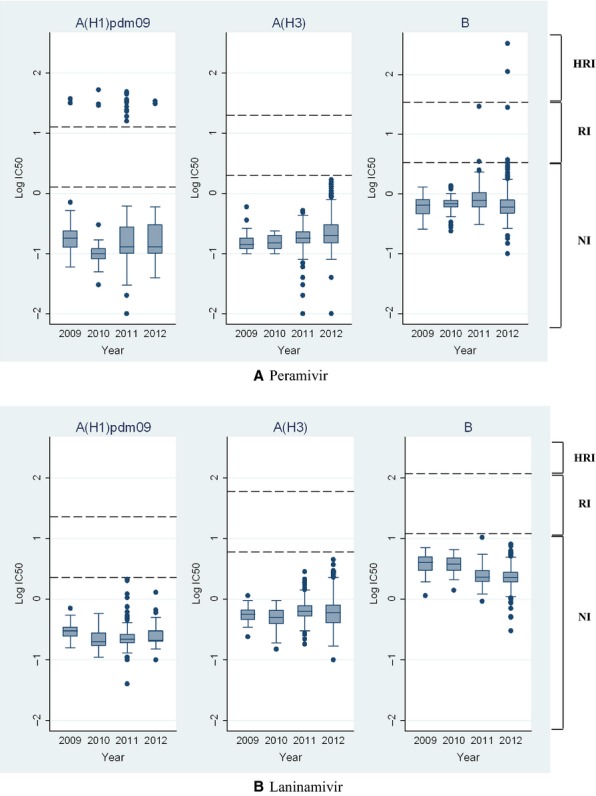
Box-and-whisker plots comparing the distribution of (A) peramivir IC50 and (B) laninamivir IC50 values (log10 transformed) of A(H1N1)pdm09, A(H3N2) and influenza B viruses from 2009 to 2012. The boxes represent the 25th to 75th percentiles, with horizontal lines within each box representing the median IC50 values. The whiskers represent the highest and the lowest values situated within the 1·5 IQR plus 75th percentile and the 1·5 IQR minus 25th percentile regions. The dashed lines define the regions “normal inhibition” (NI); “reduced inhibition” (RI); and “highly reduced inhibition” (HRI).
Six influenza B virus isolates were identified as having reduced or highly reduced peramivir inhibition (Figure 1, Table 2). The following influenza B residues are numbered based on straight influenza B NA amino acid numbering starting from the first methionine residue, GISAID accession numbers for sequences of the variant viruses are listed in Table 2. B/Malaysia/210/2012 contained two novel NA mutations Y142H and G145R, with the resulting isolate demonstrating a 487-fold increase in peramivir IC50 (Table 2). Y142H is located on the surface of the NA active site and could indirectly affect the binding pocket scaffold loop region including G145R (Figure 2). This may explain how G145R together with Y142H have a strong additive inhibitory effect. Other novel substitutions located in a framework residue (D432G) and outside the active site (K360E and A395E) (Figure 2) were also identified in three influenza B viruses from Thailand and Malaysia with reduced or highly reduced inhibition. B/Bangkok/29/2012, which contained A395E, had a minor five-fold increase in peramivir IC50, while B/Malaysia/283/2012 and B/Malaysia/221/2012, which contained K360E and D432G NA mutations, respectively, had 165- and 41-fold increases in peramivir IC50 (Table 2). All five of these B variants had normal laninamivir, oseltamivir and zanamivir inhibition, apart from B/Bangkok/29/2012 (A395E NA mutation) which had a five-fold increase in oseltamivir IC50. The final two B strains with reduced or highly reduced peramivir inhibition, B/Waikato/21/2011 and B/Wellington/39/2011, have previously been reported to have reduced inhibition to zanamivir and/or oseltamivir.17 B/Waikato/21/2011 contained an A245T NA mutation and demonstrated a five-fold increase in peramivir IC50, while B/Wellington/39/2011 contained an I221T mutation which resulted in a 43-fold increase in peramivir IC50 (Table 2). Variant viruses with either an I221T or I221V NA mutation have also been reported in a number of B viruses from USA and China.18,19 Compared with wild-type viruses, the I221T variant reported here had a much greater increase in peramivir IC50 (43-fold), than reported for the I221V variants from the USA, which exhibited an eight-fold increase.19 I221T and A245T are both located near the substrate binding site of the NA (Figure 2). Apart from reductions in peramivir sensitivity, the I221T B variant also demonstrated reduced oseltamivir inhibition17, while the A245T mutation was found to affect sensitivity to three of the four NA inhibitors, oseltamivir (20-fold reduction), zanamivir (32-fold reduction) and peramivir (five-fold reduction), even though the residue is not located within the NA active site. The original clinical specimens of many of these isolates were not available to the WHO Collaborating Centre for Reference and Research on Influenza, Melbourne, for sequence analysis (details listed in Table 2) as clinical specimens are often discarded by submitting laboratories once virus isolates are cultured. Therefore, we were unable to investigate whether the mutations had arisen during cell culture, as has been the case for some NAI-resistant variants previously reported.20
Table 2.
Influenza B viruses with reduced or highly reduced peramivir inhibition
| Designation | GISAID isolate ID | Lineage | Mutation (s) | Inhibition category | Peramivir IC50 (nm)* (fold difference†) | Laninamivir IC50 (nm)* (fold difference†) |
|---|---|---|---|---|---|---|
| B/Waikato/21/2011 | EPI_ISL_118616 | B/Victoria | A245T*** | Reduced inhibition | 3·48 ± 1·14 (5) | 7·30 ± 1·35 (3) |
| B/Bangkok/29/2012 | EPI_ISL_134483 | B/Victoria | A395E*** | Reduced inhibition | 3·73 ± 1·57 (5) | 3·81 ± 0·47 |
| B/Malaysia/221/2012 | EPI_ISL_122586 | B/Victoria | D432G*** | Reduced inhibition | 28·01 ± 8·75 (41) | 1·14 ± 0·33 |
| B/Wellington/39/2011 | EPI_ISL_118617 | B/Victoria | I221T** | Reduced inhibition | 29·35 ± 8·65 (43) | 2·53 ± 0·22 |
| B/Malaysia/283/2012 | EPI_ISL_128716 | B/Victoria | K360E*** | Highly reduced inhibition | 112·09 ± 37·94 (165) | 1·97 ± 0·57 |
| B/Malaysia/210/2012 | EPI_ISL_128715 | B/Victoria | G145R***; Y142H*** | Highly reduced inhibition | 331·37 ± 262·20 (487) | 3·26 ± 1·06 |
Mean IC50 ± SD (nm), each virus tested in three independent assays.
Detected in clinical specimen and virus isolate.
Clinical specimen not available.
Fold difference based on comparison with the influenza B median IC50 of viruses with normal inhibition as displayed in Table 1 (only fold difference >3 are listed).
Figure 2.
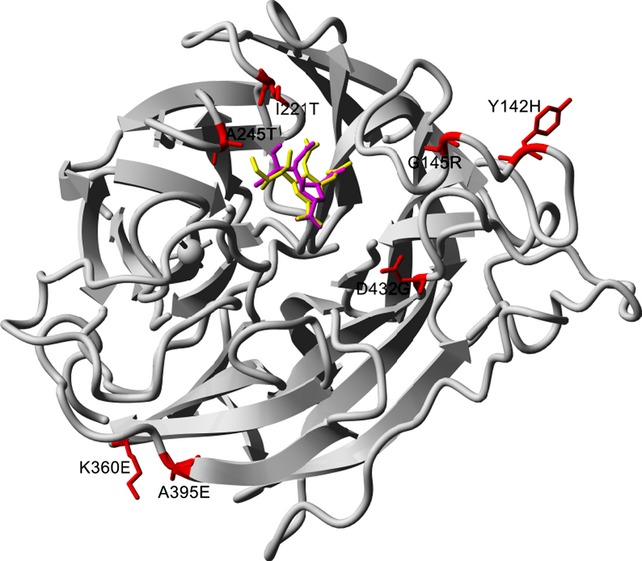
Neuraminidase residue substitutions associated with highly reduced or reduced peramivir susceptibility. The structure is from B/Perth/211/2001 (PDB ID: 3K37, http:\\www.rcsb.org) and visualized with YASARA (http:\\www.yasara.org) Peramivir is represented in magenta and laninamivir (superimposed from PDB ID: 3TIA) in yellow, the mutations are red with wild-type residue shown.
Laninamivir susceptibility
All 511 A(H1N1)pdm09, 1950 A(H3N2) viruses and 1238 influenza B had IC50 values that fell within the normal laninamivir inhibition range. The mean (±standard deviation) laninamivir IC50 values for A(H1N1)pdm09 and A(H3N2) viruses were 0·27 ± 0·05 nm and 0·62 ± 0·05 nm, respectively. The mean (±standard deviation) laninamivir IC50 for the 1238 influenza B isolates was 3·26 ± 0·26 nm, 5–12-fold higher than the mean IC50 of the influenza A(H1N1)pdm09 and A(H3N2) viruses. Again, no difference was observed between the laninamivir susceptibility of the two B lineages.
Conclusion
Although peramivir and laninamivir are currently only licensed in Japan (and in the case of peramivir, also in South Korea and China), approval in other countries is likely to follow as late-phase clinical trials are completed. Of the viruses analysed, none of the A(H1N1)pdm09, A(H3N2) and influenza B viruses had reduced or highly reduced laninamivir inhibition, while a small number of A(H1N1)pdm09 (3·2%) and B (0·5%) viruses had reduced peramivir susceptibility. Of the viruses considered to have normal inhibition, the mean and median values IC50 for peramivir were lower than those for laninamivir for all of the three influenza types/subtypes tested. The clinical implications of these differences, and for the H275Y variant viruses with highly reduced peramivir inhibition, are currently unknown and therefore require further study. However, given that the high concentration of peramivir achieved in the blood following intravenous administration21, it could be expected that the H275Y and influenza B variants detected here would be inhibited. However, clinical studies have suggested that peramivir is less effective when treating H275Y variant viruses compared with viruses with normal inhibition.22
This study has found no evidence of widespread emergence of viruses with highly reduced peramivir or laninamivir inhibition since the market launch in 2010, although the increased number of community cases of A(H1N1)pdm09 viruses with H275Y NA mutation in 2011 (which had highly reduced peramivir inhibition) was concerning. However, a limitation of this study is that the majority of the viruses tested were from regions where peramivir or laninamivir has not been approved for use. Therefore, although our data suggest that peramivir- and laninamivir-resistant viruses are not spreading from regions where the drugs are being used, further studies are required to assess the susceptibility of Japanese and South Korean viruses collected from both drug-treated and untreated patients.
Acknowledgments
The authors thank all the laboratories that submit specimens and isolates to the Melbourne WHO CCRRI for antiviral sensitivity testing. Peramivir (BCS-1812) was kindly provided by BioCryst Pharmaceuticals Inc, NC, USA and, laninamivir (R-125489) was kindly provided by Daiichi-Sankyo, Tokyo, Japan. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing. This work was partially supported by the Australian NHMRC and A*STAR Singapore through the joint grant 12/1/06/24/5793 to ACH and SMS.
References
- 1.Centers for Disease Control and Prevention. 2012. Influenza antiviral medications: summary for clinicians. Available at http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (Accessed 22 May 2013)
- 2.Shetty AK, Peek LA. Peramivir for the treatment of influenza. Expert Rev Anti Infect Ther. 2012;10:123–143. doi: 10.1586/eri.11.174. [DOI] [PubMed] [Google Scholar]
- 3.BioCryst Pharmaceuticals I. 2013. Peramivir (Neuraminidase Inhibitor). Available at http://www.biocryst.com/peramivir (Accessed 22 May 2013)
- 4.ChinaBioToday. 2013. China's SFDA ready to fast-track approvals of peramivir, a flu treatment. Available at http://www.chinabiotoday.com/articles/20130408_1 (Accessed 22 May 2013)
- 5.Birnkrant D, Cox E. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009;361:2204–2207. doi: 10.1056/NEJMp0910479. [DOI] [PubMed] [Google Scholar]
- 6.Koyama K, Takahashi M, Oitate M, et al. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long-retention profile in the mouse respiratory tract. Antimicrob Agents Chemother. 2009;53:4845–4851. doi: 10.1128/AAC.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka H, Yoshiba S, Yoshihara K, Okabe H. Assessment of the effects of renal impairment on the pharmacokinetic profile of laninamivir, a novel neuraminidase inhibitor, after a single inhaled dose of its Prodrug, CS-8958. J Clin Pharmacol. 2011;51:243–251. doi: 10.1177/0091270010361914. [DOI] [PubMed] [Google Scholar]
- 8.IMSHealth. Available at http:\\www.imshealth.com/ (Accessed 18 April 2013)
- 9.Dapat C, Kondo H, Dapat IC, et al. Neuraminidase inhibitor susceptibility profile of pandemic and seasonal influenza viruses during the 2009–2010 and 2010–2011 influenza seasons in Japan. Antiviral Res. 2013;99:261–269. doi: 10.1016/j.antiviral.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Takashita E, Fujisaki S, Kishida N, et al. Characterization of neuraminidase inhibitor-resistant influenza A(H1N1)pdm09 viruses isolated in four seasons during pandemic and post-pandemic periods in Japan. Influenza Other Respir Viruses. 2013 doi: 10.1111/irv.12132. doi: 10.1111/irv.12132. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okomo-Adhiambo M, Sleeman K, Lysen C, et al. Neuraminidase inhibitor susceptibility surveillance of influenza viruses circulating worldwide during the 2011 Southern Hemisphere season. Influenza Other Respir Viruses. 2013;7:645–658. doi: 10.1111/irv.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer A, Lackenby A, Hungnes O, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurt AC, Ernest J, Deng YM, et al. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 2009;83:90–93. doi: 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hurt AC, Okomo-Adhiambo M, Gubareva LV. The fluorescence neuraminidase inhibition assay: a functional method for detection of influenza virus resistance to the neuraminidase inhibitors. Methods Mol Biol. 2012;865:115–125. doi: 10.1007/978-1-61779-621-0_7. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Meetings of the WHO working group on surveillance of influenza antiviral susceptibility-Geneva, November 2011 and June 2012. Wkly Epidemiol Rec. 2012;39:369–374. [PubMed] [Google Scholar]
- 16.Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med. 2011;365:2541–2542. doi: 10.1056/NEJMc1111078. [DOI] [PubMed] [Google Scholar]
- 17.Leang SK, Deng YM, Shaw R, et al. Influenza antiviral resistance in the Asia-Pacific region during 2011. Antiviral Res. 2013;97:206–210. doi: 10.1016/j.antiviral.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Sleeman K, Huang W, et al. Neuraminidase inhibitor susceptibility testing of influenza type B viruses in China during 2010 and 2011 identifies viruses with reduced susceptibility to oseltamivir and zanamivir. Antiviral Res. 2013;97:240–244. doi: 10.1016/j.antiviral.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Sleeman K, Sheu TG, Moore Z, et al. Influenza B viruses with mutation in the neuraminidase active site, North Carolina, USA, 2010-11. Emerg Infect Dis. 2011;17:2043–2046. doi: 10.3201/eid1711.110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okomo-Adhiambo M, Nguyen HT, Sleeman K, et al. Host cell selection of influenza neuraminidase variants: implications for drug resistance monitoring in A(H1N1) viruses. Antiviral Res. 2010;85:381–388. doi: 10.1016/j.antiviral.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 21.FDA. Emergency use authorization of peramivir IV fact sheet for health care providers November 2009. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM187811.pdf (Accessed 07 August 2013)
- 22.Memoli MJ, Hrabal RJ, Hassantoufighi A, Eichelberger MC, Taubenberger JK. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin Infect Dis. 2010;50:1252–1255. doi: 10.1086/651605. [DOI] [PMC free article] [PubMed] [Google Scholar]


