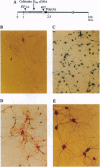
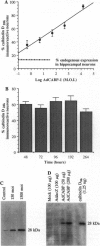
Abstract
Distinct subpopulations of neurons in the brain contain one or more of the Ca(2+)-binding proteins calbindin D28k, calretinin, and parvalbumin. Although it has been shown that these high-affinity Ca(2+)-binding proteins can increase neuronal Ca2+ buffering capacity, it is not clear which aspects of neuronal physiology they normally regulate. To investigate this problem, we used a recently developed method for expressing calbindin D28k in the somatic and synaptic regions of cultured hippocampal pyramidal neurons. Ninety-six hours after infection with a replication-defective adenovirus containing the calbindin D28k gene, essentially all cultured hippocampal pyramidal neurons robustly expressed calbindin D28k. Our results demonstrate that while calbindin D28k does not alter evoked neurotransmitter release at excitatory pyramidal cell synapses, this protein has a profound effect on synaptic plasticity. In particular, we show that calbindin D28k expression suppresses posttetanic potentiation.
Full text
PDF
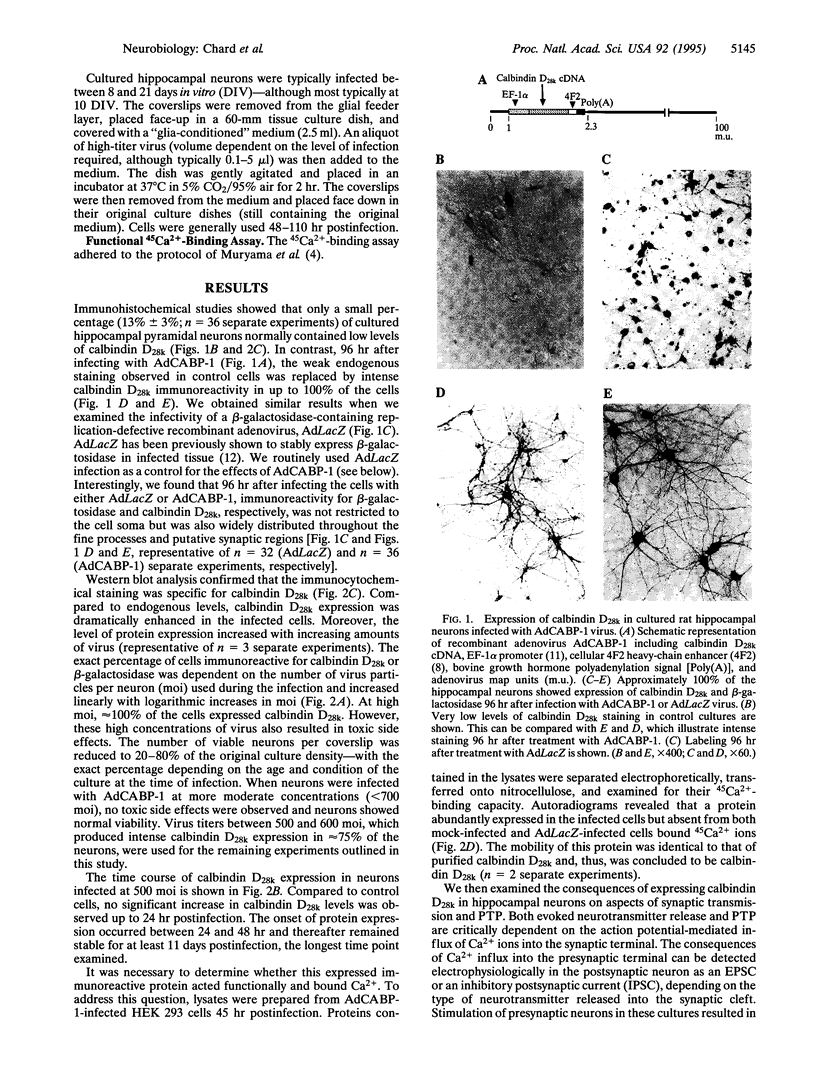
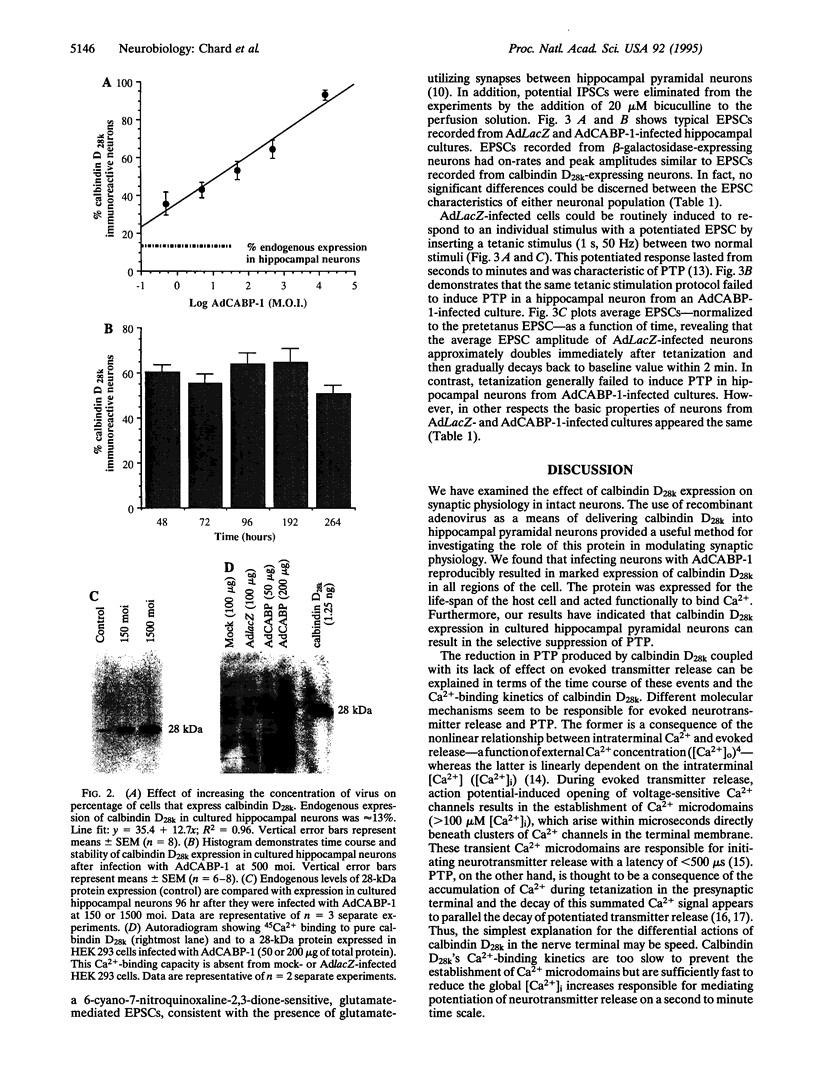

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J., Parkes C. O. Calcium-binding protein distribution in the rat brain. Brain Res. 1982 May 13;239(2):519–525. doi: 10.1016/0006-8993(82)90526-1. [DOI] [PubMed] [Google Scholar]
- Barr E., Carroll J., Kalynych A. M., Tripathy S. K., Kozarsky K., Wilson J. M., Leiden J. M. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994 Jan;1(1):51–58. [PubMed] [Google Scholar]
- Caillaud C., Akli S., Vigne E., Koulakoff A., Perricaudet M., Poenaru L., Kahn A., Berwald-Netter Y. Adenoviral vector as a gene delivery system into cultured rat neuronal and glial cells. Eur J Neurosci. 1993 Oct 1;5(10):1287–1291. doi: 10.1111/j.1460-9568.1993.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Chard P. S., Bleakman D., Christakos S., Fullmer C. S., Miller R. J. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J Physiol. 1993 Dec;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Christakos S., Mattson M. P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994 Jan;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Eliot L. S., Kandel E. R., Hawkins R. D. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci. 1994 May;14(5 Pt 2):3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Fullmer C. S., Wasserman R. H. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol. 1992 Feb;262(2 Pt 1):C517–C526. doi: 10.1152/ajpcell.1992.262.2.C517. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Karpinski B. A., Yang L. H., Cacheris P., Morle G. D., Leiden J. M. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol Cell Biol. 1989 Jun;9(6):2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Kandel E. R. Post-tetanic potentiation at an identified synapse in Aplysia is correlated with a Ca2+-activated K+ current in the presynaptic neuron: evidence for Ca2+ accumulation. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5430–5434. doi: 10.1073/pnas.79.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Lowenstein D. H., Miles M. F., Hatam F., McCabe T. Up regulation of calbindin-D28K mRNA in the rat hippocampus following focal stimulation of the perforant path. Neuron. 1991 Apr;6(4):627–633. doi: 10.1016/0896-6273(91)90065-8. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Ebisawa K., Nonomura Y. Purification of vitamin D-dependent 28,000-Mr calcium-binding protein from bovine cerebellum and kidney by calcium-dependent elution from DEAE-cellulose DE-52 column chromatography. Anal Biochem. 1985 Nov 15;151(1):1–6. doi: 10.1016/0003-2697(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. Analysis of adenosine actions on Ca2+ currents and synaptic transmission in cultured rat hippocampal pyramidal neurones. J Physiol. 1991 Apr;435:373–393. doi: 10.1113/jphysiol.1991.sp018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D., Hans M., Zipser K., Augustine G. J. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991 Dec;7(6):915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]