Abstract
Background and objectives
The term “nondisease-specific” has been used to describe problems that cross multiple domains of health and are not necessarily the result of a single underlying disease. Although individuals with reduced eGFR and elevated albumin-to-creatinine ratio have many comorbidities, the prevalence of and outcomes associated with nondisease-specific problems have not been well studied.
Design, setting, participants, & measurements
Participants included 3557 black and white United States adults ≥75 years of age from the Reasons for Geographic and Racial Differences in Stroke Study. Nondisease-specific problems included cognitive impairment, depressive symptoms, exhaustion, falls, impaired mobility, and polypharmacy. Hazard ratios for mortality over a median (interquartile range) of 5.4 (4.2–6.9) years of follow-up associated with one, two, or three to six nondisease-specific problems were calculated and stratified by eGFR (≥60, 45–59, and <45 ml/min per 1.73 m2) and separately, albumin-to-creatinine ratio (<30, 30–299, and ≥300 mg/g). Secondary outcomes included hospitalizations and emergency department visits over 1.8 (0.7–4.0) and 2.3 (0.9–4.7) years of follow-up, respectively.
Results
The prevalence of nondisease-specific problems was more common at lower eGFR and higher albumin-to-creatinine ratio levels. Within each eGFR and albumin-to-creatinine ratio strata, the risk for mortality was higher among those with a greater number of nondisease-specific problems. For example, among those with an eGFR=45–59 ml/min per 1.73 m2, the multivariable adjusted hazard ratios (95% confidence intervals) for mortality associated with one, two, or three to six nondisease-specific problems were 1.17 (0.78 to 1.76), 1.95 (1.24 to 3.07), and 2.44 (1.39 to 4.27; P trend <0.001). Risk for hospitalization and emergency department visits was higher among those with more nondisease-specific problems within eGFR and albumin-to-creatinine ratio strata.
Conclusions
Among older adults, nondisease-specific problems commonly co-occur with reduced eGFR and elevated albumin-to-creatinine ratio. Identification of nondisease-specific problems may provide mortality risk information independent of measures of kidney function.
Keywords: geriatric nephrology, epidemiology and outcomes, CKD
Introduction
The prevalence of moderate to severe CKD defined as an eGFR<60 ml/min per 1.73 m2 increases with age (1). Several studies have shown that, among older adults, an eGFR<60 ml/min per 1.73 m2 is associated with an increased prevalence of concurrent CKD complications, including hypertension, anemia, and bone and mineral disease, and an increased risk for adverse health outcomes, including mortality, and cardiovascular disease (2–4). However, because of the high burden of comorbidities and heterogeneity in life expectancy in this population, an approach to older adults that focuses only on kidney-specific biomarkers or complications may have limited efficacy in improving health outcomes (5,6).
A geriatric approach to patient care emphasizes the evaluation for problems that cross multiple domains of health and do not fit into discrete disease categories but affect function and quality of life (7–9). The term nondisease-specific has been used to describe health conditions or problems that involve multiple organ systems and are not necessarily the result of a single underlying disease (10). They include symptoms and impairments that may not meet diagnostic thresholds, such as cognitive impairment, depressive symptoms, exhaustion, falls, and impaired mobility, or problems that result from the overtreatment of multiple individual diseases, such as with polypharmacy (11). The terminology nondisease-specific may be preferred to geriatric, which implies that they are an inevitable consequence of aging or occur only among older adults. The evaluation for nondisease-specific problems has been used in cardiology and surgery in addition to disease-specific measures to improve prognostication (12–14); however, they are not often part of the clinical workup for patients with CKD.
Several of these problems individually have been shown to be associated with kidney disease (15–17). For example, an association between kidney disease and cognitive impairment has been consistently shown in previous studies (18–20). However, there are few data on the co-occurrence of nondisease-specific problems and the association with outcomes among older adults with CKD. Therefore, the aim of this study was to determine, among adults aged ≥75 years, the prevalence of six nondisease-specific problems in aggregate by level of eGFR and albumin-to-creatinine ratio (ACR). Nondisease-specific problems included cognitive impairment, depressive symptoms, exhaustion, falls, impaired mobility, and polypharmacy. Additionally, we assessed the risk of death, hospitalization, and emergency department (ED) visits associated with the co-occurrence of these problems stratified by level of eGFR and, separately, ACR. To do so, we analyzed data from a large United States population sample enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.
Materials and Methods
Study Participants
The REGARDS Study was designed to evaluate the excess stroke mortality among blacks compared with whites and in the stroke belt compared with the rest of the continental United States (21). The study enrolled black and white United States adults aged ≥45 years. Blacks and residents of the stroke belt were oversampled. Fifty-six percent of the sample were recruited from the eight southern United States states commonly considered as the stroke belt, including the region known as the stroke buckle (coastal North Carolina, South Carolina, and Georgia) and the remainder of North Carolina, South Carolina, and Georgia along with Alabama, Mississippi, Tennessee, Arkansas, and Louisiana. Forty-four percent of the sample were recruited from the other 40 contiguous states in the United States as well as Washington DC.
Between January of 2003 and October of 2007, 30,239 black and white United States adults were enrolled in the REGARDS Study. To focus on older adults, the analysis that we present was limited to REGARDS participants aged ≥75 years (n=5171). We excluded participants who did not have available data on eGFR (n=244), ACR (n=256), or all of the nondisease-specific problems (defined below; n=1071) as well as those on dialysis at baseline (n=6) or those missing follow-up data (n=37), leaving 3557 participants for this analysis. Among participants missing data on nondisease-specific problems, lack of available data on cognition accounted for most of the missing data (96%) because of its introduction into the REGARDS protocol 11 months after the initiation of recruitment. Participants included versus participants excluded were similar with regards to age (79.4±3.9 versus 79.5±3.9 years old, respectively), race (33.6% black versus 39.3% black, respectively), and sex (55.5% women versus 56.0% women, respectively). The REGARDS Study protocol was approved by the Institutional Review Boards at the participating institutions, and all participants provided informed consent.
Data Collection
The REGARDS Study baseline data were obtained through interview- and self-administered questionnaires and an in-home study visit. Age, sex, race, education, household income, and current cigarette smoking were obtained by self-report. Participants were also asked about history of physician-diagnosed hypertension, atrial fibrillation, coronary heart disease (CHD), stroke, and diabetes mellitus. Physical measurements and collection of a fasting blood sample were obtained. Following a standardized protocol, systolic BP and diastolic BP were measured two times with the participant in the seated position. The average of the two measurements was calculated and used for analysis. Waist circumference was measured midway between the lowest rib and the iliac crest in the standing position. Laboratory measures included serum creatinine, glucose, total and HDL cholesterol, C-reactive protein (CRP), and urinary albumin and creatinine. Diabetes was defined as taking insulin or oral hypoglycemic agents or on the basis of the baseline blood collection, a fasting blood glucose ≥126 mg/dl, or a nonfasting blood glucose ≥200 mg/dl. CRP was measured using a high-sensitivity, particle-enhanced immunonepholometric assay. Atrial fibrillation was defined as self-reported history or evidence on an electrocardiogram performed during the in-home assessment.
Measures of Kidney Function
Serum creatinine assays were performed at the University of Vermont and calibrated with an isotope dilution mass spectroscopic standard. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR, which was subsequently categorized into three levels: ≥60, 45–59, and <45 ml/min per 1.73 m2 (22). Urinary albumin was measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota using the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany) and reported as ACR (<30, 30–299, and ≥ 300 mg/g).
Six Nondisease-Specific Problems
The six nondisease-specific problems included (1) cognitive impairment, (2) depressive symptoms, (3) exhaustion, (4) history of falls, (5) impaired mobility, and (6) polypharmacy. These six problems were chosen, because they are routinely evaluated as a part of geriatric assessment and on the basis of the available data in the REGARDS Study. Cognition, depressive symptoms, exhaustion, falls, and impaired mobility were assessed by telephone. Cognitive impairment was defined as a score of four or less on the six-item screener, which evaluates global cognitive function (23). Scores on this scale range from zero to six, with lower scores indicating worse cognition. The presence of depressive symptoms was defined as a score of four or more on the four-item Center for Epidemiologic Studies Depression Scale (24). Exhaustion was defined as answering “little of the time” or “none of the time” to the Short Form-12 (SF-12) question “How much of the time during the past 4 weeks did you have a lot of energy?” History of falls was on the basis of an answer of “yes” to the question “Have you experienced a fall within the past year?” and an answer of “two or more” to the question “How many times have you fallen in the last year?” Participants were considered to have impaired mobility if they answered a lot among the options of “limited a lot,” “limited a little,” or “not limited at all” to the SF-12 question “Does your health now limit you in climbing several flights of stairs?” During the in-home visit, participants were asked to provide the bottles for all medications that they had taken in the past 2 weeks, and medication names were recorded and subsequently coded into drug classes. Polypharmacy was defined as the concurrent use of ≥10 prescription medications (21).
All-Cause Mortality
Mortality subsequent to the REGARDS in-home examination was assessed through semiannual telephone follow-up, contact with proxies provided by the participant on recruitment, and searches of the Social Security death index and the national death index. The date of death was confirmed through the Social Security death index, death certificates, or the national death index. Follow-up for this analysis was available through March 31, 2011.
Hospitalization and ED Visits
Data on hospitalizations and ED visits were obtained through the linkage of REGARDS participants to Medicare claims. REGARDS participants were linked to Medicare enrollment and claims data from 1999 to 2010 by Social Security number, sex, and date of birth. We restricted the analyses of hospitalization and ED visits to 2268 REGARDS participants who were enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage, respectively) but not Medicare Advantage at the time of their baseline study visit. For these outcomes, participants were followed until their first event (hospitalization or ED visit), loss of A+B Medicare coverage, death, or December 31, 2010.
Statistical Analyses
Within each eGFR (≥60, 45–59, and <45 ml/min per 1.73 m2) and ACR (<30, 30–299, and ≥300 mg/g) stratum, the mean levels or prevalence of baseline participant characteristics were calculated. The prevalence of each of six nondisease-specific problems was then calculated by levels of eGFR and ACR. Next, the percentage of participants with zero, one, two, or three to six problems was calculated for each level of eGFR and ACR separately. Participants with three, four, five, or six problems were grouped because of the low prevalence of three or more nondisease-specific problems after stratification by eGFR and ACR. The associations between nondisease-specific problems and mortality, hospitalization, and ED visits were calculated by eGFR and ACR categories separately. Below, we describe the analyses for eGFR categories. Identical analyses were conducted using ACR categories. Within each eGFR strata, associations between nondisease-specific problems and all-cause mortality were examined by calculating mortality rates and hazard ratios (HRs) using Cox proportional hazards models. In the Cox models, no nondisease-specific problems served as the referent category. Multivariable adjustment included age, sex, race, region of residence, education, household income, current cigarette smoking, atrial fibrillation, CHD, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and high-sensitivity CRP. Multiplicative interaction was assessed separately for eGFR and ACR by nondisease-specific problems by comparing the log likelihoods for models with and without multiplicative interaction terms. Models were then repeated for hospitalization and ED visits separately, with zero nondisease-specific problems serving as the referent within each eGFR category. Because of the smaller sample size with Medicare data, we pooled participants with eGFR<60 ml/min per 1.73 m2 in these secondary analyses. For the analyses of ACR, we pooled those with ACR≥30 mg/g and those with two or more nondisease-specific problems. Proportional hazards assumptions were confirmed by modeling disease-specific problems by time (log transformed). SAS, version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
Results
Participant Characteristics
Of 3557 participants ≥75 years old, 55.5% were women, and 33.6% were black. Baseline participant characteristics by level of eGFR and ACR are displayed in Tables 1 and 2. Compared with their counterparts with eGFR≥60 ml/min per 1.73 m2, those with eGFR<45 ml/min per 1.73 m2 were more likely to be women and black. Participants with higher ACR were less likely to be women and more likely to be black. Lower eGFR and higher ACR were associated with a higher prevalence of smoking, hypertension, atrial fibrillation, CHD, and diabetes mellitus. Additionally, a lower eGFR or higher ACR was associated with higher waist circumference and high-sensitivity CRP. The prevalence of each of six nondisease-specific problems by level of eGFR and ACR separately is displayed in Tables 1 and 2. There was a graded increase in the prevalence of cognitive impairment, exhaustion, impaired mobility, and polypharmacy at lower eGFR levels. At higher levels of ACR, there was a higher prevalence of cognitive impairment and polypharmacy.
Table 1.
Baseline characteristics of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study population ≥75 years old by eGFR
| Participant Characteristics | eGFR, ml/min per 1.73 m2 | ||
|---|---|---|---|
| ≥60 | 45–59 | <45 | |
| n | 2616 | 606 | 335 |
| Age, yr | 79.0±3.7 | 80.2±4.2 | 80.6±4.2 |
| Women | 54.8 | 53.6 | 64.2 |
| Black | 33.3 | 32.3 | 38.5 |
| Less than a high school education | 15.5 | 20.1 | 21.5 |
| Household income <$20,000 | 21.1 | 20.5 | 30.5 |
| Geographic region | |||
| Stroke belt | 30.3 | 29.9 | 27.5 |
| Stroke buckle | 18.9 | 22.6 | 21.5 |
| Other region | 50.8 | 47.5 | 51.0 |
| Current smoker | 5.2 | 6.0 | 7.2 |
| Hypertension | 64.6 | 78.0 | 82.0 |
| Atrial fibrillation | 12.3 | 15.1 | 25.0 |
| Coronary heart disease | 25.0 | 35.4 | 35.9 |
| Stroke | 7.5 | 10.4 | 10.6 |
| Diabetes mellitus | 17.3 | 25.9 | 30.2 |
| Systolic BP (mmHg) | 131.2±17.4 | 131.9±18.0 | 129.7±17.2 |
| Diastolic BP (mmHg) | 74.5±9.4 | 73.4±9.8 | 71.5±10.0 |
| Waist circumference (cm) | 92.9±14.9 | 94.4±13.7 | 95.0±13.4 |
| HDL cholesterol (mg/dl) | 54.0±16.8 | 51.8±17.4 | 50.1±15.9 |
| Total cholesterol (mg/dl) | 185.9±39.0 | 181.7±39.4 | 180.0±37.8 |
| High-sensitivity C-reactive protein (mg/L) | 1.8 (0.9–4.2) | 2.0 (0.9–4.5) | 2.8 (1.3–6.1) |
| Cognitive impairment | 13.2 | 15.2 | 18.8 |
| Depressive symptoms | 9.8 | 9.7 | 9.0 |
| Exhaustion | 14.6 | 18.0 | 23.3 |
| Falls (≥2 within the past year) | 9.3 | 8.9 | 10.8 |
| Impaired mobility | 17.7 | 21.3 | 28.4 |
| Polypharmacy (≥10 medications) | 19.8 | 27.6 | 34.6 |
Numbers are percentages or means±SDs except for high-sensitivity C-reactive protein, which is medians (25th–75th quartiles).
Table 2.
Baseline characteristics of the REGARDS Study population ≥75 years old by albumin-to-creatinine ratio
| Participant Characteristics | Albumin-to-Creatinine Ratio, mg/g | ||
|---|---|---|---|
| <30 | 30–299 | ≥300 | |
| n | 2781 | 658 | 118 |
| Age, yr | 79.2±3.8 | 79.9±4.1 | 79.7±3.9 |
| Women | 57.2 | 50.2 | 44.9 |
| Black | 30.9 | 41.5 | 52.5 |
| Less than a high school education | 16.2 | 20.7 | 12.7 |
| Household income<$20,000 | 21.0 | 24.5 | 28.0 |
| Geographic region | |||
| Stroke belt | 30.2 | 30.1 | 24.6 |
| Stroke buckle | 20.2 | 19.5 | 12.7 |
| Other region | 49.7 | 50.5 | 62.7 |
| Current smoker | 4.7 | 7.6 | 13.6 |
| Hypertension | 65.8 | 77.4 | 82.2 |
| Atrial fibrillation | 12.6 | 19.1 | 19.0 |
| Coronary heart disease | 26.1 | 33.5 | 34.8 |
| Stroke | 7.4 | 11.9 | 11.0 |
| Diabetes mellitus | 16.1 | 31.1 | 47.9 |
| Systolic BP (mmHg) | 129.6±16.6 | 136.1±19.4 | 139.8±20.9 |
| Diastolic BP (mmHg) | 73.7±9.3 | 75.1±10.4 | 76.6±10.5 |
| Waist circumference (cm) | 92.8±14.3 | 95.1±15.5 | 97.4±13.1 |
| HDL cholesterol (mg/dl) | 54.0±17.0 | 50.6±16.1 | 52.2±16.5 |
| Total cholesterol (mg/dl) | 185.4±39.0 | 180.8±38.7 | 188.3±38.8 |
| High-sensitivity C-reactive protein (mg/L) | 1.8 (0.9–4.0) | 2.4 (1.0–5.3) | 4.6 (1.5–8.2) |
| Cognitive impairment | 12.9 | 16.9 | 23.7 |
| Depressive symptoms | 9.8 | 9.7 | 8.5 |
| Exhaustion | 15.4 | 18.4 | 17.8 |
| Falls (≥2 within the past year) | 8.4 | 12.9 | 11.9 |
| Impaired mobility | 18.1 | 24.0 | 23.7 |
| Polypharmacy (≥10 medications) | 21.4 | 25.7 | 31.4 |
Numbers are percentages or means±SDs except for high-sensitivity C-reactive protein, which is medians (25th–75th quartiles).
Nondisease-Specific Problems and All-Cause Mortality
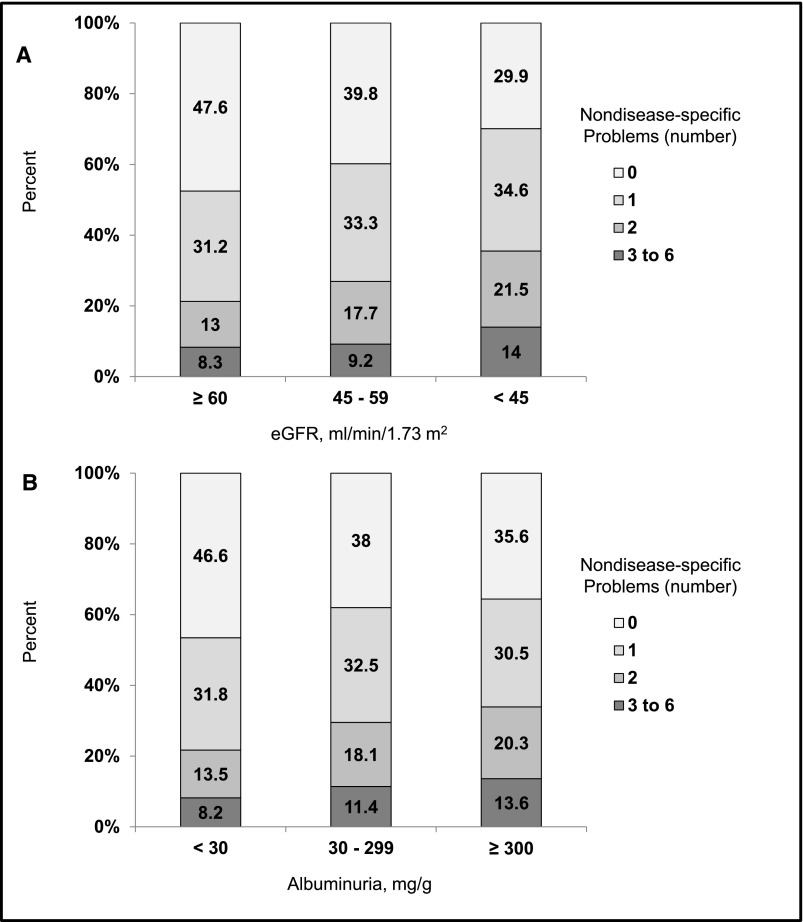
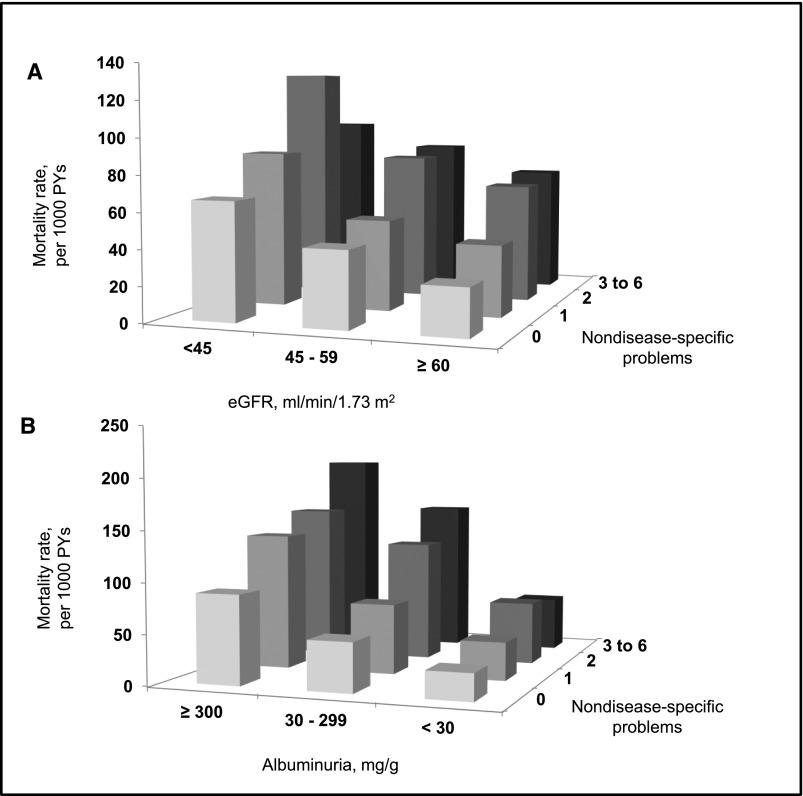
Participants with lower eGFR or higher ACR had more nondisease-specific problems (Figure 1). The prevalences of three to six nondisease-specific problems were 8.3%, 9.2%, and 14.0% among those with eGFR levels ≥60, 45–59, and <45 ml/min per 1.73 m2, respectively. Similarly, the prevalences of three to six nondisease-specific problems were 8.2%, 11.4%, and 13.6% at ACR levels <30, 30–299, and ≥300 mg/g, respectively. There were 857 deaths over a median of 5.4 years (interquartile range [IQR]=4.2–6.9) of follow-up. All-cause mortality rates were higher with an increasing number of nondisease-specific problems and at lower eGFR and higher ACR levels (Figure 2, Supplemental Table 1). These associations remained present after multivariable adjustment (Tables 3 and 4). For example, among those with an eGFR=45–59 ml/min per 1.73 m2, the multivariable adjusted HRs (95% confidence intervals) for mortality associated with one, two, and three to six nondisease-specific problems were 1.17 (0.78 to 1.76), 1.95 (1.24 to 3.07), and 2.44 (1.39 to 4.27; P trend <0.001), respectively. Among those with ACR=30–299 mg/g, adjusted HRs (95% confidence intervals) for mortality associated with one, two, and three to six nondisease-specific problems were 1.30 (0.90 to 1.85), 2.16 (1.47 to 3.18), and 2.64 (1.70 to 4.12), respectively (P trend <0.001). Interactions between eGFR level and number of nondisease-specific problems and between ACR level and number of nondisease-specific interactions were not statistically significant (each P for interaction >0.05).
Figure 1.
Participants with lower eGFR or higher ACR had more nondisease-specific problems. Prevalence of nondisease-specific problems by level of (A) eGFR and (B) albuminuria in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.
Figure 2.
All-cause mortality rates were higher with an increasing number of nondisease-specific problems and at lower eGFR and higher ACR levels. All-cause mortality rates associated with level of (A) eGFR and (B) albuminuria and number of nondisease-specific problems in the REGARDS Study. PY, person-years.
Table 3.
Multivariable adjusted hazards ratios (95% confidence intervals) for all-cause mortality associated with number of nondisease-specific problems among the REGARDS Study population ≥75 years old by eGFR
| No. of Nondisease-Specific Problems | eGFR, ml/min per 1.73 m2 | ||
|---|---|---|---|
| ≥60 | 45–59 | <45 | |
| n | 2616 | 606 | 335 |
| 0 | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 1.34 (1.08 to 1.67) | 1.17 (0.78 to 1.76) | 1.52 (0.93 to 2.49) |
| 2 | 1.91 (1.47 to 2.47) | 1.95 (1.24 to 3.07) | 1.90 (1.12 to 3.22) |
| 3–6 | 2.28 (1.67 to 3.10) | 2.44 (1.39 to 4.27) | 1.64 (0.88 to 3.07) |
| P trend | <0.001 | <0.001 | 0.04 |
Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed).
Table 4.
Multivariable adjusted hazards ratios (95% confidence intervals) for all-cause mortality associated with number of nondisease-specific problems among the REGARDS Study population ≥75 years old by albumin-to-creatinine ratio
| No. of Nondisease-Specific Problems | Albumin-to-Creatinine Ratio, mg/g | ||
|---|---|---|---|
| <30 | 30–299 | ≥300 | |
| n | 2781 | 658 | 118 |
| 0 | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 1.35 (1.09 to 1.68) | 1.30 (0.91 to 1.85) | 1.42 (0.68 to 2.99) |
| 2 | 1.88 (1.45 to 2.44) | 2.16 (1.47 to 3.18) | 2.12 (0.94 to 4.79) |
| 3–6 | 1.91 (1.38 to 2.65) | 2.64 (1.70 to 4.12) | 2.77 (1.07 to 7.16) |
| P trend | <0.001 | <0.001 | 0.02 |
Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed).
Nondisease-Specific Problems and Secondary Outcomes
During follow-up, 1447 participants were hospitalized over a median of 1.8 years (IQR=0.7–4.0 years), and 1661 participants had an ED visit over a median of 2.3 years (IQR=0.9–4.7). Hospitalization and ED visit rates were higher with an increasing number of nondisease-specific problems and at lower eGFR and higher ACR levels (Supplemental Tables 2 and 3). More nondisease-specific problems were associated with higher multivariable adjusted HRs for hospitalization and ED visits within each eGFR and ACR strata (Tables 5, 6, 7, and 8).
Table 5.
Multivariable adjusted hazards ratios (95% confidence intervals) for hospitalization associated with number of nondisease-specific problems among the REGARDS Study population ≥75 years old by eGFR
| No. of Nondisease-Specific Problems | eGFR, ml/min per 1.73 m2 | |
|---|---|---|
| ≥60 (n=1648) | <60 (n=620) | |
| 0 | 1 (reference) | 1 (reference) |
| 1 | 1.17 (1.00 to 1.36) | 1.48 (1.16 to 1.90) |
| 2 | 1.59 (1.30 to 1.94) | 1.49 (1.10 to 2.02) |
| 3–6 | 1.78 (1.41 to 2.25) | 2.23 (1.57 to 3.17) |
| P trend | <0.001 | <0.001 |
Restricted to 2268 REGARDS participants who were enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage, respectively) but not Medicare Advantage at the time of their baseline study visit. Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed). Because of the smaller sample size with Medicare data, we pooled participants with eGFR < 60 ml/min per 1.73 m2.
Table 6.
Multivariable adjusted hazards ratios (95% confidence intervals) for hospitalization associated with number of nondisease-specific problems among REGARDS Study population ≥75 years old by albumin-to-creatinine ratio
| No. of Nondisease-Specific Problems | Albumin-to-Creatinine Ratio, mg/g | |
|---|---|---|
| <30 (n=1795) | ≥30 (n=473) | |
| 0 | 1 (reference) | 1 (reference) |
| 1 | 1.25 (1.08 to 1.45) | 1.23 (0.93 to 1.62) |
| 2 | 1.62 (1.34 to 1.96) | 1.62 (1.21 to 2.17) |
| 3–6 | 1.75 (1.40 to 2.20) | |
| P trend | <0.001 | 0.001 |
Restricted to 2268 REGARDS participants who were enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage, respectively) but not Medicare Advantage at the time of their baseline study visit. Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed). Because of the smaller sample size with Medicare data, we pooled those with ACR ≥ 30 mg/g and those with two or more nondisease-specific problems.
Table 7.
Multivariable adjusted hazards ratios (95% confidence intervals) for emergency department visits associated with number of nondisease-specific problems among the REGARDS Study population ≥75 years old by eGFR
| No. of Nondisease-Specific Problems | eGFR, ml/min per 1.73 m2 | |
|---|---|---|
| ≥60 (n=1648) | <60 (n=620) | |
| 0 | 1 (reference) | 1 (reference) |
| 1 | 1.11 (0.97 to 1.28) | 1.35 (1.07 to 1.70) |
| 2 | 1.32 (1.09 to 1.60) | 1.30 (0.98 to 1.72) |
| 3–6 | 1.61 (1.28 to 2.01) | 1.82 (1.31 to 2.54) |
| P trend | <0.001 | <0.001 |
Restricted to 2268 REGARDS participants who were who were enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage, respectively) but not Medicare Advantage at the time of their baseline study visit. Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed). Because of the smaller sample size with Medicare data, we pooled participants with eGFR < 60 ml/min per 1.73 m2.
Table 8.
Multivariable adjusted hazards ratios (95% confidence intervals) for emergency department visits associated with number of nondisease-specific problems among REGARDS study population ≥75 years old by albumin-to-creatinine ratio
| No. of Nondisease-Specific Problems | Albumin-to-Creatinine Ratio, mg/g | |
|---|---|---|
| <30 (n=1795) | ≥30 (n=473) | |
| 0 | 1 (reference) | 1 (reference) |
| 1 | 1.18 (1.03 to 1.35) | 1.27 (0.97 to 1.67) |
| 2 | 1.34 (1.12 to 1.60) | 1.48 (1.11 to 1.97) |
| 3–6 | 1.54 (1.24 to 1.90) | |
| P trend | <0.001 | 0.007 |
Restricted to 2268 REGARDS participants who were who were enrolled in Medicare parts A and B (fee-for-service hospital and outpatient coverage, respectively) but not Medicare Advantage at the time of their baseline study visit. Multivariable adjustment includes age, race, sex, education, income, region of residence, current cigarette smoking, atrial fibrillation, coronary heart disease, stroke, diabetes, systolic BP, diastolic BP, waist circumference, HDL cholesterol, total cholesterol, and C-reactive protein (log transformed). Because of the smaller sample size with Medicare data, we pooled those with ACR ≥ 30 mg/g and those with two or more nondisease-specific problems.
Discussion
In this study of United States adults ≥75 years of age, the presence of multiple nondisease-specific problems, including cognitive impairment, depressive symptoms, exhaustion, falls, impaired mobility, and polypharmacy, was higher at lower eGFR and higher ACR levels, and there was a higher risk for death, hospitalization, and ED visits among participants with more of these problems. These findings suggest that the evaluation for nondisease-specific problems using brief assessments, single-item questions, or review of medication pill bottles can provide information on risk for adverse events in older adults with CKD.
Prior studies have evaluated the association of CKD with individual nondisease-specific problems. For example, in separate cross-sectional analyses of the Chronic Renal Insufficiency Cohort Study, associations between reduced kidney function and frailty/poor physical performance, depressive symptoms, and cognitive impairment have been shown (19,20). A higher prevalence and incidence of cognitive impairment among those with CKD have been reported in several other population-based studies (17–20,25). In a previous analysis of the REGARDS Study, an 11% increase in the odds of prevalent cognitive impairment was shown for every 10-ml/min per 1.73 m2 incremental decrease in eGFR (18). Depressive symptoms have also been evaluated in association with reduced eGFR (16). Exhaustion, a key component of the frailty phenotype, has been reported to be more prevalent at lower levels of reduced eGFR (26). Reduced eGFR has also been shown to be associated with falls, incident ambulatory mobility impairment, and a more rapid decline in community mobility (15,27). Additionally, polypharmacy is common among individuals with CKD. In a prior study, participants with CKD were prescribed an average of eight medications (28).
Prior studies have conceptualized nondisease-specific problems as a consequence of kidney disease. In this study, we determined the risk for mortality, hospitalization, and ED visits among those with reduced eGFR or elevated ACR co-occurring with nondisease-specific problems. Understanding the impact of the coexisting chronic disease and nondisease-specific problems is important for guiding appropriate clinical care to older adults. The presence of coexisting chronic conditions or multimorbidity has been shown to be associated with adverse health outcomes and is common among those with CKD (6,29). We found that the presence of multiple nondisease-specific problems provided complementary information to eGFR and ACR on risk for adverse events.
Despite the advantages of using the large Unites States population–based sample from the prospective REGARDS Study, this analysis has possible and known limitations. As with other observational studies, causal associations between nondisease-specific problems and mortality could not be determined. Participants did not undergo a formal geriatric assessment. Data were not available on some nondisease-specific problems, including incontinence, dizziness, or vision and hearing impairment. Several of the problems studied have been shown to be dynamic. However, with the exception of cognitive function, longitudinal assessments of these problems were not conducted in the REGARDS Study, and we could not account for changes in these problems over time. Few cases of ESRD among those ≥75 years old have occurred over the follow-up period, and therefore, we could not examine the association between nondisease-specific problems and this outcome. Although data on cognition and depressive symptoms were obtained by validated measures and polypharmacy was assessed by a pill bottle review during an in-home examination, exhaustion, falls, and impaired mobility were obtained by self-report. More objective measures for some of the nondisease-specific problems, such as mobility (e.g., gait speed and life-space mobility), were not available. However, this study suggests that, despite one-time measures, these brief assessments and self-reported problems were associated with mortality independent of several mortality risk factors, including CHD, stroke, and diabetes. Because these measures are brief, they may be incorporated into electronic medical records to provide clinicians with real-time information about the presence of the problems.
Our findings may have important clinical implications. Because old age is a period of marked heterogeneity in life expectancy, functional status, and response to treatment, caring for older adults is challenging (5,30). Clinical evaluation for the presence of multiple nondisease-specific problems studied here may provide important context in which to interpret markers of kidney function and risk-stratify patients. More research is necessary to determine if an evaluation for nondisease-specific problems can be used to guide individualized treatment plans, improve prognostication, or facilitate discussion about treatment decisions for older adults with CKD. Additionally, studies may identify those who could benefit from a formal interdisciplinary geriatric assessment or whether interventions targeting nondisease-specific problems can reduce hospitalizations or ED visits.
In conclusion, in this study, nondisease-specific problems, including cognitive impairment, depressive symptoms, exhaustion, falls, impaired mobility, and polypharmacy, were common among older adults with CKD. The presence of nondisease-specific problems was associated with the risk of mortality, hospitalization, and ED visits. Future studies should determine if routine clinical evaluation for multiple nondisease-specific problems is helpful for risk-stratifying older adults with CKD or guiding individualized treatment plans.
Disclosures
O.M.G. has had past research support from Amgen. D.G.W. is a member of the Amgen National Nephrology Advisory Board. P.M. served on an Amgen National Nephrology Advisory Board and as a consultant to Amgen. None of the other authors declare any conflict of interest.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study for their valuable contributions.
This research project is supported by Cooperative Agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Additional support was provided by National Institute on Aging Grant R03AG042336-01, the T. Franklin Williams Scholarship Award (funding provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, the American Society of Nephrology, and the American Geriatrics Society), and US Department of Veterans Affairs Grant 1IK2CX000856-01A1.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00880114/-/DCSupplemental.
See related editorial, “Lower Physical Activity and Depression Are Associated with Hospitalization and Shorter Survival in CKD,” on pages 1669–1670.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bowling CB, Inker LA, Gutiérrez OM, Allman RM, Warnock DG, McClellan W, Muntner P: Age-specific associations of reduced estimated glomerular filtration rate with concurrent chronic kidney disease complications. Clin J Am Soc Nephrol 6: 2822–2828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drawz PE, Babineau DC, Rahman M: Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc 60: 310–315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 167: 2490–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bowling CB, O’Hare AM: Managing older adults with CKD: Individualized versus disease-based approaches. Am J Kidney Dis 59: 293–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA: Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55[Suppl 2]: S23–S33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuben DB, Rosen S: Principles of geriatric assessment. In: Hazzard's Geriatric Medicine and Gerontology, 6th Ed., edited by Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, New York, McGraw-Hill, 141–152, 2009 [Google Scholar]
- 8.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS: Geriatric conditions and disability: The Health and Retirement Study. Ann Intern Med 147: 156–164, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Flacker JM: What is a geriatric syndrome anyway? J Am Geriatr Soc 51: 574–576, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Fried T: The end of the disease era. Am J Med 116: 179–185, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bowling CB, Booth JN, 3rd, Safford MM, Whitson HE, Ritchie CS, Wadley VG, Cushman M, Howard VJ, Allman RM, Muntner P: Nondisease-specific problems and all-cause mortality in the REasons for Geographic and Racial Differences in Stroke study. J Am Geriatr Soc 61: 739–746, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry SI, Wang Y, Gill TM, Krumholz HM: Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol 55: 309–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PG, Cigolle C, Blaum C: The co-occurrence of chronic diseases and geriatric syndromes: The health and retirement study. J Am Geriatr Soc 57: 511–516, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Sánchez E, Vidán MT, Serra JA, Fernández-Avilés F, Bueno H: Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart 97: 1602–1606, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, Harris T, Newman AB: Chronic kidney disease and functional limitation in older people: Health, aging and body composition study. J Am Geriatr Soc 54: 750–756, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kop WJ, Seliger SL, Fink JC, Katz R, Odden MC, Fried LF, Rifkin DE, Sarnak MJ, Gottdiener JS: Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol 6: 834–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurella Tamura M, Muntner P, Wadley V, Cushman M, Zakai NA, Bradbury BD, Kissela B, Unverzagt F, Howard G, Warnock D, McClellan W: Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 58: 756–763, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, Messé SR, Sehgal AR, Kusek J, DeSalvo KB, Cornish-Zirker D, Cohan J, Seliger SL, Chertow GM, Go AS: Vascular risk factors and cognitive impairment in chronic kidney disease: The Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol 6: 248–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G, Go AS, Chronic Renal Insufficiency Cohort Investigators : Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58: 338–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G: The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology 25: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC: Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 40: 771–781, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Radloff L: The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1: 385–401, 1977 [Google Scholar]
- 25.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Dalrymple LS, Katz R, Rifkin DE, Siscovick D, Newman AB, Fried LF, Sarnak MJ, Odden MC, Shlipak MG: Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol 8: 2091–2099, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowling CB, Muntner P, Sawyer P, Sanders PW, Kutner N, Kennedy R, Allman RM: Community mobility among older adults with reduced kidney function: A study of life-space. Am J Kidney Dis 63: 429–436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailie GR, Eisele G, Liu L, Roys E, Kiser M, Finkelstein F, Wolfe R, Port F, Burrows-Hudson S, Saran R: Patterns of medication use in the RRI-CKD study: Focus on medications with cardiovascular effects. Nephrol Dial Transplant 20: 1110–1115, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Wolff JL, Starfield B, Anderson G: Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 162: 2269–2276, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Uhlig K, Boyd C: Guidelines for the older adult with CKD. Am J Kidney Dis 58: 162–165, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.