Abstract
Background and objectives
A proposed histopathologic classification for ANCA-associated GN is predictive of long-term renal outcome in adult populations. This study sought to validate this system in a pediatric cohort.
Design, setting, participants, & measurements
This was a retrospective, single-center, cohort study of 40 children diagnosed and followed until their transition to adult care at one institution between 1987 and 2012. Renal biopsy specimens were reviewed by a pathologist blinded to patient outcome and were classified using the new histopathologic classification system of focal, crescentic, mixed, and sclerotic groups. Time to the composite outcome of CKD stages 3 and 4 (determined by eGFR with repeated creatinine measures using the Schwartz equation) or ESRD (defined as dialysis dependence or transplantation) were ascertained.
Results
The study population consisted of 40 children (70% female), followed for a median of 2.4 years. The biopsy specimens were categorized as focal in 13 patients (32.5%), crescentic in 20 (50%), mixed in two (5%), and sclerotic in five (12.5%). Mixed and crescentic were combined for analyses. Survival analysis of time to the composite renal endpoint of at least 3 months of eGFR<60 ml/min per 1.73 m2 or ESRD differed significantly among the three biopsy groups log-rank P<0.001), with an adjusted hazard ratio of 3.14 (95% confidence interval, 0.68 to 14.4) in the crescentic/mixed group and 23.6 (95% confidence interval, 3.9 to 144.2) in the sclerotic category compared with the focal category. The probability of having an eGFR>60 ml/min per 1.73 m2 at 2 years was 100% for the focal, 56.5% for the crescentic/mixed, and 0% for the sclerotic biopsy categories.
Conclusions
This study showed the clinical utility of this histopathologic classification system and its ability to discriminate renal outcomes among children with ANCA GN.
Keywords: ANCA, glomerulonephritis, pediatric nephrology, renal biopsy, vasculitis
Introduction
ANCA-associated vasculitides (AAV) include granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis. Although AAV are rare in childhood, renal involvement is common, occurring in 60%–90% of reported pediatric cases (1,2), and are most frequently associated with MPA (3). The most severe form of renal involvement in AAV is the rapidly progressive ANCA-associated necrotizing crescentic GN; among children, 30%–40% will progress to CKD and about 10% to ESRD by adulthood (2,4).
In 2010, an international working group of renal pathologists developed a new histopathologic classification system for ANCA GN and validated it in a population of 100 adults with ANCA GN from two European Vasculitis Study Group trials (5–7). Preceding that, various pathologic criteria, including percentage of normal glomeruli, degree of chronic (sclerotic) lesions, and active (crescentic) lesions predicted kidney outcomes in several studies (8–10). Collating these data, the working group proposed a novel and intuitive schema classifying the renal histopathologic features into four distinct categories: focal, crescentic, mixed, and sclerotic. The biopsy category is reliable in predicting long-term renal outcome (eGFR) at both 1 and 5 years (5).
Any newly devised classification system needs external validation in the pediatric population to confirm both relevance and reproducibility as a prognostic tool, especially because pediatric populations may differ in both disease severity and outcomes. For example, when the predictive value of the Oxford classification system for IgA nephropathy was applied to children, there was inconsistent association of the pathologic criteria with renal outcomes (11–15). Therefore, we sought to validate this new pathologic classification of ANCA GN in cases with childhood onset and determine the association with renal outcomes.
Materials and Methods
This was a nonconcurrent, single-center cohort study of consecutive children diagnosed with pauci-immune necrotizing GN in association with granulomatosis with GPA or MPA at the Hospital for Sick Children. A complete population was ascertained as previously described; the study included 24 new patients and extended follow-up of the 16 patients described in an earlier study (2). Children were diagnosed and followed up until their transfer to an adult center from January 1, 1987, to August 31, 2012. All patients met classification criteria of the American College of Rheumatology or Chapel Hill Classification systems for GPA or MPA and satisfied the European League Against Rheumatism and the Pediatric Rheumatology European Society criteria for the classification of childhood vasculitides (16). The study included children age≤18 years of age who had an initial renal biopsy at the Hospital for Sick Children. Patients were excluded if they had an overlap syndrome, such as anti-glomerular basement membrane/ANCA-positive vasculitis, or the biopsy specimen was unavailable or deemed inadequate for histopathologic classification by the pathologist. The Research Ethics Board at the Hospital for Sick Children approved the study, and we adhered to the Declaration of Helsinki.
Data Collection
All data were collected from the electronic patient record or the archived clinical chart. Data were collected on demographic characteristics, clinical symptoms, laboratory data, and kidney function. eGFR was calculated using the modified Schwartz equation (17). Classification of CKD grade did not differ with the change of creatinine assay to an isotope dilution mass spectrometry reference method in 2008. Treatment data included use of plasma exchange, antihypertensive medications or angiotensin-converting enzyme inhibitors, and type of immunosuppressive therapy used (including steroids, cyclophosphamide, methotrexate, mycophenolate mofetil, and rituximab).
Renal Histopathology
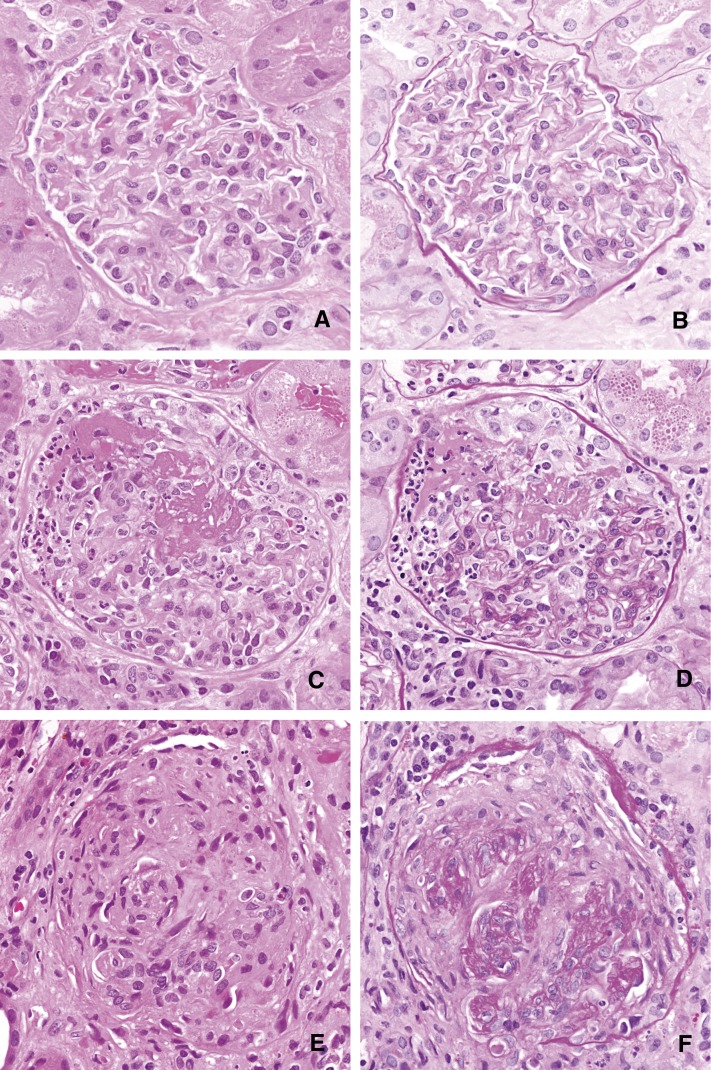
All renal biopsy specimens were re-reviewed by a single pediatric renal pathologist, blinded to patient outcome. For light microscopy, all biopsy specimens had been fixed in 10% formalin, routinely processed, and sectioned at 2 microns. Fifteen levels were stained on each specimen; three each were stained with hematoxylin and eosin, hematoxylin-phloxine-safranin, periodic acid–Schiff, periodic acid ammoniacal silver, and Masson trichrome. Biopsy specimens were then classified using the published histopathologic schema (5) as focal, crescentic, mixed, or sclerotic on the basis of the light microscopic findings (Figure 1).
Figure 1.
Illustration of the histopathological scoring system for ANCA-associated GN. Glomeruli were categorized as normal (A and B), those with cellular crescents (C and D), or sclerotic (E and F). The left column (A, C, E) illustrates a representative glomerulus in each category, stained with hematoxylin and eosin. In the right column (B, D, F), glomeruli have been stained with periodic acid–Schiff to highlight the basement membranes in the vascular tuft, making the crescent easier to distinguish from the glomerulus in the case of the cellular crescent, and to demonstrate the obliteration of the glomerulus in the sclerotic category. (A–F, original magnification, ×400.)
Outcomes
The primary outcomes measured were (1) time to the composite outcome of CKD stages 3 and 4 (defined as an eGFR 15–59 ml/min per 1.73m2) or ESRD (defined as having an eGFR of <15 ml/min per 1.73 m2), dialysis dependence or transplantation; (2) time to CKD stages 3 and 4; and (3) the percentage of patients reaching CKD or ESRD at 6 months and longest follow-up. Secondary outcomes included degree of proteinuria, presence of hypertension, and number of relapses (defined as an increase in immunosuppressive treatment due to renal disease, suggested by an increase in serum creatinine or proteinuria and confirmed by renal biopsy). Data were obtained at 6 months and last follow-up. In addition, all serum creatinine measures from diagnosis to last follow-up were collected from the electronic patient record.
Statistical Analyses
Summary statistics were used to summarize patient demographic characteristics and clinical features at presentation. Normally distributed data are presented as mean±SD; otherwise, medians with interquartile range are provided. We presented the mean eGFR per 1-month period over an 18-month follow-up time. The mean number of distinct creatinine values per patient was 94 (range, 7–753). Urinary protein-to-creatinine ratios and eGFR for the remainder of analyses were log transformed. Comparisons are made of baseline information by biopsy category using appropriate tests for continuous or categorical measures.
We determined time to CKD by eGFR with repeated creatinine measures using the Schwartz equation, or ESRD as defined as dialysis dependence or transplantation. Kaplan–Meier survival of time to ESRD or the composite outcome of CKD (eGFR< 60 ml/min per 1.73 m2) or ESRD and linear regression analyses of the slope of eGFR were performed. All patients who received dialysis therapy at dialysis initiation were set to ESRD if they remained on maintenance dialysis for at least 3 months. We also conducted a Cox regression analysis to determine the risk of biopsy category and CKD/ESRD. A P value of 0.05 indicated a statistically significant difference. Data were analyzed using Stata software, version 11.
Results
The study population consisted of 40 children with ANCA GN seen from January 1, 1987, to August 31, 2012, consecutively. There were an equal number of patients with GPA and MPA. The median age of the patients at renal diagnosis was 12 (range, 3.6–17.3) years, and 70% of the cohort was female.
Renal Pathology
Upon classification as per the new schema (outlined in Table 1), there were 13 (32.5%) cases in the focal category, 20 (50%) in the crescentic category, two (5%) in the mixed category, and five (12.5%) in the sclerotic category. For the remainder of the analyses, we combined the two children with mixed pathologic features into the crescentic group (Table 2). The eGFR at baseline in the mixed group ranged from 87–138.7 ml/min per 1.73 m2. By definition, patients in the mixed group had <50% normal, <50% crescentic, and <50% globally sclerotic glomeruli. A glomerulus was described as being sclerotic when >80% of the glomerulus was sclerosed. Across categories from focal to sclerotic, serum creatinine, albumin, eGFR, hemoglobin, erythrocyte sedimentation rate, urine protein-to-creatinine ratio and need for dialysis at baseline varied significantly.
Table 1.
Pathologic classification schema
| Biopsy Category | Definition |
|---|---|
| Focal | ≥50% normal glomeruli |
| Crescentic | ≥50% glomeruli with cellular crescents |
| Mixed | <50% normal, <50% crescentic, <50% globally sclerotic glomeruli |
| Sclerotic | ≥50% globally sclerotic glomeruli |
Data obtained from reference 5.
Table 2.
Baseline demographic and clinical characteristics of 40 children with ANCA GN from 1987 to 2012
| Characteristic | Focal ( n=13) | Crescentic/Mixed (n=22) | Sclerotic (n=5) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| Male | 2 | 9 | 1 | 0.74 |
| Age at ANCA GN diagnosis (yr) | 13.5 (7–15) | 11 (8–15) | 11.2 (11.1–13.2) | 0.84 |
| Time to ANCA GN Diagnosis (d) | 109 (34–163) | 26 (10–51) | 24 (14–86) | 0.16 |
| Diagnosis | ||||
| MPA | 4 (30.7) | 13 (59.1) | 4 (80) | 0.05 |
| GPA | 8 (66.7) | 10 (43.5) | 1 (20) | 0.05 |
| ANCA positive | 9 (81.8) | 18 (85.7) | 4 (80) | 0.79 |
| ANCA specificity | ||||
| p-ANCA | 4 (36.4) | 11 (57.9) | 4 (80) | 0.29 |
| c-ANCA | 5 (45.5) | 6 (31.6) | 0 | — |
| PR3 | 5 (55.5) | 10 (62.5) | 3 (100) | 0.37 |
| MPO | 4 (44.5) | 6 (37.5) | 0 (0) | |
| ELISA titer | ||||
| 1/80 | 2 (22.2) | 3 (17.7) | 0 | 0.76 |
| 1/160 | 2 (22.2) | 6 (35.3) | 1 (25) | – |
| 1/320 | 3 (33.3) | 2 (11.8) | 1 (25) | – |
| 1/640 | 2 (22.2) | 6 (35.3) | 2 (50) | – |
| Kidney status at presentation | ||||
| Creatinine (mg/dl) | 0.6 (0.5–0.9) | 2.8 (1.1–6.4) | 3.5 (3.1–9.7) | 0.01 |
| eGFR (ml/min per 1.73 m2) | 106.3 (84.8–126.6) | 19.6 (10.7–57.7) | 19.4 (15–20.0) | <0.001 |
| Proteinuria non-nephrotic (n) | 10 | 12 | 1 | 0.01 |
| Nephrotic (n) | 1 | 8 | 2 | – |
| Protein-to-creatinine ratio (mg/g) | 695 (265–1425) | 1894 (1327–3735) | 24,925 (13,000–36,849) | 0.02 |
| Need for dialysis at diagnosis | 0 | 9 (39) | 3 (60) | 0.01 |
| Other | ||||
| Hemoglobin (g/dL) | 11.7 (11.0–11.8) | 9.85 (8.1–10.8) | 8.5 (6.5–9.3) | 0.02 |
| Platelets (×109/L) | 291 (240–355) | 361 (294–416) | 391 (238–438) | 0.42 |
| Neutrophils (×109/L) | 7.4 (4.4–8.8) | 9.7 (6.8–12.6) | 5.5 (4.4–9.6) | 0.23 |
| Albumin (g/dl) | 3.6 (3.5–3.9) | 3.1 (2.7–3.4) | 2.6 (2.6–3.1) | 0.01 |
| ESR (mm/hr) | 32 (12–90) | 103 (54–133) | 93 (78–110) | 0.02 |
| CRP (mg/L) | 3.4 (0.9–22.2) | 55.5 (7.3–183) | 59.3 (0.6–118) | 0.32 |
| C3 (g/L) | 1.2 (0.99–1.3) | 1.3 (1.1–1.5) | 0.97 (0.85–1.0) | 0.39 |
| IgA (mg/dl) | 150 (120–200) | 150 (100–280) | 220 (130–290) | 0.71 |
| IgM (mg/dl) | 120 (80–170) | 90 (60–130) | 125 (120–150) | 0.53 |
| IgG (mg/dl) | 1050 (720–1330) | 990 (620–1490) | 1300 (920–1590) | 0.79 |
| Treatment of ANCA GN | ||||
| Corticosteroids | 11 (84.6) | 22 (100) | 5 (100) | 0.14 |
| Cyclophosphamide | 5 (38.5) | 17 (77.3) | 2 (40) | 0.05 |
| Plasma exchange | 1 (7.7) | 4 (18.1) | 1 (10) | 0.68 |
| Azathioprine | 2 (15.4) | 0 | 0 | 0.12 |
| Methotrexate | 4 (30.8) | 0 | 0 | 0.03 |
| Rituximab | 2 (15.4) | 0 | 0 | 0.12 |
Continuous variables were analyzed using one-way ANOVA and categorical variables by chi- square analyses. Unless otherwise noted, values are expressed as number (percentage) of patients or median (range). MPA. microscopic polyangiitis, GPA, granulomatosis with polyangiitis; p-ANCA, perinuclear ANCA; c-ANCA, cytoplasmic ANCA; PR3, proteinase 3; MPO, myeloperoxidase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Outcomes
The median duration of follow-up was 883 (interquartile range, 95–1480) days. Four of the 13 (31%) patients requiring dialysis at diagnosis were dialysis independent at 6 months. Mean duration of dialysis in those that came off dialysis was 9.7 days (range, 7–30). No patients died during the study period.
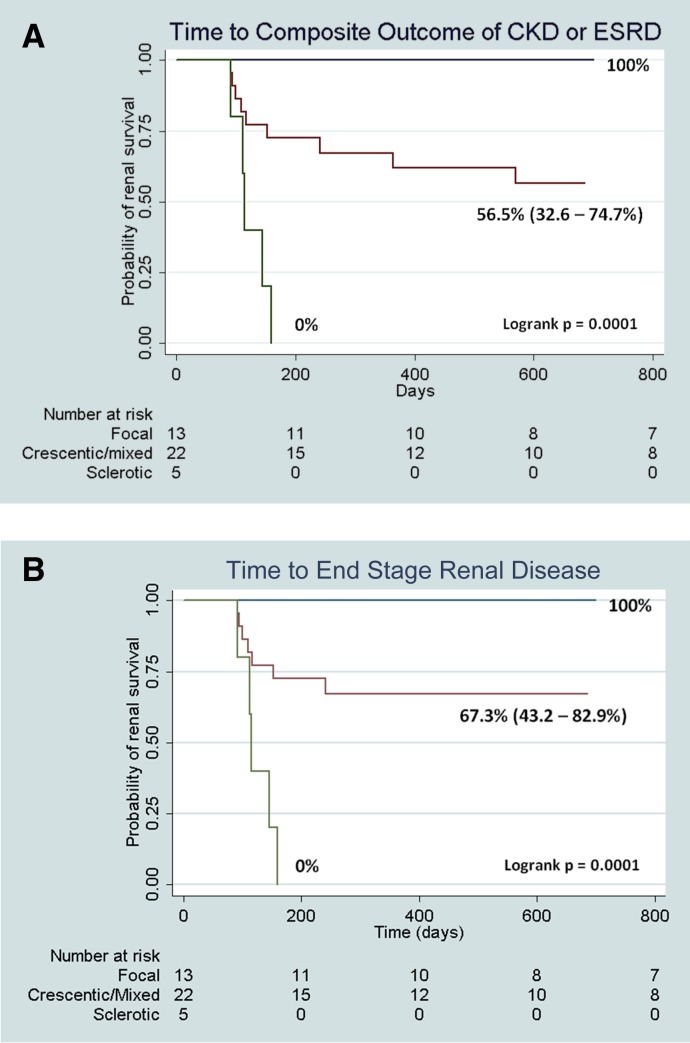
We conducted a Kaplan–Meier survival analysis of time to the composite renal endpoint of at least 3 months of eGFR<60 ml/min per 1.73 m2 or ESRD (dialysis or transplantation) by the three biopsy groups (log-rank P <0.001). We limited follow-up to 2 years as seven children transitioned to adult care. Probability (95% confidence interval [95% CI]) of eGFR>60 ml/min per 1.73 m2 at 2 years after diagnosis was 100% for focal, 56.5% (95% CI, 32.6% to 74.7%) for crescentic/mixed, and 0% for sclerotic biopsy categories (Figure 2A). Probability of not reaching ESRD (dialysis or transplantation) at 2 years after diagnosis was 100% for focal, and 67.3% (95% CI, 43.2% to 82.9%) for crescentic/mixed. Those with sclerosis all progressed to ESRD (Figure 2B).
Figure 2.
Renal survival by biopsy category. (A) Time to composite outcome of CKD/ESRD and probability of renal survival at 2 years after diagnosis of ANCA GN. Probability (95% confidence interval [95% CI]) of renal survival at 2 years after diagnosis by kidney biopsy category. Survival functions for all biopsy categories differed significantly (log-rank P<0.001). (B) Time to ESRD and probability of renal survival at 2 years after diagnosis of ANCA GN. Probability (95% CI) of eGFR>60 ml/min per 1.73 m2 (excluding patients who underwent transplantation) at 2 years after diagnosis by kidney biopsy category. Survival functions for all biopsy categories differed significantly (log-rank P<0.01).
By Cox regression analyses, we demonstrated that children in the crescentic/mixed groups were three times more likely to develop the composite endpoint of CKD/ESRD (adjusted hazard ratio, 3.14; 95% CI, 0.68 to 14.4); however, the confidence interval crossed 1. Those with sclerosis were at 23.6 times higher risk than those with focal pathology (95% CI, 3.9 to 144.2) while controlling for age at diagnosis and sex. On the basis of the small sample size, we had wide CIs; however, there was a consistently higher risk for progression in both the crescentic and sclerotic group than in the focal group (P for trend <0.001). Age at diagnosis and sex were not associated with higher risk. Additionally, we could not adjust for urinary protein-to-creatinine ratio or eGFR because they were tightly correlated (R=−0.62; P<0.001). For every increase in the natural log of urinary protein-to-creatinine, there was a higher risk of progression (adjusted hazard ratio of 5.36; 95% CI, 1.99 to 14.42) while controlling for age at diagnosis and log of eGFR at baseline. Similarly, for every increase in the natural log of eGFR at baseline, there was a 90% lower risk of progression to CKD and ESRD (adjusted hazard ratio, 0.12; 95% CI, 0.04 to 0.39) after adjustment for age and urinary protein-to-creatinine ratio. All models were limited by small sample size and thus were minimally adjusted. Any effect related to therapy could not be assessed.
None of the focal group developed CKD stages 3 or 4 or ESRD by 6 months or longest follow-up (Table 3); however, two focal patients had a reduced eGFR of 60–90 ml/min per 1.73 m2 at last follow-up but did not meet CKD criteria as defined by stages 3 and 4 CKD. By last follow-up, within the mixed combined category, both children with mixed pathologic type had normal renal function. Among the 20 participants with crescentic pathologic features alone, eight had an eGFR> 60 ml/min per 1.73 m2, 3 had CKD stages 3 and 4, and nine had reached ESRD.
Table 3.
Outcomes of 40 children with ANCA GN from 1987 to 2012
| Outcome | Focal (n=13) | Crescentic/Mixed (n=22) | Sclerotic (n=5) |
|---|---|---|---|
| 6-mo follow-up | |||
| eGFR (ml/min per 1.73 m2) | 109.9 (100.6–115.4) | 57.8 (7.8–97) | 10.4 (7.5–11.5) |
| CKD (eGFR < 60 ml/min per 1.73 m2) | 0 | 4 | 0 |
| ESRD | 0 | 6 | 5 |
| Urine protein-to-creatinine ratio (mg/g) | 162 (130–212) | 1261 (673–2832) | 951 (248–2456) |
| Last follow-up | |||
| Duration of follow-up (mo) | 71.5 (19.5–91.5) | 28.5 (25–47) | 79 (42.5–86.5) |
| eGFR (ml/min per 1.73 m2) | 106.2 (99.9–116.1) | 72.4 (32.1–94.5) | 6.8 (5.7–87.3) |
| CKD (eGFR < 60 ml/min per 1.73 m2) | 0 | 3 | 0 |
| ESRD | 0 | 9 | 5 |
| Urine protein-to-creatinine Ratio (mg/g) | 236 (73–381) | 235 (142–820) | – |
Values are expressed as the number of patients or the median (interquartile range).
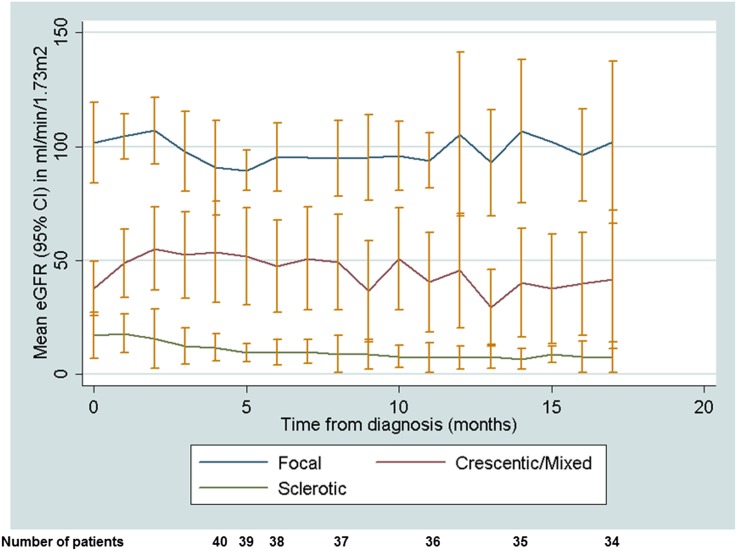
Mean eGFR (95% CI) variation over time by biopsy category is presented in Figure 3. By histopathology, there is a clear separation of the mean eGFR for each month over the 18 months since diagnosis. Those in the focal or crescentic/mixed group had an increase in eGFR over the first 3 months after diagnosis, with a slow decline evident in the crescentic/mixed group and no decline evident in the focal group. Linear regression analysis demonstrated an association with slope of (ln transformed) eGFR for the combined crescentic/mixed categories with baseline (ln transformed) protein-to-creatinine ratio (P=0.05) and need for dialysis at presentation (P=0.05) after adjustment.
Figure 3.
Mean eGFR (95% CI) variation over time by biopsy category. With use of repeated measures of serum creatinine to calculate eGFR, the mean eGFR per 1-month period was calculated and plotted over an 18-month follow-up period.
Clinical outcomes at the end of follow-up, and before transition to adult care, demonstrated that 42.4% of the overall cohort had CKD and 33.3% had progressed to ESRD. Among all children, 36.7% were hypertensive and half were prescribed angiotensin-converting enzyme inhibition for proteinuria. Nine (24.3%) children in total were dialysis dependent from diagnosis, and six underwent transplantation (without recurrence) during the study period.
Among a subset of children with focal and crescentic disease, biopsies were repeated to determine progression or because of relapses. Among children with crescentic disease who had a repeat biopsy (n=10), eight progressed to sclerotic disease out of the original 20 children with crescentic disease. Among the focal group, only one had repeat biopsy and was reclassified as mixed.
Median time to diagnosis of AAV from time of symptoms was 15 days (range, 32–109 years). Children with renal AAV almost uniformly received steroids (95%), and 32 children received additional therapies, including rituximab (n=3), methotrexate (n=4), cyclophosphamide (n=26), azathioprine (n=3), or plasma exchange (n=6). Clinical management varied according to the year of diagnosis with availability of steroid-sparing medications, consensus guidelines, and physician practice. Only six children received plasma exchange, with a mean eGFR of 20.3 (range, 6–56) ml/min per 1.73 m2; however, the specific indication for initiation of plasma exchange was difficult to ascertain retrospectively.
Discussion
Childhood-onset ANCA GN poses a significant risk for progressive disease. In our study, 42.4% of children with ANCA GN progressed to CKD, and 33.3% progressed to ESRD in a median of 2.4 years. Our data support the use of this histopathologic classification schema to provide important prognostic information in childhood-onset ANCA GN. By histopathologic classification, patients with sclerotic disease on biopsy fared poorly, with rapid progression to ESRD in less than 6 months. Those children with focal disease presented with mild disease and had a higher baseline eGFR. The children with crescentic/mixed disease had initial improvement in eGFR and a slow decline over 2 years. Half of those with crescentic disease had a renal relapse, and this was associated with progression to sclerotic category on rebiopsy in 80%. Baseline eGFR was an additional predictor for ESRD in the original study of ANCA GN in adults; however, proteinuria was not included in the study (5). We were also able to demonstrate that within the crescentic/mixed category, the need for dialysis at diagnosis of ANCA GN and degree of proteinuria predicted a greater decline in renal function.
The pathologic classification initially developed and validated in adults can now be extended for use in children. The classification not only can predict discrete outcomes of ESRD but also can delineate GFR decline over follow-up and risk of CKD. Thus, at diagnosis, important prognostic information is available for counseling of children and families. Currently, therapies are decided on the basis of extrarenal manifestations and crescentic disease; however, it may be that further discerning protocols based on pathologic features can be considered. A most striking example is that all children in the focal category on biopsy had an eGFR>60 ml/min per 1.73 m2, whereas all sclerotic patients progressed to ESRD despite aggressive early therapy. We also observed that patients initially in the crescentic category who relapsed invariably progressed to the sclerotic category.
Substantial morbidity is associated with the treatments for AAV occurring in childhood, including adverse effects of steroids and an increased rate of infections (7-fold increase with steroid and cyclophosphamide used in combination), as well as the more long-term risks of infertility (1,4). Our data suggest that for the sclerotic category, intensive immunosuppressive therapy is unlikely to result in renal function recovery.
Several validation studies of the new pathologic criteria have been published and have demonstrated reliability in predicting outcomes for patients with focal or sclerotic disease in ANCA GN (18–21). One consistent finding, however, in contrast to the original study, has been that the mixed category confers better renal survival than does crescentic disease (5,19,20). We have a small number of children with mixed pathologic features in contrast to all the adult studies but a similar number with crescentic pathologic features (50%); this is more in keeping with the European and Chinese cohorts (47%–55%) and dissimilar to findings in Japan, where only 8% of the population had crescentic disease. This could reflect varying disease processes as all the patients in the Japanese study had antibodies to myeloperoxidase (5,19).
Although outcomes for patients with ANCA GN have improved substantially over the last decade, attributable to earlier diagnosis and better therapeutic strategies, renal involvement in AAV is common, occurring in about 88% of childhood cases and 80% of adult cases (2,20,22). Compared with reported studies of children with ANCA GN, we demonstrated a higher proportion of children reaching CKD or ESRD. We have extended a prior study conducted at the Hospital for Sick Children that found 40% of the first 22 children with ANCA GN progressed to CKD and 12% to ESRD (2). Arulkumaran et al. collated all the pediatric literature on a total of 86 children and found that although the number of children progressing to CKD is similar for both GPA (29%) and MPA (27%), the progression to ESRD is more likely with MPA (35%) than with GPA (5%) (4). Our study could not demonstrate an association between diagnosis of GPA versus MPA and renal outcomes given the small sample size. Overall, renal survival in children (66.7% at longest follow-up for our cohort) appears better than that reported in adults with renal survival estimates of 53.5% at 5 years (20). No child from our cohort died, in stark contrast to the outcomes seen with adult-onset disease.
Our study has limitations, including a small number of children; however, it is one of the largest pediatric cohorts with ANCA GN. We also could not assess the effect of individual therapies on our outcomes of interest given the small sample size and variability in therapy. Given the large time frame over which children were included in the study, and the fact that treatment regimens have changed during this time, our results will need to be validated prospectively, with modern treatment regimens. We also have many children who have transitioned into adulthood and therefore have very little follow-up information after age 18 years. Our study also lacked power to ascertain other factors from within the crescentic/mixed group apart from need for dialysis and degree of proteinuria, which may provide further prognostic information. Perhaps other pathologic factors, such as tubular atrophy and interstitial fibrosis or the degree of CD3+ tubulitis, may help in this regard in future studies (9,23–25).
The clinical utility of this histopathologic classification system and its ability to clearly discriminate kidney outcomes among ANCA GN patients has been demonstrated in adults. This study of 40 children with ANCA GN provides supportive evidence that this classification system is similarly predictive of outcome as in adults and can likely be extended to the pediatric population. This classification will enhance communication to patients and families, clinicians, and researchers. Developing studies to prospectively evaluate treatments by pathologic diagnosis will permit optimization of our treatment strategies and ultimately lead to better evidence for the treatment of this severe disease.
Disclosures
None.
Acknowledgments
D.G.N. is supported by a fellowship from the Transplant Centre at the Hospital for Sick Children and a Postgraduate Medical Education, Restracomp award from the Hospital for Sick Children. Part of this work was submitted in abstract form at the International Pediatric Nephrology Association meeting in 2013.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rottem M, Fauci AS, Hallahan CW, Kerr GS, Lebovics R, Leavitt RY, Hoffman GS: Wegener granulomatosis in children and adolescents: Clinical presentation and outcome. J Pediatr 122: 26–31, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Akikusa JD, Schneider R, Harvey EA, Hebert D, Thorner PS, Laxer RM, Silverman ED: Clinical features and outcome of pediatric Wegener’s granulomatosis. Arthritis Rheum 57: 837–844, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Peco-Antic A, Bonaci-Nikolic B, Basta-Jovanovic G, Kostic M, Markovic-Lipkovski J, Nikolic M, Spasojevic B: Childhood microscopic polyangiitis associated with MPO-ANCA. Pediatr Nephrol 21: 46–53, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Arulkumaran N, Jawad S, Smith SW, Harper L, Brogan P, Pusey CD, Salama AD: Long- term outcome of paediatric patients with ANCA vasculitis. Pediatr Rheumatol Online J 9: 12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël L-H, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, Ekstrand A, Gaskin G, Gregorini G, de Groot K, Gross W, Hagen EC, Mirapeix E, Pettersson E, Siegert C, Sinico A, Tesar V, Westman K, Pusey C, European Vasculitis Study Group : A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 349: 36–44, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO, Sinico RA, Stegeman CA, Westman KW, van der Woude FJ, de Lind van Wijngaarden RA, Pusey CD, European Vasculitis Study Group : Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 18: 2180–2188, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM: Renal histopathology and clinical course in 94 patients with Wegener’s granulomatosis. Nephrol Dial Transplant 16: 953–960, 2001 [DOI] [PubMed] [Google Scholar]
- 9.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, Noël LH, Ferrario F, Waldherr R, Hagen EC, Bruijn JA, Bajema IM: Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Determinants of outcome in ANCA-associated glomerulonephritis: A prospective clinico-histopathological analysis of 96 patients. Kidney Int 62: 1732–1742, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA, Fervenza FC, Cattran DC: Validation of the Oxford classification of IgA nephropathy. Kidney Int 80: 310–317, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, Kaito H, Sako M, Iijima K, Yoshikawa N: Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol 27: 783–792, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Le W, Zeng CH, Liu Z, Liu D, Yang Q, Lin RX, Xia ZK, Fan ZM, Zhu G, Wu Y, Xu H, Zhai Y, Ding Y, Yang X, Liang S, Chen H, Xu F, Huang Q, Shen H, Wang J, Fogo AB, Liu ZH: Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC Nephrol 13: 158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, Feehally J, Roberts IS, Amore A, Alpers CE, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator SN, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo AB, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int 77: 921–927, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC, Kawasaki T, Lindsley C, Petty RE, Prieur AM, Ravelli A, Woo P: EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 65: 936–941, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang DY, Wu LH, Liu G, Chen M, Kallenberg CGM, Zhao MH: Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: A study of 121 patients in a single center. Nephrol Dial Transplant 27: 2343–2349, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Muso E, Endo T, Itabashi M, Kakita H, Iwasaki Y, Tateishi Y, Komiya T, Ihara T, Yumura W, Sugiyama T, Joh K, Suzuki K: Evaluation of the newly proposed simplified histological classification in Japanese cohorts of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in comparison with other Asian and European cohorts. Clin Exp Nephrol 17: 659–662, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilhorst M, Wilde B, van Paassen P, Winkens B, van Breda Vriesman P, Cohen Tervaert JW, Limburg Renal Registry : Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: A 30-year follow-up study. Nephrol Dial Transplant 28: 373–379, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Togashi M, Komatsuda A, Nara M, Omokawa A, Okuyama S, Sawada K, Wakui H: Validation of the 2010 histopathological classification of ANCA-associated glomerulonephritis in a Japanese single-center cohort [published online ahead of print April 12, 2013]. Mod Rheumatol doi: 10.1007/s10165-013-0877-0 [DOI] [PubMed] [Google Scholar]
- 22.Renaudineau Y, Le Meur Y: Renal involvement in Wegener’s granulomatosis. Clin Rev Allergy Immunol 35: 22–29, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA: Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 56: 1751–1758, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int 61: 80–89, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Berden AE, Jones RB, Erasmus DD, Walsh M, Noël LH, Ferrario F, Waldherr R, Bruijn JA, Jayne DR, Bajema IM, European Vasculitis Society : Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol 23: 313–321, 2012 [DOI] [PubMed] [Google Scholar]