Abstract
With increasing emphasis among health care providers and funders on patient-centered care, it follows that patients and their caregivers should be included when priorities for research are being established. This study sought to identify the most important unanswered questions about the management of kidney failure from the perspective of adult patients on or nearing dialysis, their caregivers, and the health care professionals who care for these patients. Research uncertainties were identified through a national Canadian survey of adult patients on or nearing dialysis, their caregivers, and health care professionals. Uncertainties were refined by a steering committee that included patients, caregivers, researchers, and clinicians to assemble a short-list of the top 30 uncertainties. Thirty-four people (11 patients; five caregivers; eight physicians; six nurses; and one social worker, pharmacist, physiotherapist, and dietitian each) from across Canada subsequently participated in a workshop to determine the top 10 research questions. In total, 1570 usable research uncertainties were received from 317 respondents to the survey. Among these, 259 unique uncertainties were identified; after ranking, these were reduced to a short-list of 30 uncertainties. During the in-person workshop, the top 10 research uncertainties were identified, which included questions about enhanced communication among patients and providers, dialysis modality options, itching, access to kidney transplantation, heart health, dietary restrictions, depression, and vascular access. These can be used alongside the results of other research priority–setting exercises to guide researchers in designing future studies and inform health care funders.
Keywords: Dialysis, research priorities, patient preferences
Introduction
The traditional approach to identifying research priorities in health care has not involved patients (1). However, with increasing emphasis among health care providers and funders on patient-centered care, defined as care that is respectful of patient preferences and in which patient values guide clinical decisions (2), it follows that patients and their caregivers should be included when establishing priorities for research in health care.
Because they live with their disease, people with kidney failure receiving dialysis, those with severe kidney disease who are likely to soon require RRT, and their caregivers become “experts” in their disease. Quality of life for people undergoing dialysis is severely impaired and is rated similarly to that among patients with metastatic cancer (3). Considerable responsibility is also placed on the patients and their caregivers, given the ongoing need for dialysis (regardless of the dialysis modality that is selected) and the required modifications to diet and fluid intake. For these reasons, involving patients, caregivers, and clinicians in setting research priorities for people receiving dialysis is particularly important.
The National Institute of Diabetes and Digestive and Kidney Diseases recently completed a research prioritization exercise in which they asked the scientific community to formulate and prioritize research objectives to improve understanding of kidney function and disease (4), including for specific areas, such as dialysis therapies (5). This process did not specifically seek input from patients (only 7% of responses were received from individuals identifying themselves as lay-people), and no patients were involved in the second phase of work, during which the top priorities were selected. Indeed, few studies have elicited the research priorities of patients receiving dialysis (6), and none have done so systematically or have attempted to yield questions that relate to all facets of the disease (7,8).
We used the method established by the James Lind Alliance (9) to identify the most important unanswered questions (or uncertainties) about the management of kidney failure (i.e., in terms of diagnosis, prognosis, and treatment) from the perspective of adult patients receiving (or approaching the need for) dialysis, their caregivers, and the health care professionals who care for these patients. The goal of the James Lind Alliance is to develop patient-centered priorities. However, the method uses a shared process for identifying research priorities that includes patients, caregivers, and clinicians throughout the priority-setting exercise, with the expectation that this will increase the support for the priorities identified compared with an exercise that includes only patients. To clarify whether priorities varied across respondent types, we also compared the research priorities elicited across the different types of survey respondents.
Materials and Methods
As outlined in detail in the Supplemental Material, research uncertainties relevant to patients undergoing or nearing dialysis were identified through four key steps: identification and invitation of potential partners, collection of research uncertainties through a national survey, refinement and prioritization of uncertainties to assemble a list of the top 30 uncertainties, and an in-person workshop to determine the top 10 research uncertainties.
The priority-setting process was initiated in July 2012, with the formation of an 11-person Steering Group that included patients, a caregiver, clinicians, an employee of the Kidney Foundation of Canada, and an expert in the James Lind Alliance approach (see www.CANN-NET.ca for a list of members). The Steering Group held biweekly conference calls from July 2012 to June 2013 to oversee the process.
We developed a survey to identify uncertainties, consisting of broad questions about the overall management of severe kidney failure and dialysis, including diagnosis, prognosis, and treatment issues (Supplemental Material). Patients, caregivers, and clinicians were invited to complete the online survey through communications from our partner organizations (e.g., the Kidney Foundation of Canada), and emails to members of the Canadian Society of Nephrology and Canadian Association of Nephrology Nurses and Technologists. We also distributed paperbased surveys in 10 Canadian hemodialysis centers and three severe CKD and peritoneal dialysis clinics. Finally, we searched the most recent guidelines relevant to the care of patients receiving dialysis to identify research topics suggested by the guideline developers.
The uncertainties identified by survey respondents and from guidelines were combined, and those deemed not relevant to adult patients undergoing dialysis (e.g., prevention of kidney failure, management of a patient with a kidney transplant, issues exclusive to pediatric patients) were eliminated. Steering Group members then worked in pairs (a clinician paired with a patient or caregiver) to assign uncertainties into similar groups, with the goal of identifying a summary question for each group of uncertainties.
A summary document was prepared with all of the uncertainties, including the number of times the uncertainty was identified by patients, caregivers, and clinicians; and whether it was identified from a clinical practice guideline. The document was circulated to Steering Group members to facilitate an interim ranking exercise. Over the course of four Steering Group conference calls, the relative importance and wording of the uncertainties that were most highly ranked by committee members were discussed. The product was a short-list of 30 uncertainties to be considered at the workshop (Supplemental Material).
Finally, 34 people from across Canada participated in a 1-day workshop: 11 patients; five caregivers; eight physicians; six nurses; and one social worker, pharmacist, physiotherapist, and dietitian each (Supplemental Material). The workshop used a nominal group technique approach and a combination of small- and large-group exercises (9). A consensus approach (with voting when needed) was used to identify the top 10 research uncertainties.
To assess differences in research uncertainties across groups, we ranked the frequency with which research uncertainties from the survey were identified by patients, caregivers, physicians, and nurses.
Results
Characteristics of Survey Respondents
In total, we collected 1820 uncertainties from 317 respondents. The characteristics of the survey respondents are provided in Table 1. Most respondents expressed more than one uncertainty; the mean number of uncertainties per respondent was 5.5. One hundred seventy-three respondents (54.6%) were patients, 37 (12%) were caregivers, and 107 were health care professionals (34%; of these, 25 [23%] were physicians and 38 [36%] were nurses). Most patients were receiving in-center hemodialysis (53%), and 32% were undergoing home hemodialysis or peritoneal dialysis. Most respondents (75%) were from Western Canada or Ontario and identified themselves as white (79%). Forty-eight percent of patient respondents were age 50–69 years, similar to the average age of people starting dialysis in Canada (63 years). Only 1% of patients were Aboriginal, a rate lower than expected given that Aboriginal (10) peoples are three times as likely to be receiving treatment for ESRD as non-Aboriginals (11).
Table 1.
Profile of all survey respondents
| Variable | Patients (n=173) | Health Care Professionals (n=107) | Caregivers (n=37) |
|---|---|---|---|
| Patient receiving in-center hemodialysis | 92 (53) | ||
| Patient receiving home hemodialysis | 18 (10) | ||
| Patient receiving peritoneal dialysis | 38 (22) | ||
| Patient not receiving dialysis | 25 (14) | ||
| Physician | 25 (23) | ||
| Nurse | 38 (36) | ||
| Dietitian | 6 (1) | ||
| Social worker | 6 (1) | ||
| Member of an organizationa | 5 (5) | ||
| Other health care professional | 27 (25) | ||
| Age | |||
| 18–29 yr | 5 (3) | 5 (6) | 3 (10) |
| 30–39 yr | 15 (10) | 20 (23) | 3 (10) |
| 40–49 yr | 24 (16) | 26 (30) | 6 (21) |
| 50–59 yr | 34 (22) | 24 (28) | 6 (21) |
| 60–69 yr | 43 (25) | 10 (11) | 10 (34) |
| 70–79 yr | 22 (18) | 2 (2) | 1 (3) |
| ≥80 yr | 11 (6) | 0 | 0 |
| Sex | |||
| Male | 80 (52) | 59 (68) | 25 (86) |
| Female | 73 (48) | 28 (32) | 4 (14) |
| Ethnicity | |||
| Aboriginal | 2 (1) | 0 | 1 (3) |
| Asian | 9 (5) | 12 (11) | 1 (3) |
| Black | 8 (5) | 0 | 1 (3) |
| Mixed | 3 (2) | 2 (2) | 1 (3) |
| Other | 7 (4) | 1 (1) | 0 |
| White | 119 (69) | 70 (65) | 23 (62) |
| Prefer not to say | 25 (14) | 22 (21) | 10 (27) |
| Province | |||
| Atlantic | 28 (18) | 9 (10) | 4 (14) |
| British Columbia | 6 (4) | 5 (6) | 3 (10) |
| Ontario | 55 (36) | 24 (28) | 13 (45) |
| Prairies (Alberta, Saskatchewan, Manitoba) | 58 (38) | 45 (52) | 9 (31) |
| Quebec | 5 (3) | 4 (5) | 0 |
| Territories | 1 (1) | 0 | 0 |
Values are expressed as the number (percentage) of patients. Because not all of the demographic questions were mandatory, the categories do not all add to their respective denominators.
Typically represented members or volunteers of the Kidney Foundation of Canada, who advertised the web survey on their website and Facebook page.
Uncertainties
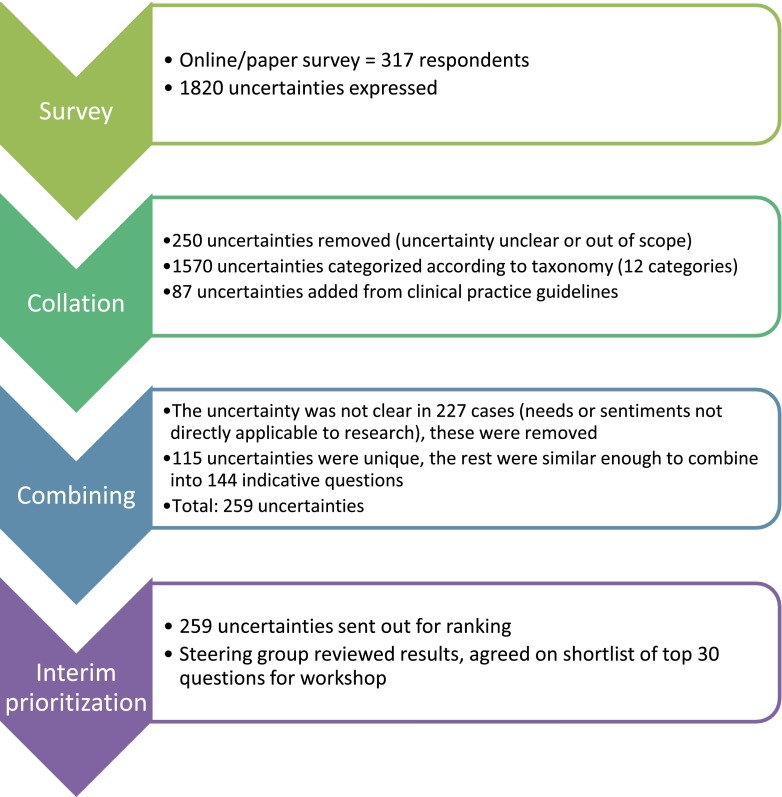
Of the 1820 uncertainties, 91 were eliminated because they were deemed not relevant to adult patients receiving or nearing dialysis, and 159 were eliminated because the uncertainty was not clear (Figure 1). From the remaining 1570 in-scope uncertainties, we identified 144 summary questions across the 12 categories (Table 2) as well as 85 unique questions. Eighty-seven uncertainties from relevant clinical practice guidelines were also included, 57 of which were combined into summary questions identified from our survey and 30 that were unique.
Figure 1.
The top thirty research uncertainties were developed through a national survey, followed by a process to combine and prioritize research uncertainties.
Table 2.
Frequency of in-scope uncertainties, by category
| Taxonomy Category | Participants, n (%) (n=1570) |
|---|---|
| Severe kidney disease (not yet receiving dialysis) | 302 (19.2) |
| Peritoneal dialysis | 39 (2.5) |
| Hemodialysis | 168 (10.7) |
| Hemodialysis vascular access | 170 (10.8) |
| Chronic complications | 76 (4.8) |
| Diet | 161 (10.3) |
| Symptoms | 274 (17.5) |
| Health system services | 71 (4.5) |
| Communication | 43 (2.7) |
| Education | 67 (4.3) |
| End of life | 7 (0.4) |
| Other | 192 (12.2) |
As a result of the process described above, a summary document was prepared with the 259 uncertainties identified within the survey and the guidelines, from which the Steering Group chose the top 30 uncertainties to be considered at the in-person workshop (Supplemental Material).
At the conclusion of the workshop, the top 10 research uncertainties were identified (Table 3). These included questions about the optimal communication methods between patients and health care professionals, the optimal dialysis modality, and the best way to manage a variety of symptoms due to kidney failure or its treatment. The uncertainties that were ranked 11–15 are identified in the Supplemental Material.
Table 3.
Top 10 research uncertainties
| Research Uncertainty | Source,a Themes, and Uncertainties Encompassed |
|---|---|
| 1. What is the best way to enhance communication between health care professionals and patients and to maximize patient participation in decision making with regard to the advantages and disadvantages of different forms of dialysis, and access to test results to facilitate self-management? | Source: Mostly patients, but also some health professionals and caregivers |
| Themes | |
| Inform decision making about dialysis treatment options | |
| Need for improved communication among all parties (doctors, nurses, patients, etc.) | |
| Potential for patients to be more engaged in their own care (e.g., by means of having access to test results, information about blood-work and effects of medications) | |
| Having access to information about others’ experiences (and what the pros/cons were) in the context of decision making | |
| Combined the following uncertainties: | |
| “What is the best way of informing patients with kidney failure about the advantages and disadvantages of different forms of dialysis; and how can we ensure that people get the right information, at the right time, and in the right way to ensure informed decision-making?” | |
| “How can communication between patients with kidney failure and health care providers be improved, and does enhanced communication (including providing test results) increase patients’ ability to participate in the management of their condition?” | |
| 2. How do different dialysis modalities compare in terms of their effect on quality of life, mortality, and patient acceptability, and are there specific patient factors that make one modality better for some patients with kidney failure than others? | Source: Noted by a similar proportion of patients and health professionals, and some caregivers |
| Themes | |
| Uncertainties by health professionals were mostly related to comparison of dialysis modalities (PD versus HD, nocturnal HD versus short/frequent HD, home versus hospital HD) in terms of quality of life and mortality | |
| Many patients submitted uncertainties about determining the optimal length of time and frequency of HD for individual patients, and their impact on outcomes, with the potential that patients’ quality of life could be improved with shorter HD sessions | |
| This question was combined with a second uncertainty noted within the top 30: | |
| “How can hemodialysis be tailored to a patient [in terms of: length, frequency, location and schedule (e.g. day/nighttime)] to enhance effectiveness and quality of life?” | |
| 3. What are the causes and effective treatment(s) of, and ways to prevent, itching in dialysis patients? | Source: Mostly patients |
| Themes | |
| Causes of itchy skin | |
| Best treatment for itching | |
| Availability of improved treatments | |
| 4. What is the best strategy to increase kidney transplantation, including access to transplantation, increasing the efficiency of the recipient workup, and increasing the availability of donor kidneys? | Source: Mostly health professionals, but also some patients and care providers |
| Themes | |
| Improving access to donor kidneys and transplantation | |
| How transplantation workup could be more efficient | |
| 5. What is the psychological and social impact of kidney failure on patients, their family, and other caregivers, and can this be reduced? | Source: Mostly health professionals, but also by patients |
| Themes | |
| Impact of dialysis on caregivers (particularly in the case of home dialysis patients) | |
| Impact of dialysis on patients and the family unit and close friends | |
| Potential interventions to reduce the burden of dialysis | |
| 6. What are the best ways to promote heart health in dialysis patients, including management of BP? | Source: Noted by a similar proportion of patients and health professionals |
| Themes | |
| Identification of treatments that would reduce the effect of heart disease in people with kidney failure receiving dialysis | |
| Identification of appropriate BP target(s) for dialysis patients | |
| Management of elevated BP | |
| Concern about damage to organs and arteries if BP is not controlled properly | |
| 7. For people with kidney failure, what is the effect of each of the dietary restrictions (sodium, potassium, phosphate) separately, and when taken in combination, on important outcomes, including quality of life? | Source: Mostly patients, but also health professionals and some caregivers |
| Themes | |
| Benefits associated with strict dietary restriction | |
| Whether adherence to a renal diet improves health outcome(s) in dialysis patients | |
| Whether dietary restrictions could be relaxed in some way because they have a significant effect on quality of life | |
| 8. What are the best ways to manage symptoms in people receiving or nearing dialysis, including poor energy, nausea, cramping, and restless legs? | Source: Mostly patients, some health professionals and a few care providers |
| Themes | |
| Complications that arise with dialysis treatment (e.g., headaches, nausea, cramping, and poor energy) and how to effectively treat them | |
| Optimal method to determine the amount of fluid to remove, so as to prevent low BP and fatigue | |
| Combined | |
| “What are the causes and effective treatment(s) of poor energy in dialysis patients?” | |
| “What are the best ways to manage or prevent complications that occur during or shortly after the hemodialysis treatment itself (i.e. low blood pressure, cramping, nausea, headaches)?” | |
| “What are the causes and effective treatment(s) of, and ways to prevent, cramping in dialysis patients?” | |
| 9. What are the causes and effective treatment(s) of depression in dialysis patients? | Source: Noted by a similar proportion of patients and health professionals |
| Themes | |
| Emotional effect of dialysis on the patient | |
| How to manage mood changes and depression, and what may be responsible for depression (i.e., the adverse effects of medications or other treatments, or kidney disease itself) | |
| 10. What is the best vascular access (among both new and existing types of access) for people receiving hemodialysis? | Source: Mostly health professionals, although some patients and care providers |
| Themes | |
| Identification of the best vascular access options across different patient types | |
| How the access should be placed and by whom, and how it should be managed | |
| Which access option offers patients the best quality of life, which one lasts the longest, and which one has the fewest complications | |
| Potential for less invasive methods of access in the near future |
PD, peritoneal dialysis; HD, hemodialysis.
“Source” refers to the group that noted the uncertainty within the survey.
Comparing the Priorities Identified by Patients, Caregivers, Physicians, and Nurses in the Survey
Some uncertainties were identified by all four groups of respondents to the survey (e.g., how to determine the optimal duration of hemodialysis and the best dialysis modality), but there were also key differences across the four groups (Table 4). Patients were more likely to identify research to address symptoms that may be difficult to manage (e.g., itching, cramping, restless legs, poor energy) than were the other groups; six, three, two, and zero of the top 10 questions were related to symptoms for patients, nurses, physicians, and caregivers respectively (Table 4). In general, the priorities ranked in the top 10 by the James Lind Alliance process (Table 3) reflected a mix of priorities identified by all four groups.
Table 4.
Comparing the top 10 uncertainties identified by patients, caregivers, physicians, and nurses who responded to the survey
| Rank | Question |
|---|---|
| Patients | |
| 1 | How does one determine the optimal length of time and frequency of HD for a particular patient and how can dialysis be tailored so each patient gets effective dialysis in the shortest possible time? |
| 2 | What are the cause, prevention and treatment of itching in dialysis patients? |
| 3 | What are the causes and treatments of poor energy in dialysis patients? |
| 4 | What options or strategies (i.e., financial, etc.) are available to assist the ability of a dialysis patient to travel? |
| 5 | What are the causes and treatment of sleep disorders in dialysis patients? |
| 6 | What is the cause and treatment of depression in dialysis patients? |
| 6 | What is the effect of exercise on a dialysis patient's health? |
| 8 | What is the frequency, causes and treatment of restless leg syndrome? |
| 9 | What are the most effective means (including medications, supplements, diet, exercise and other lifestyle factors) of preventing or slowing the progression of chronic kidney disease? |
| 9 | What is the best treatment for cramping in dialysis patients? |
| Caregivers | |
| 1 | What are the most effective means (including medications, supplements, diet, exercise and other lifestyle factors) of preventing or slowing the progression of chronic kidney disease? |
| 1 | How could dialysis delivery be improved to offer patients the best possible quality of life? |
| 1 | Are there strategies to reduce wait times (for a transplant) and what is the best method of working people up for transplantation? |
| 1 | For how long is dialysis an effective treatment and what happens after dialysis is no longer an appropriate treatment? |
| 1 | Can a smaller, portable and more efficient dialysis machine be developed? |
| 1 | What are the benefits and risks of grafts versus fistulae versus catheter? |
| 1 | Does following the renal diet improve outcome? (outcomes include slowing progression of kidney disease, increasing survival, life expectancy, health) |
| 2 | Is dialysis modality going to impact how long I live, how well I live (i.e., my quality of life), and which modality has a higher success rate on a per-patient basis? |
| 2 | What is the best strategy to maximize the availability/supply of kidneys? |
| 2 | When is the optimal time to get on the wait list (for a transplant)? |
| 2 | How does one determine the optimal length of time and frequency of HD for a particular patient and how can dialysis be tailored so each patient gets effective dialysis in the shortest possible time? |
| 2 | Can the length of HD be shortened? |
| 2 | [What are the] gaps in information regarding food preparation and food content for a renal diet? |
| 2 | What is the effect of exercise on a dialysis patient's health? |
| 2 | What are the effects of prescribed drugs (i.e., cardiovascular drugs, vitamin D, antidepressants) on dialysis patients? |
| Physicians | |
| 1 | What are the benefits and risks of grafts versus fistulae versus catheter? |
| 1 | What are the complications and side effects of treatment for calcium and phosphate imbalance? |
| 3 | Is dialysis modality going to impact how long I live, how well I live (i.e., my quality of life), and which modality has a higher success rate on a per-patient basis? |
| 3 | What is the cause, prevention and treatment of itching in dialysis patients? |
| 5 | When is the optimal time to initiate dialysis and what is the role of lab testing in the decision to initiate dialysis? |
| 5 | How could dialysis delivery be improved to offer patients the best possible quality of life? |
| 7 | How does one determine the optimal length of time and frequency of HD for a particular patient and how can dialysis be tailored so each patient gets effective dialysis in the shortest possible time? |
| 7 | What is the impact of phosphate restriction on health outcome(s)? |
| 7 | What is the best treatment for cramping in dialysis patients? |
| 7 | What are the effects of prescribed drugs (i.e., cardiovascular drugs, vitamin D, antidepressants) on dialysis patients? |
| Nurses | |
| 1 | When is the optimal time to initiate dialysis and what is the role of lab testing in the decision to initiate dialysis? |
| 1 | How does one determine the optimal length of time and frequency of HD for a particular patient and how can dialysis be tailored so each patient gets effective dialysis in the shortest possible time? |
| 3 | Is dialysis modality going to impact how long I live, how well I live (i.e., my quality of life), and which modality has a higher success rate on a per-patient basis? |
| 4 | What is the frequency, causes and treatment of restless leg syndrome? |
| 5 | What are the most effective means (including medications, supplements, diet, exercise and other lifestyle factors) of preventing or slowing the progression of chronic kidney disease? |
| 6 | How could dialysis delivery be improved to offer patients the best possible quality of life? |
| 6 | What are the eligibility criteria for transplantation and how can we improve access and the selection process for transplantation? |
| 6 | What are the benefits and risks of grafts versus fistulae versus catheter? |
| 6 | What are the complications and side effects of treatment for calcium and phosphate imbalance? |
| 6 | What are the causes and treatments of poor energy in dialysis patients? |
| 6 | What is the cause and treatment of depression in dialysis patients? |
| 6 | [What is known about the status or state of] informed decision-making in dialysis patients; i.e., how much choice do they have when it comes to their options? |
| 6 | What are the effects of prescribed drugs (i.e., cardiovascular drugs, vitamin D, antidepressants) on dialysis patients? |
| 6 | How can medication compliance be improved? |
| 6 | Are there social barriers or other structural factors that determine choice of modality? |
HD, hemodialysis.
Discussion
Using an established method (9), we identified the top 10 research uncertainties from the perspective of patients living with severe kidney disease, their caregivers, and the health professionals that care for them. These include questions ranging from the causes and optimal management of patient-relevant symptoms, the most appropriate dialysis modality for each patient, cardiovascular health, ways to increase access to transplantation, the best method of communication with patients and families, how to address the psychological and social needs of patients, the effect of dietary restrictions, and the optimal hemodialysis access. Although it is difficult to compare these priorities to the current research being conducted in the field, these uncertainties appear to focus more on improving symptoms and optimizing communication, and less on determining how to extend life.
From the survey results, we noted differences among the priorities identified by patients, caregivers, physicians, and nurses, particularly with regard to symptoms. While we did not specifically seek priorities from researchers, the priorities we identified have some overlap with those identified by the Kidney Research National Dialogue (KRND), which was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (4). The objectives for the KRND dialysis therapies subgroup, which included 10 experts in dialysis and research into dialysis therapies, was to identify broad themes to help focus the research agenda for dialysis therapies (5). The themes identified by this conference included some topics that were similar to the priorities we identified, including “Decreasing Cardiovascular Risk in Dialysis Patients,” “Improving Vascular Access Outcomes,” and “Innovative Approaches to Reduce the Burden of Hemodialysis Treatments” as well as a broad focus on “Enhancing Patient-Centered Outcomes.” However, it also included themes that were not identified as priorities within our work, including “Identifying Uremic Toxins and Optimizing Their Clearance” and “Optimizing the Composition of Dialysate.”
We are aware of two other studies that have incorporated patient input regarding research priorities for people with severe kidney disease undergoing dialysis (6,8), although one only considered priorities to inform a social science research agenda for patients with kidney failure (8). Although the study by Tong et al. incorporated the views of patients, in general, the research priorities identified were broad compared with the research uncertainties identified by our process. Moreover, while research priorities of patients have been collected for other clinical areas, including diabetes, eczema, eye disease, and prostate cancer (12–14), we are not aware of any studies that have compared the priorities of patients, caregivers, and health care professionals.
Consistent with the study by Tong et al. (6), we found that many uncertainties submitted by patients in our survey touched on the optimal way to prevent or manage earlier forms of CKD and thus avoid the need for dialysis. These questions, while clearly important to patients and caregivers, were beyond the scope of this current project and will be considered in a future research priority–setting partnership for patients with earlier stages of kidney disease.
Our study has strengths and limitations. We conducted a national survey and applied a transparent method routinely used and successfully applied by other groups (9), which ensured that different perspectives could be incorporated. While it might be seen as a limitation that we did not exclusively consider priorities from patients alone, the James Lind Alliance uses a shared process for identifying research priorities, including patients, caregivers, and clinicians throughout the priority-setting exercise with the expectation that this will increase the support for the priorities identified, across all groups, in comparison to an exercise including each group separately. Although we have identified important priorities for research, we acknowledge that there is no gold standard for identifying research priorities and that the final priority-setting workshop exercise includes participants’ subjective viewpoints; however, the use of experienced facilitators and an established process reduced this impact. Efforts were made to survey as many patients, caregivers, and health care professionals across Canada as possible, but our responses may not be entirely representative of all patient groups (including the frail elderly, Aboriginal people, African Canadian people, and Francophone Canadians). In addition, there were only 317 respondents. Nonetheless, the average age of respondents was similar to the average age of patients starting dialysis, and with >1500 uncertainties identified we had a comprehensive list from which to prioritize.
We believe that the priority-setting process that we used gave us a robust set of research questions that researchers can address over the coming years. While we do not feel that these top 10 research uncertainties should be the sole driver of the research agenda, we do believe they should receive careful consideration by funders and researchers alike. Many (if not all) of the questions could be answered with carefully designed randomized clinical trials, with the need for basic discovery research, and mixed-methods research in some cases as well. Given that nephrology is the medical specialty with the fewest trials to guide management (15), health care funders are encouraged to offer targeted funding competitions for the questions of highest priority and to encourage researchers to use these priorities when developing their own research agendas. With the increasing emphasis on patient-centered care in the United States and elsewhere, the importance of including patients and their caregivers in establishing research priorities that inform clinical care is clear (16–18). However, identifying the best approach to engage patients and get their feedback has been a challenge for health care organizations and for funders (18).
Through a comprehensive process involving patients, caregivers, and a multidisciplinary group of health care professionals, we have assembled the top 10 priorities for new research questions relevant to patients receiving or nearing dialysis. These can be used alongside the results of other research priority–setting exercises to guide researchers in designing research studies, including justifying the importance of research questions, and can inform health care funders.
Disclosures
While none of the authors has any relevant financial conflicts of interest, given the nature of the study, full disclosure of research grants is provided. B.H. holds an investigator-initiated research grant from Hoffman La Roche unrelated to this project, and B.M. holds investigator-initiated research grants from Hoffman La Roche and Baxter Healthcare Corporation, both unrelated to this project. B.M. and B.H. hold funding from the Canadian Institutes of Health Research, Kidney Foundation of Canada and AI-HS for research investigating optimal care and management of patients with chronic diseases, and for establishing a knowledge translation network in kidney diseases. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Supplementary Material
Acknowledgments
The authors are indebted to L. Barnieh, R. Wald, and S. Soroka for their assistance in facilitating survey distribution and providing suggestions for names of patients and caregivers and to K. Born and K. Cowan for facilitating the workshop.
B.H. and B.M. are supported by Alberta Innovates–Health Solutions (AI-HS) (formerly Alberta Heritage Foundation for Medical Research) salary awards. B.M. and B.H. are supported by an alternative funding plan from the government of Alberta and the Universities of Alberta and Calgary. A.L. is supported by a Canada Research Chair in Health Policy and Citizen Engagement. This research was supported by two grants from Canadian Institutes of Health Research (operating grant number 284262, team grant number 251048), an interdisciplinary team grant from AI-HS, the Interdisciplinary Chronic Disease Collaboration, and the Kidney Foundation of Canada.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01610214/-/DCSupplemental.
References
- 1.Canada's Strategy for Patient-Oriented Research: Improving health outcomes through evidence-informed care. 2013. Available at: at http://www.cihr-irsc.gc.ca/e/44000.html Accessed September 27, 2013
- 2.Institue of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC, National Academy Press, 2001 [PubMed] [Google Scholar]
- 3.Liem YS, Bosch JL, Hunink MG: Preference-based quality of life of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health 11: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Rys-Sikora KE, Ketchum CJ, Star RA; Kidney Research National Dialogue (KRND) Editorial Board: Kidney research national dialogue overview and commentary. Clin J Am Soc Nephrol 8: 1599–1602, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrotra R, Agarwal A, Bargman JM, Himmelfarb J, Johansen KL, Watnick S, Work J, McBryde K, Flessner M, Kimmel PL; Kidney Research National Dialogue (KRND): Dialysis therapies: a national dialogue. Clin J Am Soc Nephrol 9: 812–814, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong A, Sainsbury P, Carter SM, Hall B, Harris DC, Walker RG, Hawley CM, Chadban S, Craig JC: Patients’ priorities for health research: Focus group study of patients with chronic kidney disease. Nephrol Dial Transplant 23: 3206–3214, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Murray MA, Brunier G, Chung JO, Craig LA, Mills C, Thomas A, Stacey D: A systematic review of factors influencing decision-making in adults living with chronic kidney disease. Patient Educ Couns 76: 149–158, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Schipper K, Abma TA: Coping, family and mastery: Top priorities for social science research by patients with chronic kidney disease. Nephrol Dial Transplant 26: 3189–3195, 2011 [DOI] [PubMed] [Google Scholar]
- 9.James Lind Alliance. JLA method. 2013. Available at: http://www.lindalliance.org/JLA_Method.asp Accessed August 2013
- 10.Canadian Institute for Health Information: Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada 2001 to 2010. 2011. Available at: https://secure.cihi.ca/free_products/2011_CORR_Annua_Report_EN.pdf Accessed November 1, 2013
- 11.Canadian Institute for Health Information. End-Stage Renal Disease Among Aborginal People in Canada: Treatment and Outcomes. Available at: https://secure.cihi.ca/free_products/EndStageRenalDiseaseAiB-ENweb.pdf Accessed November 1, 2013
- 12.Gadsby R, Snow R, Daly AC, Crowe S, Matyka K, Hall B, Petrie J: Setting research priorities for Type 1 diabetes. Diabet Med 29: 1321–1326, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Lophatananon A, Tyndale-Biscoe S, Malcolm E, Rippon HJ, Holmes K, Firkins LA, Fenton M, Crowe S, Stewart-Brown S, Gnanapragasam VJ, Muir KR: The James Lind Alliance approach to priority setting for prostate cancer research: An integrative methodology based on patient and clinician participation. BJU Int 108: 1040–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Batchelor J, Ridd M, Clarke T, Ahmed A, Cox M, Crowe S, Howard M, Lawton S, McPhee M, Rani A, Ravenscroft JC, Roberts A, Thomas KS: The Eczema Priority Setting Partnership: identifying and prioritizing important research questions for the treatment of eczema. A collaborative partnership between patients, carers, clinicians and researchers. Br J Dermatol 167: 51, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Strippoli GF, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Tinetti ME, Basch E: Patients’ responsibility to participate in decision making and research. JAMA 309: 2331–2332, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lloyd K, White J, Chalmers I: Schizophrenia: Patients’ research priorities get funded. Nature 487: 432, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Patient-Centered Outcomes Reserach Institute: 2013. Available at: http://pcori.org/ Accessed September 27, 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.