Abstract
Background and objectives
Studies and clinical experience suggest that kidney disease clusters in families, but few population-based studies have been performed. This study investigates risks and causes of ESRD in Norwegians with and without a first-degree relative with ESRD.
Design, setting, participants, & measurements
On the basis of data from the Norwegian Population Registry, first-degree relatives for most Norwegians were identified. All Norwegians with ESRD (defined as chronic RRT) since 1980 have been registered in the Norwegian Renal Registry. All Norwegians born in Norway who were alive in 1980 and had at least one registered relative were included. For this study, data on ESRD were available through 2009, and individuals without ESRD were censored at December 31, 2009. Data were analyzed in a cohort design, with ESRD in a first-degree relative of the included person as the main explanatory variable. Risks of ESRD and different causes of ESRD were analyzed using Cox regression statistics.
Results
In total, 5,119,134 individuals were included, of whom 8203 individuals developed ESRD during follow-up and 27,046 individuals had a first-degree relative with ESRD. Compared with individuals without a first-degree relative with ESRD, individuals with a first-degree relative with ESRD had a relative risk of ESRD of 7.2 (95% confidence interval, 6.5 to 8.1). Similar analyses showed that relative risk of ESRD caused by nonhereditary causes was 3.7 (95% confidence interval, 3.1 to 4.4), relative risk of ESRD caused by glomerular disease was 5.2 (95% confidence interval, 4.1 to 6.6), relative risk of ESRD caused by interstitial disease was 4.7 (95% confidence interval, 3.1 to 7.3), relative risk of ESRD caused by diabetic nephropathy was 2.6 (95% confidence interval, 1.6 to 4.1), and relative risk of ESRD caused by hypertensive nephrosclerosis was 2.6 (95% confidence interval, 1.6 to 4.1). Relative risk of nonhereditary parenchymal renal disease was 3.8 (95% confidence interval, 3.1 to 4.7).
Conclusions
As expected, ESRD clusters in families. Interestingly, ESRD without known hereditary cause also clusters in families.
Keywords: nephrology, family history, ESRD
Introduction
Familial clustering of kidney diseases following Mendelian laws of inheritance is well known (1,2). Few population-based studies have been performed, and few studies have investigated the extent to which other kidney diseases cluster in families.
Several new genes associated with different kidney diseases have recently been identified. These findings have greatly expanded the understanding of several nephropathies, such as FSGS (3,5,6). Similarly, genetic risk factors for diabetic nephropathy and IgA nephropathy have been identified (7,8). APOLI 1 mutations have been identified as a strong risk factor for renal disease in African Americans (9–11) and could explain the higher risks of hypertensive nephrosclerosis, FSGS, and HIV nephropathy in this population (12,13). Complex multifactorial patterns of inheritance are a common characteristic for most of these genetic risk factors.
Identification of family members at risk of ESRD permits early focus on modifiable risk factors. On the basis of data from the Norwegian Population Registry and the Norwegian Renal Registry, this study investigates the excess risk of ESRD in Norwegians with a first-degree relative with ESRD. The risks of different causes of ESRD are investigated in additional analyses. The objectives were to describe the nationwide incidence of familial clustering of ESRD in a predominantly white European population and investigate whether non-Mendelian renal disorders also cluster in families.
Materials and Methods
The study protocol was approved by the regional ethics committee.
The Norwegian Population Registry was established in 1960 and comprises an 11-digit personal identification number for all Norwegian citizens and individuals with permanent residence in Norway. Parental information has been registered for all Norwegians residing with their parents since 1970; thus, parental information is available for most individuals born after 1953. The registration of maternal identification numbers is almost complete since 1953, and the registration of paternal identification numbers is approximately 99% complete. Data collected from 1960 to June of 2009 were available for this study.
Since 1980, all individuals in Norway developing ESRD with the need for chronic RRT have been registered in the Norwegian Renal Registry, and the cause of ESRD was reported by the treating physician using the old European Renal Association-European Dialysis and Transplant Association classification (14). The diagnosis reported to the Registry may, in some cases, be inaccurate, because not all patients have undergone a kidney biopsy. Data were available through June of 2009.
The National Cause of Death Registry comprises data on all deaths; for this study, data were available through December of 2008.
On the basis of data from the Norwegian Population Registry, first-degree relatives of Norwegian citizens were identified; parents, siblings, or children were defined as first-degree relatives. Siblings were defined as individuals with the same mother and father. All individuals registered in the Norwegian Population Registry born in Norway and alive in 1980 (start of follow-up) with at least one registered first-degree relative were eligible for inclusion in this study. The 11-digit national identification number was used to link data on the included individuals with data from the Norwegian Renal Registry and the National Cause of Death Registry. Individuals with more than six siblings or more than four children were excluded to reduce the size of the data file.
Explanatory Variables
The main exposure variable is whether a first-degree relative of the included individual developed ESRD before June of 2009, which means that an individual with ESRD could be registered as a first-degree relative with ESRD for several of the included individuals. Other variables used in adjusted analyses were sex, birth year, and number of recorded first-degree relatives (categorized as one or two, three or four, five to seven, and eight or more; included in the adjusted analyses as a continuous variable).
Outcome Variables
The main outcome was ESRD; onset was defined as the date of starting dialysis treatment or undergoing renal transplantation. Individuals without ESRD were followed until June 30, 2009 or the date of death. Secondary outcomes were the different causes of ESRD and death. Cause of ESRD was categorized into the following six categories: known hereditary nephropathy, glomerular disease (primary or secondary), interstitial nephritis (including chronic pyelonephritis and obstructive disease), diabetic nephropathy, hypertensive nephropathy, and other cause of ESRD.
Statistical Analyses
Data were analyzed in a cohort design with ESRD in a first-degree relative as the main explanatory variable and development of ESRD as the main outcome variable. Relative risk (RR) of ESRD was analyzed by Cox regression analyses using age as the time variable. Analyses were adjusted by including the described variables as covariates in the Cox regression model. Death before ESRD was treated as a censoring event. Because no patients with ESRD had been registered before 1980, the statistical analyses were left truncated. Consequently, the counting process formulation of proportional hazards (Cox regression) was applied (4). This method does not include individuals in the analysis until an event could be registered (i.e., an individual born in 1960 would be included in the analyses at 20 years of age and right-censored at age 49 years if ESRD or death did not occur). Rates of ESRD and different causes of ESRD were calculated as the number of cases per million follow-up years. If not otherwise stated, means±SDs or estimates (95% confidence intervals [95% CIs]) are given. The analyses were performed with the statistical package STATA MP, edition 11.1 (StataCorp).
Results
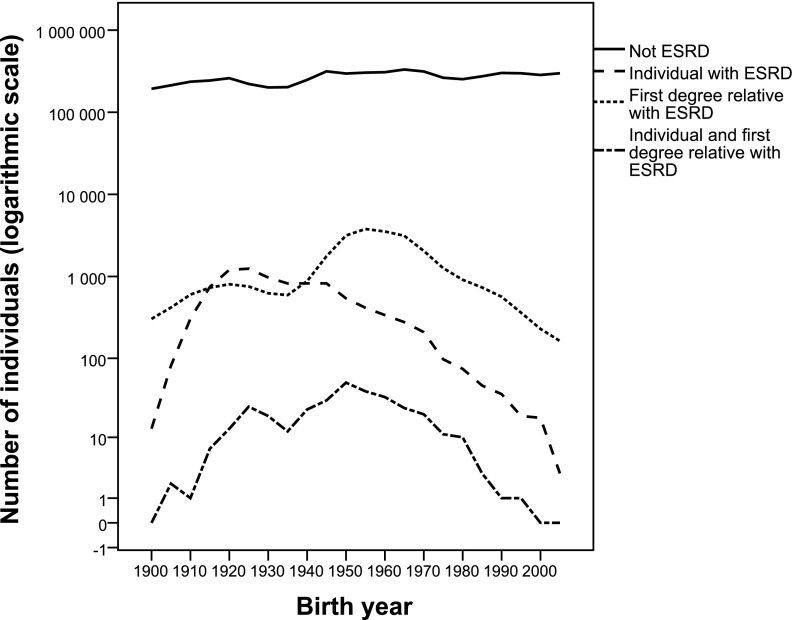
This study included 5,119,134 individuals in the analyses. During follow-up, 8203 included individuals developed ESRD, and 27,046 included individuals had at least one first-degree relative with ESRD. The included individuals who developed ESRD were older, were more often men, and had fewer recorded relatives than individuals without ESRD (Table 1). Individuals born between 1915 and 1945 were more likely to develop ESRD, and individuals born between 1940 and 1980 were more likely to have a first-degree relative with ESRD (Figure 1). Of 8203 individuals with ESRD, 313 individuals had a first-degree relative with ESRD. Of 313 individuals identified with ESRD, 282 individuals had one relative with ESRD, and 31 individuals had two relatives with ESRD. Categories and reported causes of ESRD are given in detail in Table 2.
Table 1.
Characteristics of included individuals
| Characteristic | Total Population | Born after 1952 | ||
|---|---|---|---|---|
| No ESRD | ESRD | No ESRD | ESRD | |
| N total | 5,110,934 | 8203 | 3,288,340 | 1851 |
| Sex (% men) | 51.1 | 66.6a | 51.3 | 62.8a |
| Mean (SD) age (yr) at the end of follow-up or ESRD | 43.5±25.4 | 57.7±18a | 28.1±16.4 | 32.9±12.3a |
| Mean (SD) birth yr | 1963±28 | 1940±18a | 1980±16 | 1966±11a |
| Mean (SD) age (yr) at death if first-degree relative with ESRD | 65.9±21.6 | 58.0±15.9a | ||
| Mean (SD) age (yr) at death if no first-degree relative with ESRD | 69.0±19.3 | 67.1±13.5a | ||
| N (%) with one or two recorded relatives | 1,204,727 (23.6) | 3222 (39.3)a | 345,087 (10.5) | 129 (7.0)a |
| N (%) with three or four recorded relatives | 2,414,181 (41.9) | 3059 (37.3)a | 1,545,437 (47) | 749 (42.9)b |
| N (%) with five to seven recorded relatives | 1,438,642 (28.2) | 1576 (19.2)a | 1,142,575 (34.8) | 759 (41.0)a |
| N (%) with eight or more recorded relatives | 326,381 (6.4) | 346 (4.2)a | 255,241 (7.7) | 169 (9.1) |
| Mean (SD) no. of recorded relatives | 4.0±2.1 | 3.4±2a | 4.5±2 | 4.8±2a |
| N (%) with recorded parents | 3,664,059 (71.7) | 3020 (36.8)a | 3,238,048 (98.5) | 1805 (97.5)b |
| N (%) with recorded siblings | 3,113,134 (61) | 2431 (29.6)a | 2,802,089 (85.2) | 1625 (87.8)a |
| N (%) with recorded children | 3,104,798 (60.7) | 6709 (81.8)a | 1,407,960 (42.8) | 943 (51)a |
| N (%) with first-degree relative with ESRD | 26,733 (0.5) | 313 (3.8)a | 17,983 (0.6) | 164 (8.7)a |
| N (%) with one or two parents with ESRD | 16,601 (0.3) | 110 (1.3)a | 14,267 (0.4) | 82 (4.4)a |
| N (%) with one to three siblings with ESRD | 4879 (0.1) | 124 (1.5)a | 3491 (0.1) | 85 (4.6)a |
| N (%) with one to three children with ESRD | 5421 (0.1) | 92 (1.1)a | 379 (0.01) | 6 (0.03)a |
| N (%) with first-degree relative with ESRD caused by glomerular disease | 8724 (0.2) | 76 (0.9)a | 5795 (0.2) | 36 (1.9)a |
| N (%) with first-degree relative with ESRD caused by interstitial nephritis | 2787 (0.05) | 22 (0.3)a | 1856 (0.06) | 17 (0.9)a |
| N (%) with first-degree relative with ESRD caused by hereditary diseases | 3169 (0.06) | 168 (2.0)a | 2303 (0.07) | 89 (4.8)a |
| N (%) with first-degree relative with ESRD caused by diabetes | 3941 (0.08) | 18 (0.2)a | 2443 (0.07) | 11 (0.6)a |
| N (%) with first-degree relative with ESRD caused by hypertension | 4613 (0.09) | 20 (0.2)a | 3261 (0.1) | 10 (0.5)a |
| N (%) with first-degree relative with ESRD caused by other disease | 2726 (0.05) | 6 (0.1) | 1851 (0.06) | 1 (0.05) |
P<0.001 for the comparison between ESRD and no ESRD groups.
P<0.01 for the comparison between ESRD and no ESRD groups.
Figure 1.
Number of individuals born in Norway per birth year and whether they have ESRD themselves and/or first-degree relatives with ESRD.
Table 2.
List of presumed causes of ESRD in included individuals as reported by the treating nephrologist using the old European Renal Association-European Dialysis and Transplant Association code list (14)
| Cause of ESRD | Total ESRD Cohort | First-Degree Relative with ESRD | ||
|---|---|---|---|---|
| N | Percent | N | Percent | |
| N total | 8203 | 100 | 313 | 100 |
| Hereditary disease category | 1004 | 12.1 | 173 | 55.3 |
| Adult polycystic kidney disease | 682 | 8.3 | 124 | 39.6 |
| Alport disease | 32 | 0.4 | 8 | 2.6 |
| Medullary cystic disease including nephronophtisis | 64 | 0.8 | 26 | 8.3 |
| Congenital renal hypoplasia | 35 | 0.4 | 1 | 0.3 |
| Congenital obstructive uropathy | 88 | 1.1 | 2 | 0.6 |
| Congenital renal or urinary tract dysplasia or malformations | 34 | 0.4 | 0 | 0.0 |
| Other hereditary causes | 69 | 0.8 | 12 | 3.8 |
| Glomerular disease category | 2437 | 29.7 | 73 | 23.3 |
| IgA nephropathy | 274 | 3.3 | 9 | 2.9 |
| Wegener disease | 127 | 1.5 | 1 | 0.3 |
| Systemic lupus erythematosus | 102 | 1.2 | 5 | 1.6 |
| Crescenteric nephritis | 99 | 1.2 | 1 | 0.3 |
| Membranoproliferative disease type | 82 | 1.0 | 1 | 0.3 |
| Membranous disease | 41 | 0.5 | 0 | |
| FSGS | 89 | 1.1 | 9 | 2.9 |
| Other systemic disease | 96 | 1.2 | 1 | 0.3 |
| GN histologically examined (cause not given above) | 580 | 7.1 | 17 | 5.4 |
| GN not histologically examined | 897 | 11.0 | 29 | 9.3 |
| Other GN | 50 | 0.6 | 0 | |
| Interstitial disease category | 834 | 10.2 | 21 | 6.7 |
| Pyelonephritis unspecified | 361 | 4.4 | 10 | 3.2 |
| Pyelonephritis with acquired obstructive uropathy | 229 | 2.8 | 2 | 0.6 |
| Vesicoureteric reflux | 59 | 0.7 | 3 | 0.9 |
| Interstitial nephritis, unspecified | 85 | 1.0 | 6 | 1.9 |
| Interstitial nephritis, drug related | 100 | 1.2 | 0 | 0.0 |
| Diabetes nephropathy category | 1110 | 13.5 | 18 | 5.8 |
| Diabetes mellitus type 1 | 585 | 7.1 | 12 | 3.8 |
| Diabetes mellitus type 2 | 525 | 6.4 | 6 | 1.9 |
| Hypertensive nephropathy category | 1653 | 20.2 | 19 | 6.1 |
| Renal vascular disease caused by malignant hypertension | 64 | 0.8 | 1 | 0.3 |
| Renal vascular disease caused by essential hypertension | 1467 | 17.9 | 15 | 4.8 |
| Other | 122 | 1.5 | 3 | 0.9 |
| Other causes category | 1165 | 14.2 | 9 | 2.9 |
| Ischemic disease | 92 | 1.1 | 1 | 0.3 |
| Multiple myeloma | 210 | 2.6 | 0 | 0.0 |
| Amyloidosis | 319 | 3.9 | 3 | 0.9 |
| Tubular or medullary necrosis, not reversible | 81 | 1.0 | 1 | 0.3 |
| Loss of kidney/kidney tumor | 114 | 1.4 | 1 | 0.3 |
| Other identified renal disorders | 76 | 0.9 | 0 | 0.0 |
| Unknown cause of ESRD | 273 | 3.3 | 3 | 0.9 |
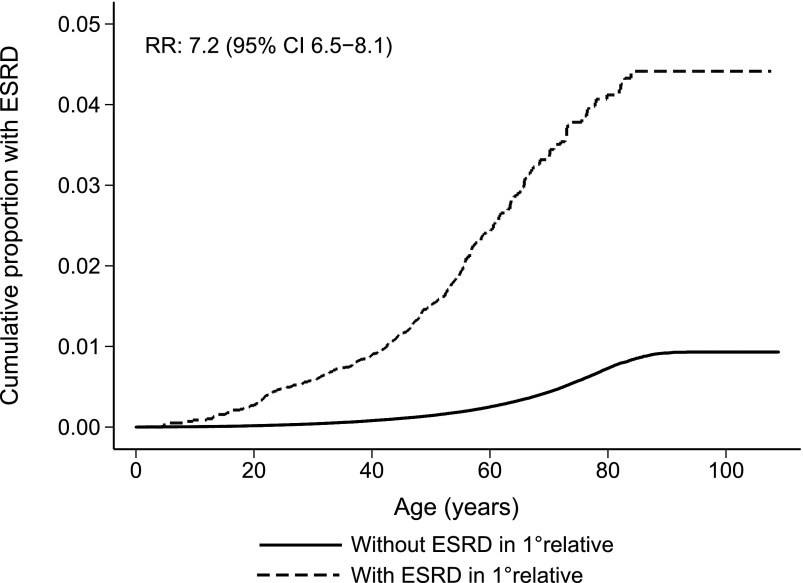
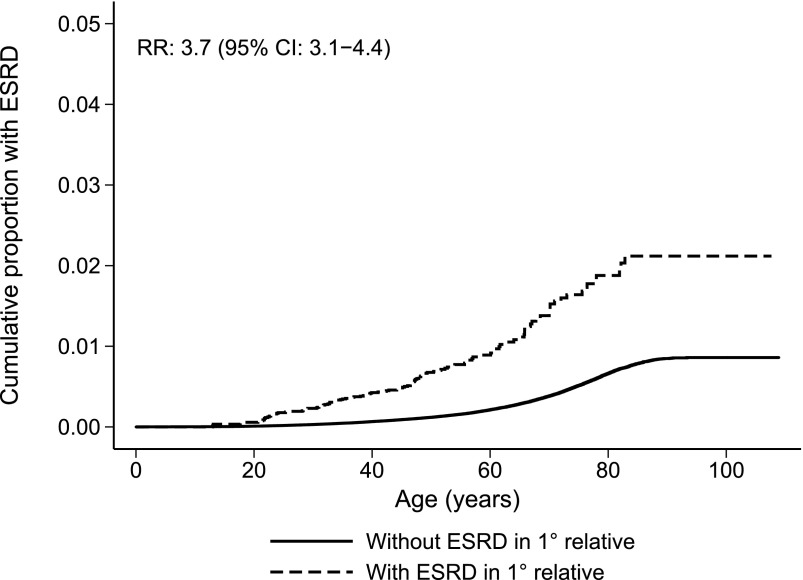
Individuals with a first-degree relative with ESRD had a significantly higher risk of developing ESRD (Figure 2). The difference was smaller but still highly significant after excluding individuals and first-degree relatives with known hereditary ESRD (Figure 3). Compared with individuals without first-degree relatives with ESRD, RR of ESRD in individuals with a first-degree relative with ESRD was 7.2 (95% CI, 6.5 to 8.1) (Table 3). In separate analyses excluding known hereditary nephropathies in the included individuals or a first-degree relative, RR was 3.7 (95% CI, 3.1 to 4.4). The RRs given above remained unchanged when adjusted for sex, birth year, and number of first-degree relatives. RR for those born after 1952 was 5.3 (95% CI, 4.1 to 6.8), and adjusted RR was 5.6 (95% CI, 4.4 to 7.3). Women tended to have a higher RR associated with having a first-degree relative with ESRD than men. In analyses where all known hereditary causes were excluded, the RR was 4.4 (95% CI, 3.3 to 5.9) for women and 3.3 (95% CI, 2.6 to 4.2) for men, and a separate analysis showed a nonsignificant trend toward an interaction with sex (P=0.06), with a stronger effect in women than men.
Figure 2.
Cumulative risk of ESRD at increasing age according to whether a first-degree relative had ESRD. CI, confidence interval; RR, relative risk.
Figure 3.
Cumulative risk of nonhereditary ESRD at increasing age according to whether a first-degree relative had ESRD.
Table 3.
Risk of ESRD and different causes of ESRD according to whether a first-degree relative had ESRD
| First-Degree Relative with ESRD | N Total Cohort | N Individuals with ESRD | Rate of ESRD (per million yr) | Total Cohort | Cohort Born after 1952 | ||
|---|---|---|---|---|---|---|---|
| Relative Risk (95% Confidence Interval) | P Value | Relative Risk (95% Confidence Interval) | P Value | ||||
| Risk of ESRD if relative with ESRD | |||||||
| No | 5,092,088 | 7890 | 69 (68–71) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 313 | 443 (395–494) | 7.2 (6.5 to 8.1) | <0.001 | 10.2 (8.7 to 12) | <0.001 |
| Risk of ESRD caused by hereditary nephropathy | |||||||
| No | 5,092,088 | 831 | 7.3 (6.8–7.8) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 173 | 245 (210–283) | 36 (30 to 42) | <0.001 | 42 (33 to 53) | <0.001 |
| Risk of ESRD caused by GN | |||||||
| No | 5,092,088 | 2364 | 21 (20–22) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 73 | 103 (81–128) | 5.2 (4.1– to 6.6) | <0.001 | 6.1 (4.4 to 8.4) | <0.001 |
| Risk of ESRD caused by interstitial nephritis | |||||||
| No | 5,092,088 | 813 | 7.1 (6.7–7.6) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 21 | 30 (18–44) | 4.7 (3.1 to 7.3) | <0.001 | 9.3 (5.4 to 16) | <0.001 |
| Risk of ESRD caused by diabetes mellitus | |||||||
| No | 5,092,088 | 1092 | 9.6 (9.0–10.2) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 18 | 26 (15–39) | 2.6 (1.6 to 4.1) | <0.001 | 2.6 (1.3 to 5.0) | <0.01 |
| Risk of ESRD caused by hypertensive nephropathy | |||||||
| No | 5,092,088 | 1634 | 14 (14–15) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 19 | 27 (16–40) | 2.6 (1.6 to 4.1) | <0.001 | 2.0 (0.5 to 8.1) | 0.30 |
| Risk of ESRD caused by other causes | |||||||
| No | 5,092,088 | 1156 | 10 (9.6–11) | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 27,046 | 9 | 13 (5.8–22) | 1.6 (0.8 to 3.1) | 0.20 | 2.1 (0.7 to 6.6) | 0.20 |
Because kidney diseases differ in their hereditary patterns, risk of ESRD was analyzed using the different categories of ESRD as end points (Table 3). In these analyses, having a first-degree relative with ESRD was associated with an RR of developing ESRD caused by hereditary causes of 36 (95% CI, 30 to 42), an RR of ESRD caused by glomerular disease of 5.2 (95% CI, 4.1 to 6.6), an RR of ESRD caused by interstitial disease of 4.7 (95% CI, 3.1 to 7.3), an RR of ESRD caused by diabetic nephropathy of 2.6 (95% CI, 1.6 to 4.1), an RR of ESRD caused by hypertensive nephrosclerosis of 2.6 (95% CI, 1.6 to 4.1), and an RR of ESRD caused by other causes of 1.6 (95% CI, 0.8 to 3.1). When excluding individuals and first-degree relatives with ESRD caused by hereditary, congenital, obstructive, or interstitial disease, RR of ESRD was 3.7 (95% CI, 3.0 to 4.6) in the unadjusted analyses and 3.8 (95% CI, 3.1 to 4.7) after adjustments for sex, birth year, and number of first-degree relatives. RRs for the different causes of ESRD in this cohort are shown in Table 3. Because of the low number of outcomes, some risk estimates are imprecise.
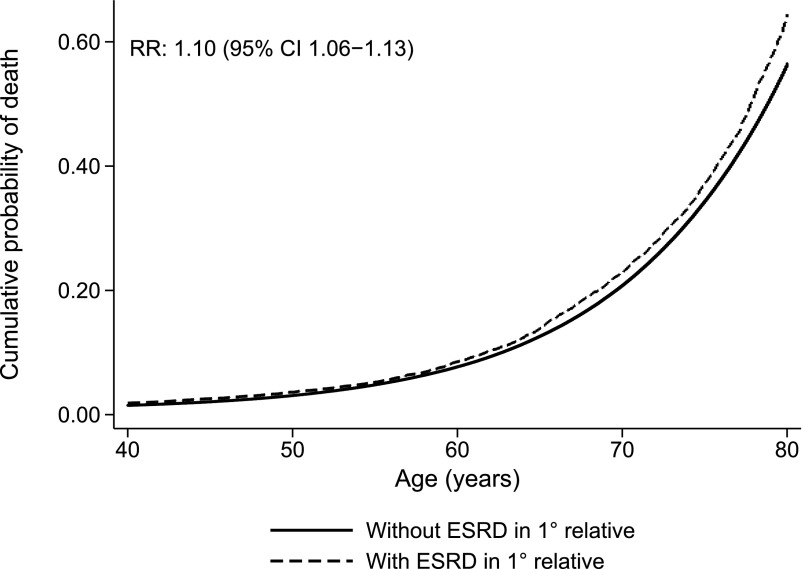
Because data on the cohort were nearly complete for individuals born after 1952, the analyses in Table 3 were repeated for this part of the cohort, which included about 60% of the total cohort but only 22.6% of those who developed ESRD (Table 1). The RR of overall ESRD in individuals with a first-degree relative with ESRD in this analysis was 10.2 (95% CI, 8.7 to 12); RR after exclusion of all patients with known hereditary disease was 5.3 (95% CI, 4.1 to 6.8), and RR was 4.6 (95% CI, 3.4 to 6.2) when excluding hereditary, congenital, obstructive, and interstitial disease. Additional analyses of all individuals who did not develop ESRD showed that average age of death was 65.9±21.6 years for individuals with a first-degree relative with ESRD compared with 69.0±21.6 years for individuals without a first-degree relative with ESRD (P<0.001) (Table 1). Cox regression statistics using death as the primary end point showed that having a first-degree relative with ESRD was associated with an RR of death of 1.10 (95% CI, 1.06 to 1.13; P<0.001) (Figure 4). The RR of death remained unchanged after adjustments for sex, birth year, and number of first-degree relatives.
Figure 4.
Cumulative probability of death at increasing age according to whether a first-degree relative had ESRD.
Discussion
This study shows that individuals of a predominantly Caucasian population with a first-degree relative with ESRD have a seven times higher risk of developing ESRD. As expected, the risk was highest for development of ESRD caused by hereditary causes. When all patients with known hereditary nephropathies were excluded, the RR decreased to 3.7 (95% CI, 3.1 to 4.4), which is still highly significant. Interestingly, having a first-degree relative with ESRD was associated with a five times higher risk of developing ESRD caused by GN or interstitial nephritis and a two-to-three times higher risk of developing ESRD caused by hypertensive nephrosclerosis or diabetic nephropathy.
The 3-fold increase in RR for nonhereditary ESRD in this study is similar to findings from a Canadian study from the 1990s, where 1.2% of first-degree relatives of individuals with ESRD developed renal failure, a three times higher risk than individuals who did not have a first-degree relative with ESRD (1). In a more recent United States study, approximately 23% of patients on incident dialysis reported a family history of ESRD (2). In both studies, the contribution from non-Mendelian kidney diseases was important. Risk factors of familial clustering were women, young age at ESRD onset, and black race (2). A study including Caucasian Americans in North Carolina also reported a nearly 3-fold increase in the risk of ESRD (15). There are, however, important design differences. This study has a national cohort design, and family history of ESRD was obtained through data from the national registry, whereas the Canadian and North Carolinian studies were case-controlled studies. Apart from known hereditary nephropathies, this study found glomerular disease to be associated with the highest RR of familial ESRD, which was followed by interstitial disease. This finding contrasts the Canadian study, which found hypertensive nephrosclerosis to be associated with the highest risk of familial ESRD. In the study from North Carolina, persons with chronic GN and diabetes nephropathy had the highest percentages of positive family history for ESRD (15). There is no clear explanation for these differences, but because the numbers with specific causes of ESRD and family history of ESRD are small in all studies, the differing results may rely on small differences in the renal disease spectrum as well as a possible difference in diagnostic tradition between populations. Differences in design may also be important. The case-controlled studies included individuals on dialysis as patients, and because patients with hypertensive nephrosclerosis are more often older, they would be more likely to have at least one relative with ESRD than younger patients with GN.
In this study, 1.8% of individuals with nonhereditary ESRD had a first-degree relative with ESRD, similar to the finding of 1.2% in the Canadian study. In a study from the southeastern United States, 23% of patients reported a close relative with ESRD (2). The high percentage in the United States study could be explained by several factors. Family history included first- and second-degree relatives, and about 50% of patients did not report information on family history and are probably less likely to have a family history of ESRD. Importantly, the background risk of ESRD is also much higher in the southeastern United States compared with Norway (348 versus 102 per million inhabitants) (16,17). The incidence of ESRD in African Americans is even greater. Absolute incidence of ESRD caused by diabetes mellitus or hypertension is more frequent in American populations compared with this Norwegian cohort. The difference in background risk between the Norwegian and American populations was also reflected in the findings in the work by McClellan et al. (18), which found that 6.4% of the Caucasian population in a United States study reported a family history of ESRD. This finding contrasts this study, which reports ESRD in a first-degree relative in 0.5% of individuals who did not develop ESRD themselves. RR of ESRD in this study did not change after adjustments for sex, birth year, and number of identified relatives. When RR was analyzed separately by sex, there was a trend toward a stronger association with family history in women, although it was not significant in an interaction analysis (P=0.06). Stronger association with a positive family history for ESRD in women has also been described previously (1,18,19). The explanation is unknown, but McClellan et al. (18) have attributed it to women being more aware of their family history. Although statistical significance was not reached in this study, the fact that the trend persisted in this registry-based cohort study may indicate a stronger association that should be further investigated.
In recent years, studies have shown genetic risk factors for several diseases previously not thought to have genetic origins or contributions (9,10,20). Different genetic risk factors have been shown to have variable penetrance ranging from clinically nonsignificant risk factors to clear disease-causing genes. Examples of glomerular disorders shown to have strong hereditary patterns in selected families include FSGS (3,21) and IgA nephropathy (8). Much of the higher risk of ESRD caused by glomerular and interstitial disease can likely be explained by unknown disease-causing or predisposing mutations in affected families. Part of the excess risk is probably explained by multifactorial inheritance that increases risk of either hypertension/diabetes (9,22,23) or progressive renal fibrosis (24).
It should also be acknowledged that individuals who share genetic risk factors often also share environmental risk factors. This study shows a higher risk of ESRD in individuals with first-degree relatives with ESRD. The higher risk may be monogenic or multifactorial. It has not been possible to quantify environmental factors or the interplay between genes and environment. The take-home messages are, nevertheless, that ESRD clusters in families and that screening of first-degree relatives of patients with ESRD with regard to renal dysfunction might be considered.
In this study, individuals with a first-degree relative with ESRD had a significantly higher risk of death, with an average age at death that was 3 years younger than in individuals without a first-degree relative with ESRD. This finding argues for additional hereditary risks in this group, which need to be addressed in future studies.
The main strengths of this study are that it is a national cohort study and that the main risk factor for having a first-degree family member with ESRD was ascertained through longstanding national registry data. The major weakness is that data are not complete for the whole period of follow-up. Family data were only complete for those born after 1952, and ESRD was not registered until 1980. The fact that family histories were incomplete before 1953 would lead to an underestimation of the probability of having a relative with ESRD; however, it is unlikely to have significantly affected the estimates of excess risk. This result is supported by the even higher RR seen in the cohort born after 1952. The fact that outcomes were not registered until 1980 was accounted for by the counting processes modifications of the Cox regression statistics, which does, however, rely on the assumption that the effect of the predictor variable was mostly unchanged across different time periods. It would, for example, assume that the excess risk attributed to having a relative with ESRD was similar for a 40-year-old individual in both 1980 and 2005. In our opinion, this assumption is fair and unlikely to have affected the results significantly. Another weakness is that the cause of ESRD was reported to the Norwegian Renal Registry by the treating nephrologist at the time of ESRD for the given individual, and it has not been possible to review these diagnoses. ESRD caused by hypertensive or diabetic renal disease accounted for the lowest number of outcomes, and, therefore, reclassification of just a few such patients could alter the results for these categories. Because similar trends were seen for all causes of ESRD, we do not believe that it would affect our main results. Another important factor is that the reporting nephrologists are more likely to report a hereditary disease as the cause of ESRD if more family members have developed ESRD than if the patient is the first family member with ESRD.
In conclusion, this study has quantified the proportion of Norwegians with a family history of ESRD as well as the excess risks and causes of ESRD in these individuals. Because several genetic risk factors for renal disease have been described in recent years, the excess risk was not unexpected, and it also confirms findings of previous studies (1,2). In our opinion, the findings of higher risks of hypertensive and diabetic nephropathy argue for the importance of multifactorial genetic risk factors that also could include potentially modifiable risk factors for more rapid progression of renal fibrosis. In the coming decades, more diseases will likely be identified with genetic causes, and it will be important to repeat this kind of study to better quantify the importance of novel disease-causing mutations and multifactorial inheritance, which increase overall risk of renal disease development and/or progression.
Disclosures
B.E.V. has been an adjunct professor at the University of Bergen, a position partly financed by Amgen Inc. since July 2012. The other authors report no conflict of interest.
Acknowledgments
The authors are grateful to the Norwegian nephrologists who report data to the Norwegian Renal Registry. This study has been supported by grants from the Western Norway Regional Health authority funds.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.O’Dea DF, Murphy SW, Hefferton D, Parfrey PS: Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. Am J Kidney Dis 32: 794–801, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Freedman BI, Volkova NV, Satko SG, Krisher J, Jurkovitz C, Soucie JM, McClellan WM: Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol 25: 529–535, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, Needham AW, Lazarus R, Pollak MR: A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen PK, Gill RD: Cox's regression model for counting processes: A large sample study. Ann Stat 10(4): 1100–1120, 1982 [Google Scholar]
- 5.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Spink C, Stege G, Tenbrock K, Harendza S: The CTLA-4 +49GG genotype is associated with susceptibility for nephrotic kidney diseases. Nephrol Dial Transplant 28: 2800–2805, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Freedman BI, Bostrom M, Daeihagh P, Bowden DW: Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2: 1306–1316, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ, AASK Study Investigators. CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369[Feldman]: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB, SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman BI, Murea M: Target organ damage in African American hypertension: Role of APOL1. Curr Hypertens Rep 14: 21–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dijk PCW, Jager KJ, de Charro F, Collart F, Cornet R, Dekker FW, Grönhagen-Riska C, Kramar R, Leivestad T, Simpson K, Briggs JD, ERA-EDTA registry : Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 16: 1120–1129, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Spray BJ, Atassi NG, Tuttle AB, Freedman BI: Familial risk, age at onset, and cause of end-stage renal disease in white Americans. J Am Soc Nephrol 5: 1806–1810, 1995 [DOI] [PubMed] [Google Scholar]
- 16.United States Renal Data System: 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Volume 2. Available at: www.usrds.org/atlas.aspx2013 Accessed December 10, 2013
- 17.Leivestad T: Annual Report 2012, The Norwegian Renal Registry. Available at: http://www.nephro.no/nnr/AARSM2012.pdf2012 Accessed September 13, 2013
- 18.McClellan W, Speckman R, McClure L, Howard V, Campbell RC, Cushman M, Audhya P, Howard G, Warnock DG: Prevalence and characteristics of a family history of end-stage renal disease among adults in the United States population: Reasons for Geographic and Racial Differences in Stroke (REGARDS) renal cohort study. J Am Soc Nephrol 18: 1344–1352, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Freedman BI, Soucie JM, McClellan WM: Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol 8: 1942–1945, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Freedman BI, Langefeld CD, Lu L, Divers J, Comeau ME, Kopp JB, Winkler CA, Nelson GW, Johnson RC, Palmer ND, Hicks PJ, Bostrom MA, Cooke JN, McDonough CW, Bowden DW: Differential effects of MYH9 and APOL1 risk variants on FRMD3 Association with Diabetic ESRD in African Americans. PLoS Genet 7: e1002150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp JB, Smith MW, Nelson GW,Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR: The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn M, Angelico MC, Warram JH, Krolewski AS: Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39: 940–945, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]