Abstract
Background and objectives
Despite the benefits of kidney transplantation, the total number of transplants performed in the United States has stagnated since 2006. Transplant center quality metrics have been associated with a decline in transplant volume among low-performing centers. There are concerns that regulatory oversight may lead to risk aversion and lack of transplantation growth.
Design, setting, participants, & measurements
A retrospective cohort study of adults (age≥18 years) wait-listed for kidney transplantation in the United States from 2003 to 2010 using the Scientific Registry of Transplant Recipients was conducted. The primary aim was to investigate whether measured center performance modifies the survival benefit of transplantation versus dialysis. Center performance was on the basis of the most recent Scientific Registry of Transplant Recipients evaluation at the time that patients were placed on the waiting list. The primary outcome was the time-dependent adjusted hazard ratio of death compared with remaining on the transplant waiting list.
Results
Among 223,808 waitlisted patients, 59,199 and 32,764 patients received a deceased or living donor transplant, respectively. Median follow-up from listing was 43 months (25th percentile=25 months, 75th percentile=67 months), and there were 43,951 total patient deaths. Deceased donor transplantation was independently associated with lower mortality at each center performance level compared with remaining on the waiting list; adjusted hazard ratio was 0.24 (95% confidence interval, 0.21 to 0.27) among 11,972 patients listed at high-performing centers, adjusted hazard ratio was 0.32 (95% confidence interval, 0.31 to 0.33) among 203,797 patients listed at centers performing as expected, and adjusted hazard ratio was 0.40 (95% confidence interval, 0.35 to 0.45) among 8039 patients listed at low-performing centers. The survival benefit was significantly different by center performance (P value for interaction <0.001).
Conclusions
Findings indicate that measured center performance modifies the survival benefit of kidney transplantation, but the benefit of transplantation remains highly significant even at centers with low measured quality. Policies that concurrently emphasize improved center performance with access to transplantation should be prioritized to improve ESRD population outcomes.
Keywords: renal transplantation, ESRD, survival, outcomes
Introduction
Kidney transplantation extends the life expectancy of patients with ESRD relative to chronic dialysis (1). The survival benefit of kidney transplantation persists among patients with various comorbid conditions, different forms of health care delivery, and varying levels of donor quality (2–7). Kidney transplantation also improves quality of life and reduces overall health care expenditures (8–11). The number of candidates added to the waiting list to receive a kidney transplant has more than doubled in the United States between 1995 (n=17,885) and 2012 (n=36,457) (12).
Despite the benefits of kidney transplantation, national kidney transplantation volume in the United States has stagnated between its peak in 2006 (n=17,095) and 2012 (n=16,485) (13). This trend is particularly notable given that transplant volume had increased in each consecutive year between 1989 and 2006 (13). Although there are likely multiple causes explaining this stagnation, one potential contributing factor relates to increased oversight of transplant centers (14–16). In 2007, the Centers for Medicare and Medicaid Services (CMS) issued a Conditions of Participation (COP), which explicitly linked performance of transplant centers, as measured by risk-adjusted post-transplant outcomes, with access to reimbursement (17). Since publication of the COP, centers with low-rated performance have had significant decline in transplant volume, whereas transplants have continued to increase among other centers (16). Although the specific mechanisms for this association have not been comprehensively evaluated, it is plausible that centers that receive low-performance evaluations have become increasingly risk averse (14,15,18). However, quality oversight may have substantial benefits for identification and improvement efforts for centers with suboptimal outcomes (14,19). An important unresolved issue relates to understanding what the potential tradeoff is for candidate outcomes if performance oversight leads to reduced transplant volume.
In this study, the primary aim was to examine the survival benefit of kidney transplantation on the basis of a transplant center’s measured performance. Our hypothesis was that transplantation is efficacious for waitlisted patients with ESRD across the continuum of center performance but that measured performance may alter the degree of survival benefit.
Materials and Methods
Data and Study Population
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere (20). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The study was approved by the Cleveland Clinic Institutional Review Board.
Center performance statistics were derived from a database obtained from the SRTR. These data contain statistics used for biannual center performance reports (e.g., observed and expected events, standardized mortality ratios, and tests of significance for outcomes) at each United States transplant center. These data were available beginning July of 2003; thus, we used this date through December of 2010 as the study period. We used the risk-adjusted model for 1-year patient survival as the measure of center performance, which is a key metric evaluated by the CMS (17). We also used the categorical assessment of center performance consistent with historical SRTR criteria, which identifies low-performing centers on the basis of meeting each of three criteria: a standardized mortality ratio (SMR)≥1.5, observed events (either 1-year graft failure or death) exceeding expected events by three, and statistically significantly different events than expected on the basis of type I error=0.05. High-performing centers were identified on SRTR reports indicating better-than-expected survival on the basis of statistically fewer observed than expected events. These data were merged with patient-level data in the SRTR by date of listing, and the transplant center patients were placed on the waiting list. The primary exposure variable for the study was the most recent center performance at the time that patients were placed on the waiting list. We chose the time of listing for this study to simulate the period in which patients selected a center for care. For patients placed on the waiting list at multiple centers, performance of the initial center was retained for the analyses.
Statistical Methods
Descriptive comparisons of the study population by center performance were on the basis of chi-squared tests and ANOVA. The primary models were Kaplan–Meier plots and multivariable Cox nonproportional hazard models with time-varying covariate of time to transplantation (21). The inception time for survival models was the date of placement on the kidney transplant waiting list. Variables used for risk adjustment in the Cox models were candidate age, body mass index, race, sex, insurance status, panel reactive antibody level, educational attainment, active versus inactive status, time on dialysis, and primary cause of ESRD. Models were censored at the time of living transplantation or last follow-up (November 30, 2011), and receipt of a deceased donor transplant was considered a time-dependent event. For the Cox models, we included all patients at the time of listing. However, as a sensitivity analysis, to compare the transplantation directly with dialysis, we generated models limited to patients who had already initiated dialysis.
To evaluate the association of center performance with the survival benefit of transplantation, we generated nonproportional Cox models in two manners. First, we generated models with an interaction term of transplantation on the basis of the categorical SRTR center performance rating. Second, we generated models using an interaction term of transplantation with centers’ SMRs. Because patient outcomes are clustered within centers, we also generated Cox models accounting for clustering using the robust sandwich estimator. Analyses were performed with SAS (version 9.2.; SAS, Cary, NC) and R software (version 3.0.1; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
The study population included 223,808 adult patients (age≥18 years) listed for solitary kidney transplantation between July 1, 2003, and December 31, 2010, with follow-up through November 30, 2011. Of these patients, 59,199 patients received a deceased donor transplant, and 32,764 patients received living donor transplants during the study period. Median follow-up from listing was 43 months (25th percentile=25 months, 75th percentile=67 months), and there were 43,951 total patient deaths. Within the cohort, 8039 (4%) patients were placed on the waiting list at a center with lower-than-expected outcomes, 11,972 (5%) patients were listed at centers with better-than-expected outcomes, and 203,797 (91%) patients were listed at centers with outcomes as expected. Descriptive statistics of the study population by center performance are presented in Table 1. The most notable differences between groups included relatively higher proportions of patients with hypertension diagnoses and lower proportions of patients listed preemptively and initially inactive at centers with lower-than-expected outcomes. Among candidates that received a transplant during the study period, recipients were less likely to receive deceased donor kidneys, donor kidneys after cardiac death, or import donor kidneys at centers with better-than-expected outcomes.
Table 1.
Patient and center characteristics by center performance
| Patient and Center Characteristics | Rating of Transplant Center on the Basis of 1-Year Post-Transplant Patient Survival at the Time of Candidate Listing | P Value | ||
|---|---|---|---|---|
| Statistically Lower than Expected (n=8039) | As Expected (n=203,797) | Statistically Higher than Expected (n=11,972) | ||
| Candidate characteristic at the time of listing | ||||
| Age (yr), mean±SD | 49.7±13.1 | 50.5±13.2 | 50.0±13.0 | <0.001 |
| African American, % | 28 | 29 | 26 | <0.001 |
| Men, % | 59 | 60 | 60 | 0.38 |
| Diabetes as primary cause of ESRD, % | 30 | 30 | 32 | <0.001a |
| Hypertension as primary cause of ESRD, % | 25 | 21 | 17 | |
| Preemptive listing (before dialysis initiation), % | 21 | 26 | 28 | <0.001 |
| Obese,b % | 33 | 34 | 34 | <0.001 |
| Panel reactive antibody>0%,b % | 29 | 33 | 34 | <0.001 |
| Private insurance, % | 38 | 45 | 46 | <0.001 |
| Less than college education,b % | 57 | 52 | 54 | <0.001 |
| Listing after failed transplant, % | 15 | 14 | 12 | <0.001 |
| Listing as inactive status, % | 20 | 22 | 36 | <0.001 |
| Transplant characteristics, n | 2325 | 54,157 | 2717 | |
| Deceased donor recipients, % | 68 | 66 | 64 | <0.001 |
| Expanded criteria donors, % | 17 | 19 | 17 | 0.003 |
| Donation after cardiac arrest donors, % | 13 | 12 | 9 | <0.001 |
| Import donors,c % | 28 | 28 | 24 | 0.003 |
| Donors>30 h cold ischemia time, % | 7 | 8 | 6 | <0.001 |
| Number of program-specific reports for 1-yr mortality | 145 | 2969 | 82 | |
| Outcome rates (95% CIs) after candidate listing | ||||
| Pretransplant mortality (per 100 patients-yr) | 6.1 (5.7 to 6.4) | 6.1 (6.1 to 6.2) | 6.2 (5.9 to 6.4) | 0.79 |
| Overall post-transplant mortality (per 100 patients-yr) | 3.7 (3.4 to 4.1) | 3.1 (3.0 to 3.2) | 2.4 (2.2 to 2.7) | <0.001 |
| Post-transplant mortality after deceased donor transplantation (per 100 patients-yr) | 4.7 (4.2 to 5.2) | 4.0 (3.9 to 4.1) | 3.1 (2.7 to 3.5) | <0.001 |
| Deceased donor transplantation (per 100 patients-yr) | 11.6 (11.1 to 12.0) | 10.6 (10.5 to 10.7) | 8.5 (8.2 to 8.8) | <0.001 |
| Living donor transplantation (per 100 patients-yr) | 5.5 (5.2 to 5.9) | 5.9 (5.8 to 6.0) | 4.5 (4.3 to 4.7) | <0.001 |
| Waitlist removal for health deterioration (per 100 patients-yr) | 1.7 (1.5 to 1.9) | 1.5 (1.5 to 1.6) | 1.1 (1.0 to 1.2) | <0.001 |
95% CI, 95% confidence interval.
Testing the association between overall primary diagnosis and performance level.
Missing values excluded from percentage.
Defined as donations outside of center donor service area.
Center Performance Ratings and Transplant Volume
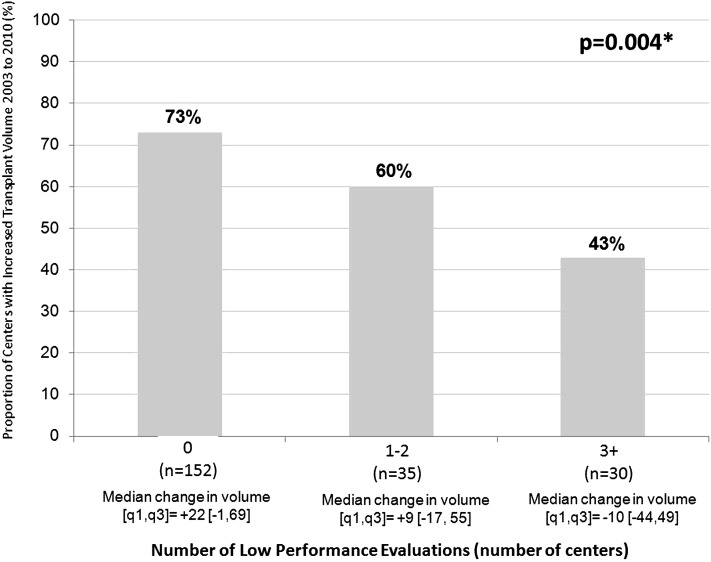
There were 3196 program-specific reports over 15 biannual periods at one of 249 transplant centers within the study period. For the primary exposure variable of 1-year mortality, 145 reports included lower-than-expected outcomes, 82 reports included higher-than-expected outcomes, and 2969 reports included no significant differences. Centers with higher-than-expected outcomes had the largest average number of candidates listed during the respective period (n=146) followed by centers with no significant differences (n=68) and lower-than-expected outcomes (n=55). There were 217 centers that had performed transplants eligible for both January of 2003 and July of 2010 SRTR program–specific reports (an additional 32 programs did not perform transplants across the period). Each report includes a 2.5-year rolling cohort of recipients. For example, the July of 2010 report includes recipients transplanted between January of 2007 and June of 2009. Among 217 centers, two thirds (67%) increased transplant volume over the study period. The proportion of centers that increased volume varied by the number of low-performance evaluations centers received during this time period. Among 152 centers without any low-performance evaluations, 73% increased volume compared with 60% of centers with one to two low-performance evaluations and 43% of centers with three or more low-performance evaluations (Figure 1) (P=0.004). The median (25th, 75th percentiles) change in volume among centers without any low-performance evaluations was +22 (−1, 69) compared with +9 (−17, 55) among centers with one to two low-performance evaluations and −10 (−44, 49) among centers with three or more low-performance evaluations.
Figure 1.
Proportion of centers with increased transplant volume by number of low-performance evaluations. *P value is on the basis of a chi-squared test comparing the proportion of centers with increased volume between the January, 2003 to July, 2010 Scientific Registry of Transplant Recipients Program-Specific Reports and number of low performance evaluations.
Unadjusted Center Outcomes and Performance
Unadjusted outcomes after listing are displayed in Table 1. There was no difference in pretransplant mortality rates between groups (P=0.79), whereas post-transplant mortality rate (including both deceased and living donor transplants; P<0.001) and the rate for deceased donor recipients alone were higher among patients in the low-performance group (P<0.001). Both deceased and living donor transplant rates were higher among lower-performing centers along with waitlist removal for health deterioration.
Unadjusted Patient Survival and Center Performance at Time of Listing
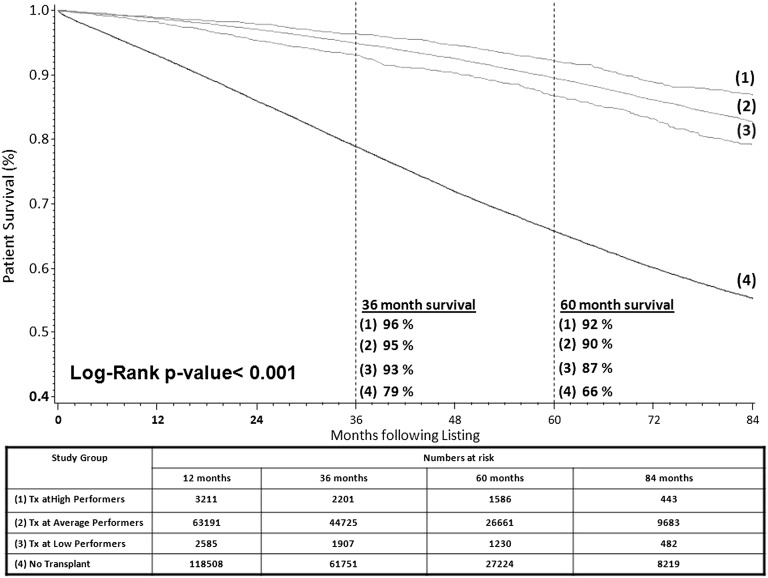
During the study period, 58% (n=129,665) of candidates did not receive a transplant, 39% (n=86,413) of patients received a transplant after listing at a center performing as expected, 2% (n=4248) of patients received a transplant after listing at a center performing better than expected, and 2% (n=3482) of patients received a transplant after listing at a center performing worse than expected. Kaplan–Meier plots for all-cause mortality from the time of placement on the waiting list on the basis of whether patients received a deceased donor transplant from a higher-, as expected, or lower-performing transplant center or did not receive a transplant during the study period are displayed in Figure 2. There was a significant difference in survival between study groups, including between groups receiving a transplant (P<0.001). Differences in survival between patients transplanted at centers with higher versus lower measured performance were approximately 1% per year (i.e., 1% and 5% at 1 and 5 years post-transplant, respectively). However, compared with patients transplanted at centers with low measured performance, patients who did not receive a transplant had substantially inferior absolute survival, including 5% and 21% at 1 and 5 years follow-up, respectively.
Figure 2.
Kaplan–Meier plot of patient survival after listing by transplant status and center quality on the basis of deceased donor transplantation at a transplant center with a given performance at the time of listing. Tx, transplantation.
Adjusted Patient Survival and Center Performance at Time of Listing
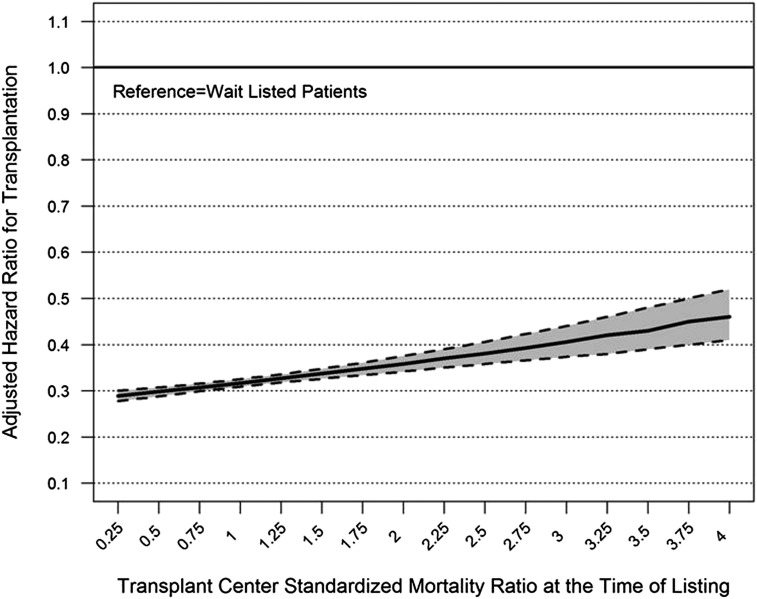
The overall risk adjusted hazard ratio (AHR) associated with transplantation relative to patients on the waiting list was 0.32 (95% confidence interval [95% CI], 0.31 to 0.32). The survival benefit of transplantation significantly varied by center performance (P value for interaction <0.001). Patients listed at centers with better-than-expected performance had an AHR associated with transplantation of 0.24 (95% CI, 0.21 to 0.27). Patients listed at centers performing as expected had an AHR of 0.32 (95% CI, 0.31 to 0.33), and patients listed at centers with lower-than-expected performance had an AHR of 0.40 (95% CI, 0.35 to 0.45). After exclusion of patients who were not on dialysis at the time of listing, the estimated hazards were similar: higher-than-expected outcomes AHR, 0.23 (95% CI, 0.20 to 0.26); as-expected AHR, 0.30 (95% CI, 0.30 to 0.31); and lower-than-expected AHR, 0.38 (95% CI, 0.34 to 0.44). Repeating the model using the SMR of the center, the interaction of SMR and transplantation was also statistically significant (P<0.001) (Figure 3), with a gradual reduction in the survival benefit of transplantation with increasing SMR. After incorporating adjustment for patient clustering within centers, the point estimates of the primary results were nearly identical, with very minor shift in confidence intervals.
Figure 3.
Adjusted hazard of mortality for kidney transplantation by standardized mortality ratio of the center at the time of listing. The hazard ratio depicts the time-dependent hazard of transplantation relative to the waiting list, and the 95% confidence interval is the shaded region.
Results of the Cox model for the survival benefit of transplantation by center performance within subsets of the candidate population are displayed in Table 2. The survival benefit of center performance was relatively consistent for patients by baseline characteristics. The exception was a greater survival benefit of center performance level among younger patients (P=0.01). Among each of the patient subsets, transplantation was associated with a statistically significant survival benefit relative to the waiting list at each center performance level.
Table 2.
Adjusted hazard of mortality for deceased donor transplantation by patient characteristics and center performance
| Candidate Characteristic/Level | Center Performance at the Time of Listing | P Value for Interaction | ||
|---|---|---|---|---|
| Higher than Expected | As Expected | Lower than Expected | ||
| All patients | 0.24 (0.21 to 0.27) | 0.32 (0.31 to 0.33) | 0.40 (0.35 to 0.45) | |
| Race/ethnicity | ||||
| Caucasian | 0.27 (0.23 to 0.32) | 0.33 (0.32 to 0.34) | 0.38 (0.32 to 0.45) | 0.50 |
| African American | 0.18 (0.14 to 0.24) | 0.32 (0.30 to 0.33) | 0.38 (0.31 to 0.48) | |
| Other | 0.21 (0.14 to 0.32) | 0.29 (0.27 to 0.30) | 0.45 (0.35 to 0.58) | |
| Sex | ||||
| Men | 0.24 (0.20 to 0.28) | 0.33 (0.31 to 0.34) | 0.42 (0.36 to 0.48) | 0.63 |
| Women | 0.25 (0.20 to 0.31) | 0.30 (0.29 to 0.32) | 0.37 (0.30 to 0.45) | |
| Body mass index | ||||
| Obesea | 0.27 (0.21 to 0.34) | 0.33 (0.31 to 0.34) | 0.39 (0.32 to 0.48) | 0.33 |
| Nonobesea | 0.24 (0.20 to 0.28) | 0.31 (0.30 to 0.32) | 0.41 (0.35 to 0.47) | |
| Cause of ESRD | ||||
| Diabetes | 0.24 (0.19 to 0.30) | 0.30 (0.29 to 0.31) | 0.42 (0.35 to 0.50) | 0.12 |
| Other | 0.24 (0.20 to 0.28) | 0.33 (0.32 to 0.34) | 0.39 (0.34 to 0.46) | |
| Primary insurance | ||||
| Private | 0.22 (0.18 to 0.27) | 0.32 (0.30 to 0.33) | 0.35 (0.28 to 0.44) | 0.06 |
| Nonprivate | 0.25 (0.21 to 0.29) | 0.32 (0.31 to 0.33) | 0.42 (0.37 to 0.48) | |
| Age, yr | ||||
| 18–40 | 0.14 (0.08 to 0.23) | 0.27 (0.25 to 0.29) | 0.37 (0.26 to 0.53) | 0.01 |
| 41–60 | 0.23 (0.19 to 0.27) | 0.30 (0.29 to 0.31) | 0.35 (0.29 to 0.41) | |
| >60 | 0.29 (0.24 to 0.36) | 0.35 (0.34 to 0.36) | 0.49 (0.41 to 0.59) | |
| Educational attainment | ||||
| College or morea | 0.24 (0.19 to 0.30) | 0.33 (0.31 to 0.34) | 0.35 (0.29 to 0.44) | 0.05 |
| Less than collegea | 0.26 (0.22 to 0.31) | 0.31 (0.29 to 0.32) | 0.41 (0.35 to 0.48) | |
Values depict the adjusted hazard ratio (95% confidence interval) of mortality for kidney transplantation relative to waitlisted patients adjusted for age, race, sex, body mass index, insurance type, panel-reactive antibody level, educational attainment, active status at listing (yes or no), primary versus retransplant status, primary diagnosis, and time to listing from ESRD onset.
Missing levels not included.
Association of Competition and the Survival Benefit of Transplantation
Among centers in the study, 3% of centers were in single–organ procurement organization (OPO) regions (low), 33% of centers shared their OPO with one to three centers (low-mid), 39% of centers shared their OPO with four to seven centers (mid-high), and 25% of centers shared their OPO with eight or more centers (high). The survival benefit associated with transplantation was similar in each level of competition (P=0.10 for the interaction of competition and transplantation): AHR, 0.35 (95% CI, 0.29 to 0.43) for low-competition regions; AHR, 0.27 (95% CI, 0.25 to 0.30) for low-mid–competition regions; AHR, 0.30 (95% CI, 0.27 to 0.32) for mid-high–competition regions; and AHR, 0.27 (95% CI, 0.24 to 0.31) for high-competition regions. There was no statistically significant association between competition level and incidence of low-performance evaluations (P=0.52). However, changes in transplant volume on the basis of incidence of low-performance evaluations differed by competition level and were only statistically significant among regions with the highest competition (P<0.01).
Discussion
There are two principal findings of this study. First, results indicate that the survival benefit of kidney transplantation is modified by the measured performance of transplant centers. Although this finding may be subject to inherent selection biases, the magnitude of differences was statistically significant and consistent within subsets of the population. These results suggest that continuing quality improvement among transplant centers is needed and that additional efforts to identify systematic characteristics of both higher- and lower-performing centers may be important. Second, transplantation is associated with a substantial survival benefit relative to chronic dialysis at each level of center performance. That is, even among centers with low measured quality, there is a marked benefit associated with transplantation, and access to transplantation remains a prominent public health priority.
Despite the significant differences in patient outcomes between centers, there are concerns about quality oversight and the potential reduction in transplant rates among low-performing centers (14,15,18,22). Concerns about performance evaluations generally derive from the fact that measures have inherent limitations (e.g., inability to account for disproportionate prevalence of underlying risk factors) that may unintentionally bias reports (22–24). As such, centers may become more reluctant to accept patients or donor organs with perceived higher risks that potentially threaten measured performance (14). Regardless of whether these concerns are valid, transplantation provides a substantial survival benefit, even among patients and donors with a higher prevalence of risk factors (5,6,25). These findings add important evidence to indicate that patients receive a significant benefit from transplantation, even among centers with lower measured performance. In fact, to place findings in perspective, the estimated absolute survival for patients who are transplanted at high- versus poorly-performing transplant centers is within 5% at 5 years post-transplant compared with a 21% difference in survival between patients transplanted at a poor-performing center and not receiving a transplant. As such, restrictions or delays to transplantation at low-performing centers that are not counterbalanced at other centers may limit overall benefits to the candidate population.
These findings support the notion that variation in quality exists between centers and, furthermore, that these differences modify the survival benefit of transplantation (26). Performance assessments may be useful to identify best practices and potentially inform patient decision-making (27). Thus, identifying centers with lower performance and providing incentives for quality improvement efforts remains important. Additional efforts to distinguish processes of care and characterize practices among centers at all levels of performance may provide important insights for quality improvement.
An unresolved question from this analysis is whether performance evaluations affect overall transplant and/or organ use rates. If these rates have been unaffected by increased oversight, then the association of transplant volume and performance may represent a shift in practice, directing patients to higher-performing centers. In contrast, if low-performing centers are more reluctant to accept patients or donor organs with higher perceived risks, performance assessments may indirectly lead to delays to transplantation. Results from this study also provide some preliminary evidence that competition may affect this relationship, with a greater change in practice in highly competitive regions. A challenge for policymakers and caregivers is to best align incentives to stimulate the growth of transplantation and simultaneously monitor center performance in a manner that maximizes the benefit of scarce donor organs to the candidate population.
The primary limitation of the study is the retrospective study design and inability to derive cause-and-effect relationships. In addition, there may be important unmeasured characteristics of patients that could affect comparisons of study groups. This study only included outcomes of deceased donor transplantation to measure the survival benefit of the procedure given that the waiting list is a reasonable reference group of patients medically cleared for the procedure; however, these results may not be consistent among living donor recipients. We chose to use center performance at the time of patient listing as the primary metric for the study on the basis of an attempt to model the time at which centers were selected for care. However, it is possible that the effect of center performance may differ, perhaps dramatically, if estimated from the time of transplantation. We also only used a single measure of risk-adjusted outcome at 2 years post-transplantation to define center quality on the basis of its use by regulators. However, there may be other factors beyond 1-year survival that are as important for patients (28,29).
In summary, findings of the study show that measured quality of kidney transplant centers in the United States has a significant association with the survival benefit of transplantation. Importantly, the benefits of transplantation persist across the spectrum of quality among transplant centers. Public policies to support the growth of transplantation and donation are critical to the ESRD population to improve patients’ longevity and quality of life. Efforts to incentivize quality improvement in a complementary manner with the growth of transplant services are particularly vital.
Disclosures
None.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR).
Funding for this study was partially supported by National Institutes of Health Grant R01-DK085185 (to J.D.S.), P60-MD00265 (to J.D.S. and A.R.S.), and UL1-TR00439 (to A.R.S.).
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The contents of this manuscript are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health or other funding entities.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Might the Current Gauge of Transplant Center Quality Result in Reducing Patient Access via Diminished Organ Utilization?,” on pages 1674–1675.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bayat S, Kessler M, Briançon S, Frimat L: Survival of transplanted and dialysed patients in a French region with focus on outcomes in the elderly. Nephrol Dial Transplant 25: 292–300, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Gill JS, Tonelli M, Johnson N, Kiberd B, Landsberg D, Pereira BJ: The impact of waiting time and comorbid conditions on the survival benefit of kidney transplantation. Kidney Int 68: 2345–2351, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, Gill J: The survival benefit of kidney transplantation in obese patients. Am J Transplant 13: 2083–2090, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK: Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589–597, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Rabbat CG, Thorpe KE, Russell JD, Churchill DN: Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol 11: 917–922, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Eggers PW: Effect of transplantation on the Medicare end-stage renal disease program. N Engl J Med 318: 223–229, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Jofré R, López-Gómez JM, Moreno F, Sanz-Guajardo D, Valderrábano F: Changes in quality of life after renal transplantation. Am J Kidney Dis 32: 93–100, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Whiting JF, Golconda M, Smith R, O’Brien S, First MR, Alexander JW: Clinical and economic outcomes of expanded criteria donors in renal transplantation. Transplant Proc 29: 3258, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Whiting JF, Golconda M, Smith R, O’Brien S, First MR, Alexander JW: Economic costs of expanded criteria donors in renal transplantation. Transplantation 65: 204–207, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network: Waiting List Additions Age by Listing Year, 2013. Available at: http://optn.transplant.hrsa.gov/latestData/rptData.asp Accessed September 14, 2013
- 13.Organ Procurement and Transplantation Network: Transplants by Donor Type, 2013. Available at: http://optn.transplant.hrsa.gov/latestData/rptData.asp Accessed September 15, 2013
- 14.Axelrod DA: Balancing accountable care with risk aversion: Transplantation as a model. Am J Transplant 13: 7–8, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schnier KE, Cox JC, McIntyre C, Ruhil R, Sadiraj V, Turgeon N: Transplantation at the nexus of behavioral economics and health care delivery. Am J Transplant 13: 31–35, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM, Soria C, Segev DL, Fung J, Goldfarb DA: The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant 13: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services: New Medicare Hospital Conditions of Participation for Transplant Centers, 2007. Available at: http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=2093&intNumPerPage=10&checkDate=&checkKey=&srchType=&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date Accessed March 22, 2007
- 18.Schold JD, Arrington CJ, Levine G: Significant alterations in reported clinical practice associated with increased oversight of organ transplant center performance. Prog Transplant 20: 279–287, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Hamilton TE: Improving organ transplantation in the United States—a regulatory perspective. Am J Transplant 8: 2503–2505, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE: Analytical methods and database design: Implications for transplant researchers, 2005. Am J Transplant 6: 1228–1242, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schemper M: Cox analysis of survival data with non-proportional hazard functions. Statistician (41): 455–465, 1992 [Google Scholar]
- 22.Howard RJ, Cornell DL, Schold JD: CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transplant 23: 778–783, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Massie AB, Segev DL: Rates of false flagging due to statistical artifact in CMS evaluations of transplant programs: Results of a stochastic simulation. Am J Transplant 13: 2044–2051, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Schold JD, Howard RJ: Prediction models assessing transplant center performance: Can a little knowledge be a dangerous thing? Am J Transplant 6: 245–246, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Wolfe RA, McCullough KP, Schaubel DE, Kalbfleisch JD, Murray S, Stegall MD, Leichtman AB: Calculating life years from transplant (LYFT): Methods for kidney and kidney-pancreas candidates. Am J Transplant 8: 997–1011, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Schold JD, Buccini LD, Heaphy EL, Goldfarb DA, Sehgal AR, Fung J, Poggio ED, Kattan MW: The prognostic value of kidney transplant center report cards. Am J Transplant 13: 1703–1712, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services HRaSAHSBDoT HRSA Transplant Center Growth and Management Collaborative: Best Practices Evaluation, 2013. Available at: ftp://ftp.hrsa.gov/organdonor/transplantctrgrowth_and_mangmtcollaborbestpracticesreport.pdf Accessed January 24, 2012
- 28.Schold JD, Harman JS, Chumbler NR, Duncan RP, Meier-Kriesche HU: The pivotal impact of center characteristics on survival of candidates listed for deceased donor kidney transplantation. Med Care 47: 146–153, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Zenios S, Atias G, McCulloch C, Petrou C: Outcome differences across transplant centers: Comparison of two methods for public reporting. Clin J Am Soc Nephrol 6: 2838–2845, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]