Abstract
Kidney disease is a significant medical and public health problem. The National Institute of Diabetes and Digestive and Kidney Diseases recently asked the community to identify research objectives, which, if addressed, could improve understanding of basic kidney function and aid in prevention, treatment, and reversal of kidney disease. The Kidney Research National Dialogue invited interested parties to submit, discuss, and prioritize ideas using an interactive website; 1600 participants posted more than 300 ideas covering all areas of kidney disease, including the cystic kidney diseases. Although much is known about the genetics and pathogenesis of cystic diseases, there remain challenges to our understanding of the fundamental mechanisms of cyst formation, what genes act as modifiers to cause variable responses in different people, and how to detect and monitor disease progression. This article summarizes key research questions for cystic kidney diseases.
Keywords: kidney disease, cysts, cilia, polycystic kidney disease
Introduction
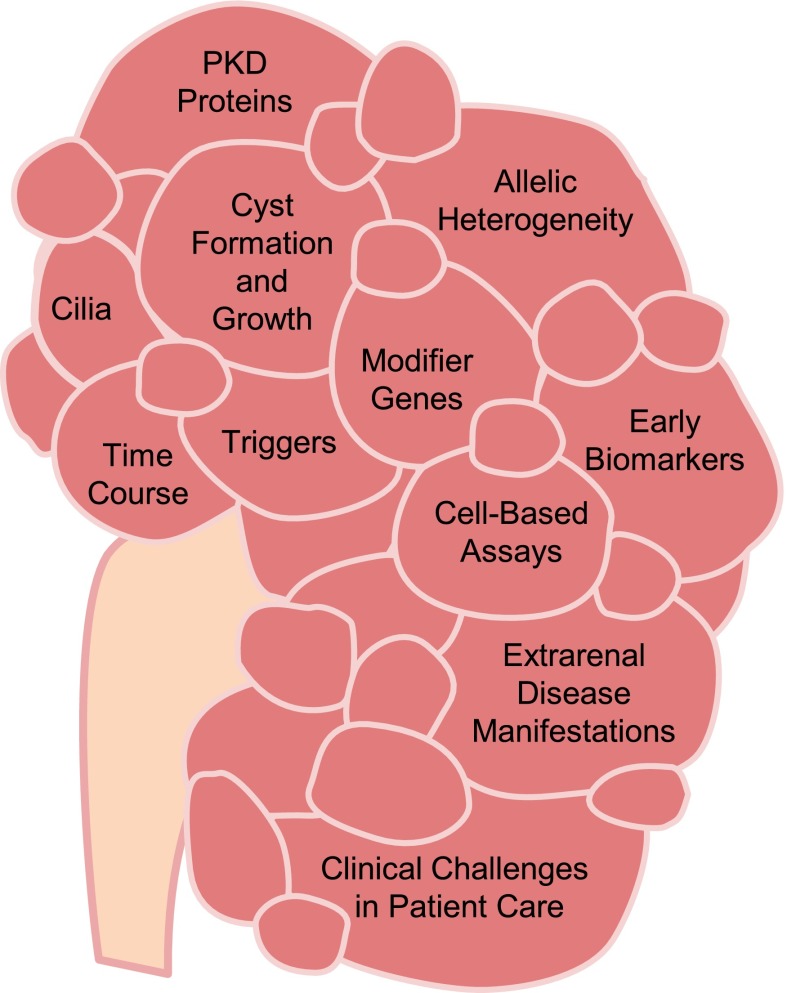
The National Institute of Diabetes and Digestive and Kidney Diseases recently asked the community to identify research objectives, which if addressed, would improve our understanding of basic kidney function and aid in the prevention, treatment, and reversal of kidney disease. The Kidney Research National Dialogue (KRND) welcomed all interested parties to submit, discuss, and prioritize ideas through an interactive website. Over 1600 participants posted ideas covering all areas of kidney disease. This commentary focuses on one important component of the KRND and highlights selected scientific opportunities in cystic kidney diseases. The most common form of cystic kidney disease is autosomal dominant polycystic kidney disease (ADPKD), which affects approximately one in 1000 individuals of all ethnicities (1). Other cystic diseases, such as autosomal recessive polycystic kidney disease (ARPKD) and nephronophthisis, are significantly less common but typically much earlier in their onset and more rapid in their progression to ESRD (2). Basic research has uncovered the genes that cause ADPKD, ARPKD, and many forms of hepatorenal fibrocystic diseases, which include Meckel–Gruber Syndrome and nephronophthisis. Several lines of investigation have implicated primary cilia in the pathogenesis of cystic kidney disease, and various signaling pathways are dysregulated in polycystic kidney disease (PKD). Although PKD researchers use a robust range of basic and clinical studies aimed at understanding, treating, and curing cystic kidney diseases, important questions remain unanswered (Figure 1).
Figure 1.
There is a continued need for basic research into cyst formation and growth, the role of cilia, and the genes that modify the course of disease. Earlier biomarkers are needed to predict prognosis and monitor disease progression. They, in turn, will help select patients for clinical trials and optimize therapeutic end points. PKD, polycystic kidney disease.
Basic Biology and Pathobiology of Cyst Formation
PKD Proteins
ADPKD is generally caused by heterozygosity for a hypomorphic or loss-of-function allele in one of two polycystin genes: PKD1 and PKD2. Recessive mutations in the fibrocystin/polyductin gene cause ARPKD. All three proteins are found in primary cilia as well as other cellular locations.
PKD1 encodes a large and complex transmembrane protein, polycystin 1. Polycystin 2, encoded by PKD2, has homology to the transient receptor potential family of cation channels and calcium channel activity. Polycystin 1 and polycystin 2 interact through their C termini and form what is proposed to be a receptor channel complex. Fibrocystin/polyductin is a type I membrane protein that interacts with polycystin 2. Polycystin 1 and fibrocystin/polyductin are cleaved, and the three proteins are packaged at high concentrations into exosome-like vesicles.
There is still much that we do not understand about the normal functions of these molecules and how mutations lead to disease. What is the significance of the various cleavage products? What activates the polycystin complex in vivo? What are the ligands for this complex? What types of cell-based assays can be developed to assess the pathogenic role of the polycystins and associated proteins?
Cilia
A convergence of data suggests that primary cilia are important in the pathogenesis of PKD, but their precise role remains unclear. It has been suggested that the PKD1/PKD2 receptor channel complex acts as a flow sensor on renal epithelial cells, perhaps by calcium signaling. However, separating flow-mediated signals from signals mediated by ligands moved under flow remains challenging. In addition, this important hypothesis leaves several questions unresolved. How do flow-mediated changes in the cilia, which seem to contain a distinct calcium compartment, influence cytoplasmic calcium and cyst formation? Loss of calcium signaling within the cilia compartment is apparently not sufficient to cause cyst formation. How do the roles of the cilium as a receptor for ligands and exosomes interact in disease pathogenesis?
The roles of cilia and polycystins are further complicated by discoveries that a large number of genes encoding cilia, transition zone, or basal body proteins causes rare recessive hepatorenal fibrocystic diseases when disrupted by mutations. Moreover, recent evidence suggests that inactivation of cilia can both stimulate and inhibit cyst growth, depending on the presence or absence of polycystins and the phase of development. We need to understand the precise relationship of cilia to cystogenesis, as well as the specific roles of the polycystins and cilia-associated protein complexes in cyst formation.
Time Course
The origin, developmental timing, and natural history of cyst initiation and progression in the cystic kidney diseases remain poorly understood. For example, in mouse models of cystic disease, renal inactivation of PKD genes after postpartum 12–postpartum 14 results in slower development of cystic disease compared with rapid disease progression associated earlier gene inactivation. What are the factors that determine the rate of cyst growth in the developing and adult kidney? Does it depend on the underlying rate of epithelial cell proliferation at the time of PKD inactivation? How do animal models relate to human PKD? Is gene inactivation occurring continuously or at key developmental windows? What is the role of noncell-autonomous factors, such as inflammation, oxidative stress, and angiogenesis in progressive cyst growth?
Proliferation of cyst-lining epithelia for renal cysts to expand is controversial and may be more important during renal development. What are the effects of maternal and fetal environments on the subsequent time course of disease? What clinical experiments can be designed to rigorously address this issue, because many antiproliferative therapies are being proposed to treat PKD?
What are the mechanistic differences between the late onset and relatively slow progression of ADPKD and the early onset and rapid progression of the various recessive hepatorenal fibrocystic diseases? What alterations take place in subcellular structure and metabolism that determine the growth and progression of cystic disease, and are those alterations similar in all cystic diseases? Do cysts regress?
Triggers
There is experimental evidence that cyst formation in ADPKD involves a second hit in epithelial cells, although little is known about the nature of those events. It is generally accepted that somatic inactivating mutations of the wild-type allele of the PKD1 or PKD2 gene play a role in the development of ADPKD. However, there is evidence that cysts develop when functional polycystin levels fall below a critical threshold for any one of a variety of reasons, including stochastic expression, differences between cells, and environmental factors, such as kidney injury. What leads to the second hit, and can it be prevented? What is the role of renal injury as an additional hit?
Allelic Heterogeneity and Modifier Genes
Many factors modify the course and severity of the cystic kidney diseases. In ADPKD, there is marked variability in disease severity among members of the same family, all of whom carry the same PKD germline mutation. Among the recessive genetic hepatorenal fibrocystic diseases, it is remarkable that different defects in the same gene can lead to clinically distinct diseases (e.g., Meckel–Gruber Syndrome, nephronophthisis, and Joubert Syndrome). This variability can be explored using well powered high-throughput sequencing and other types of genetic studies to identify new pathways and molecules that influence disease progression.
There is a widening gap between the identification of new cystic kidney disease genes and the functional characterization of the encoded proteins. More research is needed to characterize the function of these proteins and delineate the relationship between ADPKD and recessive genetic hepatorenal fibrocystic diseases.
Early Biomarkers for ADPKD
GFR decline is associated with advanced structural change in ADPKD. However, it is difficult to conduct clinical trials on the basis of GFR decline, because it occurs late in the course of disease. Moreover, clinical interventions may be more effective when initiated early (before irreparable renal damage has occurred). Thus, early biomarkers of disease progression are needed to facilitate clinical trials that may also include children.
The Consortium for Radiologic Imaging Studies of PKD (ClinicalTrials.gov identifier NCT01039987) and other studies have shown that total kidney volume (TKV) increases before GFR declines and are providing detailed longitudinal timing and variability information. It is hypothesized that strategies targeted to slow TKV increases will also slow GFR decline and delay onset of renal failure. Data from the interventional Tolvaptan Efficacy and Safety in Management of ADPKD and Its Outcomes (ClinicalTrials.gov identifier NCT00428948) and HALT-PKD (ClinicalTrials.gov identifiers NCT01885559 and NCT00283686) studies will provide important information to test this hypothesis, because both are measuring GFR and TKV over several years. However, TKV does not detect the earliest stages of cyst formation. Moreover, it is known that other processes, including inflammation and fibrosis, damage the renal parenchyma. Are there other biomarkers suitable for detection of ADPKD progression and response to therapy? Are there new imaging technologies that will allow detection of cyst formation before demonstration by magnetic resonance imaging? Are there patient-reported outcomes, such as pain or abdominal distention, that might be useful as clinical end points?
Extrarenal Disease Manifestations
ADPKD is a systemic disorder, and people with ADPKD often have cysts in other organs, such as the liver. A subset of ADPKD patients have vascular complications, including intracranial and large-vessels aneurysms. The appearance of these extrarenal symptoms, like the kidney disease, is highly variable. What genetic or physiologic factors influence the occurrence and severity of extrarenal manifestations? Is the same second-hit pathogenesis mechanism at work outside the kidney? Can these findings be translated into patient-reported outcomes for clinical trials?
Cardiovascular disease is currently the leading cause of death among patients with ADPKD. How does ADPKD affect cardiovascular disease? Are there specific interventions that may reduce the risk for cardiovascular complications?
Liver disease is more common in women with ADPKD, and estrogen has been reported to have a role in liver cystic disease. Does use of estrogen-based contraceptives increase risk for more severe liver cystic disease in women? Is the same true for hormone replacement therapy? Cyst infections are also a common complication in patients with ADPKD. What is the optimum treatment time for cyst infections to prevent recurrence? How should resolution of infection be determined?
Similarly, recessive genetic hepatorenal fibrocystic diseases often present as part of a syndrome with neurologic, ophthalmologic, and other symptoms. ARPKD patients have congenital hepatic fibrosis, which only becomes clinically significant in a subset of patients. Why do the same defective proteins function differently in different organs and cell types, and what determines the manifestation and severity of the extrarenal symptoms?
Clinical Challenges in Patient Care
There are still important questions about the optimal care of people with cystic diseases. Hypertension is one of the earliest and most common manifestations and an important cause of morbidity and mortality in ADPKD. It has been associated with more rapid renal disease progression and is the focus of the HALT-PKD study. Similarly, there is a high prevalence of systemic hypertension in children with ARPKD, and severe hypertension is often present in the first few months of life. What BP goals are appropriate for ADPKD patients with lower eGFRs? What BP goals are appropriate for children with ARPKD? Would early detection and treatment of high BP improve the outcome of ADPKD?
Exciting progress has been made over the last decade in the identification of potential treatments to delay the progression of ADPKD, such as vasopressin receptor antagonists and somatostatin analogs. However, there continues to be a gap between the identification of potential therapies in preclinical studies and their translation into clinical trials. How should clinical trials be prioritized and implemented? Because not all people with ADPKD progress to ESRD, it is also important to find markers that will identify those individuals who are most likely to benefit from future therapies.
Conclusion
In the wake of the discovery of numerous cystic kidney disease genes (e.g., PKD1, PKD2, and fibrocystin/polyductin gene) and genes for other recessive genetic hepatorenal fibrocystic diseases, there was hope that these advances would lead to a precise molecular understanding of these disorders and point the way to development of effective therapeutics. However, despite extensive research, the molecular basis of cyst formation and enlargement remains incompletely understood. Additional work is needed on early clinical end points or biomarkers as part of developing effective strategies to test promising new therapeutics. Much research remains to be done on the most important questions in cystic kidney diseases.
Disclosures
L.M.G.-W. is a consultant for Otsuka Pharmaceuticals. P.I. owns stock (<$10,000 each) in Teva Pharmaceuticals, CVS Caremark, and Johnson & Johnson; receives royalties from Elsevier; and received honoraria/consulted for Southern Society for Clinical Investigation and PKD Foundation. R.D.P. provides research support to Otsuka Pharmaceutical and is a consultant for Sanofi-Genzyme and Vertex. V.E.T. provides research support to Otsuka Pharmaceutical.
Supplementary Material
Acknowledgments
The authors thank the many members of the kidney community who participated in the Kidney Research National Dialogue (KRND). The polycystic kidney disease topic was facilitated by M.F.F. and R.S.R. The KRND was developed and implemented by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/Division of Kidney, Urologic and Hematologic Diseases staff and directed by Dr. Krystyna Rys-Sikora. The complete listing of areas of research emphasis in priority order is available on the NIDDK KRND website (http://www.niddk.nih.gov/about-niddk/offices-divisions/division-kidney-urologic-hematologic-diseases/kidney-research-national-dialogue/Pages/kidney-research-national-dialogue.aspx). Please visit this website for updates on KRND.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03410414/-/DCSupplemental.
References
- 1.Autosomal Dominant Polycystic Kidney Disease, 2011. Available at: http://www.genetests.org Accessed December 10, 2013
- 2.Kerkar N, Norton K, Suchy FJ: The hepatic fibrocystic diseases. Clin Liver Dis 10: 55–71, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.