Abstract
Background and objectives
Reduced muscle mass and strength are prevalent conditions in dialysis patients. However, muscle strength and muscle mass are not congruent; muscle strength can diminish even though muscle mass is maintained or increased. This study addresses phenotype and mortality associations of these muscle dysfunction entities alone or in combination (i.e., concurrent loss of muscle mass and strength/mobility, here defined as sarcopenia).
Design, setting, participants, & measurements
This study included 330 incident dialysis patients (203 men, mean age 53±13 years, and mean GFR 7±2 ml/min per 1.73 m2) recruited between 1994 and 2010 and followed prospectively for up to 5 years. Low muscle mass (by dual-energy x-ray absorptiometry appendicular mass index) and low muscle strength (by handgrip) were defined against young reference populations according to the European Working Group on Sarcopenia in Older People.
Results
Whereas 20% of patients had sarcopenia, low muscle mass and low muscle strength alone were observed in a further 24% and 15% of patients, respectively. Old age, comorbidities, protein-energy wasting, physical inactivity, low albumin, and inflammation associated with low muscle strength, but not with low muscle mass (multivariate ANOVA interactions). During follow-up, 95 patients (29%) died and both conditions associated with mortality as separate entities. When combined, individuals with low muscle mass alone were not at increased risk of mortality (adjusted hazard ratio [HR], 1.23; 95% confidence interval [95% CI], 0.56 to 2.67). Individuals with low muscle strength were at increased risk, irrespective of their muscle stores being appropriate (HR, 1.98; 95% CI, 1.01 to 3.87) or low (HR, 1.93; 95% CI, 1.01 to 3.71).
Conclusions
Low muscle strength was more strongly associated with aging, protein-energy wasting, physical inactivity, inflammation, and mortality than low muscle mass. Assessment of muscle functionality may provide additional diagnostic and prognostic information to muscle-mass evaluation.
Keywords: CKD, malnutrition, mortality risk, ESRD, nutrition
Introduction
CKD, especially ESRD, represents a major public health issue and an important contributor to the overall noncommunicable disease burden. Its increasing prevalence is explained to a large extent by the longer life expectancy of the community because the majority of patients initiating dialysis are in their sixth and seventh decades of life (1). Markers of both muscle mass and strength are important predictors of outcomes in this patient population (2,3) subjected to a catabolic protein-energy wasting (PEW) syndrome (4).
Etymologically, the term “sarcopenia” refers to a state of low muscle mass. However, international societies recently proposed this term to encompass the concept of limited muscle mass with limited function/mobility (5–7). The rationale is that muscle mass and muscle function do not have the same clinical relevance during the aging process (5–7). Within this study, we adhere to such terminology. Sarcopenia in these terms (i.e., reduced muscle mass with limited mobility/function) has been associated with frailty, cachexia, and functional disability, leading to worse quality of life and higher mortality rates (8). The prevalence of sarcopenia in non-CKD elderly individuals ranges from 7%–31% (8–10). CKD is considered a risk factor for accelerated aging (11), and prospective studies in older adults with CKD have quantified the risk for physical disability and cognitive decline as being approximately double that of the age-matched general population (12). Although the term sarcopenia is often used in the literature to describe muscle derangements in patients with CKD (13–15), there are only two studies that have formally assessed its prevalence and clinical correlates. Sarcopenia was more prevalent in individuals with CKD stage≥3 than in individuals with normal renal function (16), whereas it affected one third of prevalent hemodialysis patients (17).
Nonetheless, muscle strength is not solely dependent upon muscle size, and both entities may disassociate (18). As people age, the rate of decline in muscle strength is greater than the rate of loss of muscle mass (19), and muscle strength can diminish even while muscle mass is maintained or increased (20). Therefore, both conditions need to be defined independently because they may have different clinical implications. This is of particular interest because therapeutic measures to maintain or increase muscle mass or muscle strength are not necessarily the same (21). Against this background, this study assessed whether sarcopenia is common in dialysis patients, and whether these two muscle dysfunction entities (muscle mass and strength) carry additive phenotype and mortality risk associations.
Materials and Methods
Participants and Study Design
This is a post hoc cross-sectional analysis with prospective follow-up from an ongoing cohort of incident dialysis patients (22). Patient recruitment occurred between December 1994 and April 2010. Exclusion criteria included age<18 or >75 years, signs of infection, and unwillingness to participate. For this study, an additional exclusion criterion was absence of dual-energy x-ray absorptiometry (DEXA) data. Of 447 included patients, 330 patients (203 men, mean age 53±13 years, and mean residual GFR 7±2 ml/min per 1.73 m2) met this criteria and were considered in the analysis. As many as 99 patients had already initiated dialysis at the time of investigation. The median time between study inclusion and dialysis start was 8 days (10th percentile–90th percentile, 1–35). There were no differences in demographics, comorbidities, and nutritional status in included versus nonincluded patients (data not shown). The causes of CKD were diabetic nephropathy (28%), chronic GN (27%), polycystic kidney disease (13%), nephrosclerosis (6%), interstitial nephritis (2%), and other or unknown causes (24%). Diabetes mellitus was present in 31% of patients, whereas a history of cardiovascular disease (CVD; defined as cardiac, cerebrovascular, or peripheral vascular disease) was present in 36% of patients. Most patients were taking antihypertensive medications and drugs commonly used in ESRD, such as phosphate and calcium binders, diuretics, and vitamins. All patients were prospectively followed-up for ≤48 months or until death, kidney transplantation, or July 1, 2011, whichever event occurred first. The ethics committee of the Karolinska Institute approved the study protocol, and informed consent was obtained from each individual.
Biochemical Methods
Plasma and serum were extracted after an overnight fast and kept frozen at –70°C if not analyzed immediately. High-sensitivity C-reactive protein (CRP) was measured by nephelometry. IL-6 was analyzed by ELISA (Roche Diagnostics GmbH, Penzberg, Germany). TNF was analyzed at an Immulite system (Diagnostic Products Corp, Los Angeles, CA). Serum leptin levels were measured by an RIA kit (Linco Research Inc, Saint Charles, MO). The remaining biochemical analyses were performed using routine methods at the Department of Clinical Chemistry at Karolinska University Hospital at Huddinge. Residual GFR was calculated by the mean of renal urea and creatinine clearances from a 24-hour urine collection.
Body Composition and Nutritional Status Assessment
All patients underwent assessment of body composition and nutritional status, either at the time of or within 1 week of blood sampling. Body composition was assessed by means of DEXA (Lunar Corp, Madison, WI), estimating lean body mass and fat body mass. DEXA was performed within 7 days from study inclusion and after the dialysis session if dialysis was started. The lean body mass index (LBMI) and fat body mass index (FBMI) were calculated in kilograms per meters squared (23). Appendicular skeletal muscle mass was defined as the sum of the lean soft-tissue DEXA-estimated masses for the arms and legs (24). The appendicular skeletal muscle index (ASMI) was calculated as follows: ASMI=Appendicular Skeletal Muscle Mass/Height2, and is expressed in kilograms per meters squared. Midarm circumference was measured with a plastic tape measure. Midarm muscle circumference (MAMC) was calculated by the following: MAMC=Midarm Circumference−(3.1416×Triceps Skinfold Thickness/10). Handgrip strength was measured using a Harpenden Handgrip Dynamometer (Yamar, Jackson, MI) in the dominant hand or in the nonfistula hand if implanted. Each measurement was repeated three times, and the highest value was noted. Nutritional status was estimated by the subjective global assessment. PEW was defined as an subjective global assessment score>1 (25). Self-reported physical activity was recorded with the following predefined categories: performance of regular exercise, ability to perform a normal physical activity, and poor physical activity.
Definition of Sarcopenia
Presence of sarcopenia was defined from the criteria postulated by the European Working Group on Sarcopenia in Older People (7). ASMI cutoffs for low muscle mass were defined as values 2 SDs below the sex-specific mean from a young reference population (7.3 kg/m2 in men and 5.5 kg/m2 in women) (26). Handgrip strength cutoffs for low muscle strength were also defined from young reference populations (<30 kg in men and <20 kg in women) (27). Patients that fulfilled both criteria (i.e., presented concurrent low levels of muscle mass and strength) were considered sarcopenic.
Statistical Analyses
All values are expressed as the mean±SD, median (10th percentile–90th percentile), or percentage, as appropriate. A P value <0.05 was considered statistically significant. Comparisons between two groups were performed by using Wilcoxon’s rank-sum test, Fischer’s exact test, or the chi-squared test. Logistic regression models were performed to study multivariate correlates of variables assessed. A two-factor multivariate ANOVA with Wilks’ λ was used to measure the degree of correlation between the variables. The model included a test for the effect of order and tested interactions for the combined effect of low muscle mass and strength on patients’ phenotype. The general linear model procedure with least–squares means was used to identify significant interactions between factors. A chi-squared test was used for categorical variables. Survival analysis was made with the Kaplan–Meier survival curve and the Cox proportional hazard model. We included plausible clinical and biochemical confounders as multivariable covariates in the association of interest. Cox regression analyses are presented as hazard ratios with 95% confidence intervals. Biologic interactions between muscle mass and strength on mortality prediction were considered as a departure from causal additivity of effects as assessed by the relative excess risk due to interaction, the synergy index, and the attributable proportion due to interaction (28). Statistical analyses were performed using SAS software (version 9.3; SAS, Cary, NC).
Results
Baseline Characteristics
The mean age was 53±13 years, with 23% of patients aged >65 years. In addition, 203 participants (62%) were men, and the mean GFR was 7±2 ml/min per 1.73 m2. There was a positive association between handgrip strength and appendicular muscle mass (ρ=0.28; P=0.001). In adjusted logistic regression models (including age, sex, diabetes, CVD, GFR, PEW, and CRP), every SD reduction in muscle mass associated with 13% lower odds of muscle weakness (low muscle strength; odds ratio, 0.13; 95% confidence interval, 0.03 to 0.61; P=0.01; pseudo r2=0.23).
Table 1 shows patient characteristics according to the presence/absence of either low appendicular muscle mass or low muscle strength. Compared with patients with appropriate muscle stores, those with low muscle mass were older and more often men. Patients with low muscle mass more often had CVD, signs of PEW, and a nonsignificant trend toward higher diabetic comorbidity. Low muscle mass associated with lower body mass index (BMI), lower FBMI, and lower surrogates of muscle mass. In addition, a trend toward higher CRP and lower albumin, hemoglobin, and ferritin was also observed.
Table 1.
Clinical characteristics of incident dialysis patients (N=330) according to the presence of appropriate or low appendicular muscle mass and appropriate or low muscle strength, respectively
| Characteristic | Muscle Mass (N=330) | Muscle Strength (N=330) | ||||
|---|---|---|---|---|---|---|
| Appropriate | Low | P Value | Appropriate | Low | P Value | |
| Patients, n (%) | 184 (56) | 146 (44) | 212 (64) | 118 (36) | ||
| Age, yr | 53 (33–67) | 58 (35–68) | 0.003 | 51 (32–67) | 61 (44–69) | <0.001 |
| Men | 57 | 68 | 0.03 | 63 | 58 | 0.41 |
| Diabetes mellitus | 35 | 26 | 0.07 | 22 | 43 | 0.002 |
| CVD | 44 | 41 | 0.03 | 24 | 55 | <0.001 |
| PEW | 19 | 43 | <0.001 | 16 | 52 | <0.001 |
| BMI, kg/m2 | 25.8 (21.5–31.7) | 22.1 (18.8–27.0) | <0.001 | 24.1 (20.2–30.0) | 23.3 (18.5–30.8) | 0.29 |
| DEXA-LBMI, kg/m2 | 17.6 (14.4–20.5) | 15.4 (13.0–17.6) | <0.001 | 16.8 (14.0–19.8) | 15.8 (13.1–19.2) | 0.002 |
| DEXA-FBMI, kg/m2 | 7.5 (3.8–12.8) | 6.0 (2.9–10.2) | <0.001 | 6.7 (3.6–11.5) | 6.9 (3.2–11.7) | 0.34 |
| MAMC, cm | 15.5 (7.4–31.5) | 13.7 (6.1–27.2) | 0.04 | 14.8 (7.4–30.5) | 14.6 (6.2–28.5) | 0.31 |
| CO2, mmol/L | 23 (19–29) | 25 (19–30) | 0.12 | 24 (19–29) | 24 (20–30) | 0.29 |
| Serum albumin, g/dl | 3.3 (2.6–4.0) | 3.4 (2.6–4.1) | 0.06 | 3.4 (2.7–4.1) | 3.3 (2.5–3.9) | 0.02 |
| Serum creatinine, mg/dl | 8.1 (5.3–11.7) | 6.9 (4.2–10.4) | <0.001 | 8.3 (5.6–11.7) | 6.46 (4.17–10.0) | <0.001 |
| GFR, ml/min per 1.73 m2 | 7 (4–9) | 6 (4–10) | 0.80 | 7 (5–9) | 6 (4–9) | 0.27 |
| Leptin, ng/ml (n=285) | 10.0 (2.6–78.0) | 9.8 (2.6–47.8) | 0.34 | 9.0 (2.4–72.9) | 11.5 (3.6–54.0) | 0.30 |
| Cholesterol, mg/dl | 189 (116–278) | 197 (135–293) | 0.13 | 189 (123–278) | 197 (123–274) | 0.27 |
| Hemoglobin, g/L | 103 (86–121) | 108 (89–127) | 0.01 | 106 (88–123) | 104 (85–126) | 0.29 |
| Ferritin, ng/ml (n=323) | 332 (98–690) | 227 (66–715) | 0.02 | 299 (93–715) | 274 (54–684) | 0.53 |
| ACEIs/ARBs | 61 | 39 | 0.04 | 68 | 32 | 0.07 |
| PO4− binders | 57 | 43 | 0.55 | 66 | 34 | 0.28 |
| Ca2+ blockers | 57 | 43 | 0.57 | 67 | 33 | 0.24 |
| Statins | 57 | 43 | 0.89 | 62 | 38 | 0.68 |
| hsCRP, mg/L | 3.9 (0.7–25.5) | 6.8 (0.7–41) | 0.05 | 3.9 (0.6–25.2) | 6.7 (1.0–35.6) | 0.002 |
| IL-6, pg/ml (n=317) | 7.3 (2.1–52) | 7.1 (2.2–59) | 0.65 | 6.8 (2.1–50) | 9.7 (3.0–78.6) | 0.002 |
| TNF, pg/ml (n=271) | 9.8 (6.5–18.4) | 10.3 (6.6–17.9) | 0.72 | 9.7 (6.3–15.6) | 10.5 (7.2–23.9) | 0.02 |
Categorical data are shown as n (%) or %, whereas continuous data as presented as the median (10th percentile–90th percentile). CVD, cardiovascular disease; PEW, protein-energy wasting; BMI, body mass index; DEXA-LBMI, dual-energy x-ray absorptiometry total lean body mass index; DEXA-FBMI, dual-energy x-ray absorptiometry total fat body mass index; MAMC, midarm muscle circumference; ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; hsCRP, high-sensitivity C-reactive protein.
Compared with patients with appropriate muscle strength, those with low strength were older and had more comorbidities. Although BMI, FBMI, and MAMC did not differ, LBMI and serum creatinine were significantly lower in patients with low muscle strength. Three inflammatory mediators were higher, and serum albumin levels were significantly lower.
When both conditions were crosscombined, 20% of the patients were considered sarcopenic. One fourth of patients (24%) had low muscle mass but appropriate muscle strength, and 15% presented low muscle strength but had appropriate muscle mass. The remaining 41% of patients had both appropriate muscle mass and appropriate muscle strength. A multivariate logistic regression analysis (Table 2) shows that sarcopenia associated mainly with old age, low albumin, and the presence of PEW. Including IL-6 instead of CRP did not result in a statistically significant contribution to the model (data not shown).
Table 2.
ORs and 95% CIs for the presence of sarcopenia in 330 incident dialysis patients
| Parameter | OR (95% CI) | P |
|---|---|---|
| Intercept | 0.001 | |
| Age>60 yr | 2.85 (1.42 to 5.74) | 0.003 |
| Men | 1.56 (0.76 to 3.18) | 0.22 |
| Diabetes mellitus, presence | 1.05 (0.51 to 2.19) | 0.89 |
| CVD, presence | 1.48 (0.72 to 3.05) | 0.29 |
| GFR<7 ml/min/1.73 m2 | 1.40 (0.71 to 2.74) | 0.33 |
| Inflammation, CRP>10 mg/L | 1.40 (0.65 to 2.99) | 0.39 |
| Serum albumin>3.3 g/dl | 0.46 (0.21 to 0.98) | 0.04 |
| PEW, SGA>1 | 5.66 (2.81 to 11.4) | 0.001 |
Pseudo r2=0.20. eGFR and albumin were categorized according to median value in the study group. PEW was estimated by means of SGA. OR, odds ratio; 95% CI, 95% confidence interval; CRP, C-reactive protein; SGA, subjective global assessment.
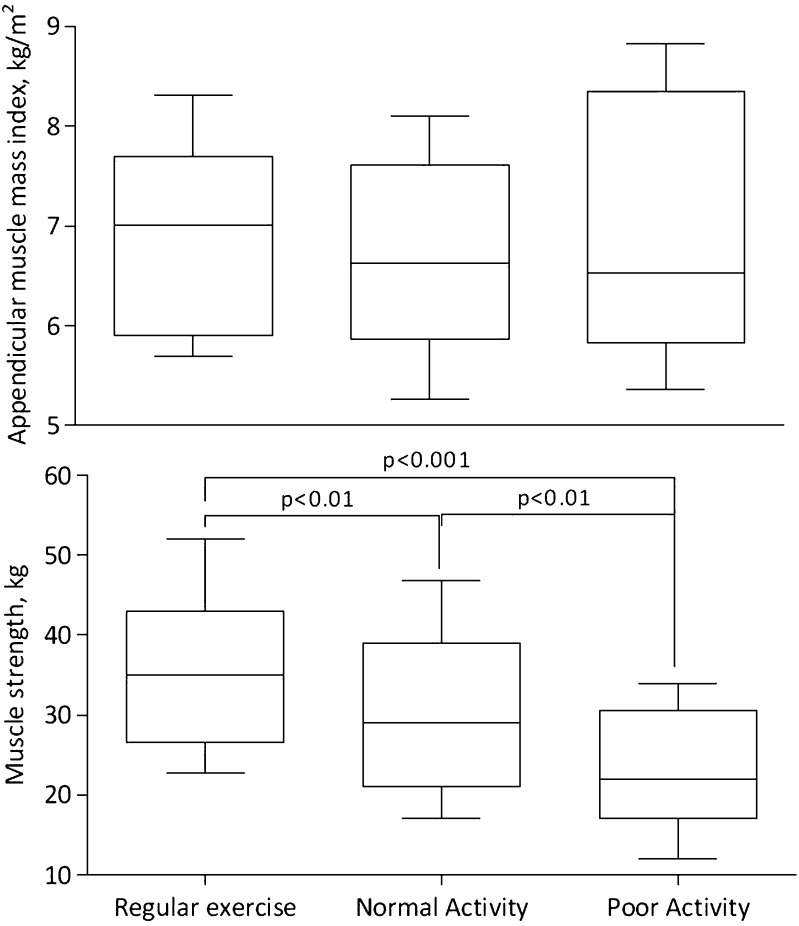
Table 3 shows patient characteristics according to the crosscombination of muscle mass and muscle strength categories. Differences in albumin, creatinine, hemoglobin, BMI, FBMI, and LBMI were associated with the low muscle mass component. Differences in age, albumin, creatinine, and inflammatory biomarkers were attributed to the effect of low muscle strength. The proportion of PEW, diabetes, and CVD was higher in patient groups with low muscle strength. Data on self-reported physical activity were available for 259 patients: 67 (26%) reported doing regular exercise, 151 (58%) reported being capable of performing normal activities, and 41 (16%) reported poor physical activity. Although there was no relation between muscle mass and physical activity level, muscle strength markedly differed among groups (Figure 1).
Table 3.
Clinical characteristics of incident dialysis patients according to the crosscombination of muscle mass and muscle strength categories
| Characteristic | Appropriate Muscle Massa | Low Muscle Massb | Multivariate ANOVAc | Chi-Square Testb | ||||
|---|---|---|---|---|---|---|---|---|
| Appropriate Muscle Strength | Low Muscle Strength | Appropriate Muscle Strength | Low Muscle Strength | Muscle Effect | Strength Effect | Interaction Term | ||
| Patients, n (%) | 134 (41) | 50 (15) | 78 (24) | 68 (20) | ||||
| Age, yr | 49 (32–66) | 60 (44–70) | 54 (32–68) | 63 (42–69) | 0.29 | <0.001 | 0.51 | |
| GFR, ml/min per 1.73 m2 | 7 (4–9) | 7 (3–9) | 7 (5–10) | 6 (4–10) | 0.43 | 0.11 | 0.75 | |
| BMI, kg/m2 | 25.2 (21.5–31.4) | 26.8 (19.2–32.6) | 22.1 (19.3–27.2) | 22.5 (18.1–26.4) | <0.001 | 0.46 | 0.11 | |
| DEXA-LBMI, kg/m2 | 17.6 (14.7–20.5) | 17.5 (14.4–21.0) | 15.9 (13.2–17.4) | 15.0 (12.6–17.8) | <0.001 | 0.13 | 0.14 | |
| DEXA-FBMI, kg/m2 | 7.1 (3.8–11.8) | 8.2 (3.2–14.2) | 5.8 (3.0–10.4) | 6.5 (3.2–9.8) | <0.001 | 0.19 | 0.20 | |
| MAMC, cm | 16.3 (7.4–31.0) | 15.2 (6.9–34.5) | 12.7 (6.1–28.5) | 14.2 (6.0–22.2) | 0.02 | 0.66 | 0.89 | |
| CO2, mmol/L | 23 (19–28) | 24 (19–32) | 24 (19–31) | 25 (20–29) | 0.44 | 0.36 | 0.15 | |
| Serum albumin, g/dl | 3.4 (2.7–4.1) | 3.2 (2.4–3.8) | 3.5 (2.7–4.2) | 3.4 (2.6–4.1) | 0.03 | 0.01 | 0.76 | |
| Serum creatinine, mg/dl | 8.7 (5.7–13.0) | 6.4 (4.2–10.6) | 7.6 (5.1–11.0) | 6.5 (4.0–9.6) | <0.001 | <0.001 | 0.58 | |
| Leptin, ng/ml (n=285) | 9.9 (2.4–78.0) | 11.1 (3.6–83.0) | 8 (2.5–47.5) | 11.5 (3.8–47.8) | 0.08 | 0.74 | 0.97 | |
| Cholesterol, mg/dl | 185 (116–278) | 201 (116–274) | 197 (135–282) | 193 (131–301) | 0.12 | 0.78 | 0.47 | |
| Hemoglobin, g/L | 103 (87–121) | 100 (84–126) | 111 (91–130) | 105 (87–126) | 0.01 | 0.16 | 0.38 | |
| Ferritin, ng/ml (n=323) | 324 (103–714) | 294 (51–648) | 224 (79–746) | 249 (58–703) | 0.37 | 0.98 | 0.67 | |
| hsCRP, mg/L | 3.5 (0.6–23) | 4.7 (1.2–32) | 4.5 (0.6–47) | 8.5 (0.9–38) | 0.26 | 0.04 | 0.33 | |
| IL-6, pg/ml (n=317) | 7.2 (2.1–50.6) | 10.7 (2.5–108) | 6.3 (2.1–57.2) | 9.4 (3.0–57.5) | 0.89 | 0.41 | 0.18 | |
| TNF, pg/ml (n=271) | 9.6 (6.3–15.8) | 11 (7.2–26.6) | 9.8 (5.3–15.6) | 10.4 (7.2–21) | 0.13 | 0.01 | 0.06 | |
| Men, % | 42 | 53 | 29 | 33 | 0.03 | |||
| Diabetes mellitus, % | 25.4 | 54.0 | 17.9 | 35.3 | <0.001 | |||
| CVD, % | 23.1 | 53.1 | 26.9 | 55.9 | <0.001 | |||
| PEW, % | 14.3 | 34.7 | 19.7 | 64.7 | <0.001 | |||
| ACEI/ARBs, % | 75 | 25 | 57 | 43 | 0.19 | |||
| PO4− binders, % | 75 | 25 | 54 | 46 | 0.12 | |||
| Ca2+ blockers, % | 77 | 23 | 54 | 46 | 0.89 | |||
| Statins, % | 72 | 28 | 48 | 52 | 0.20 | |||
Categorical data are shown as n (%) or %, whereas continuous data as presented as the median (10th percentile–90th percentile). DEXA-LBMI, dual-energy x-ray absorptiometry total lean body mass index; DEXA-FBMI, dual-energy x-ray absorptiometry total fat body mass index; ; hsCRP, high-sensitivity C-reactive protein; ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; hsCRP, high-sensitivity C-reactive protein.
For appropriate muscle mass, n=184 (56%).
For low muscle mass, n=146 (44%).
Two-factor multivariate ANOVA showing the effect (P value) attributed to the component of muscle strength, muscle mass, or the interaction term strength × mass.
For categorical values of sex through statin use, multivariate ANOVA cannot be applied and differences across the four categories were assessed by the chi-squared test.
Figure 1.
Physical activity associates with muscle strength rather than with muscle mass. The figure shows appendicular muscle mass index (upper panel) and muscle strength (lower panel) in relation to self-reported physical activity (n=259). Whiskers represent the 10th and 90th percentiles.
Associations with Mortality
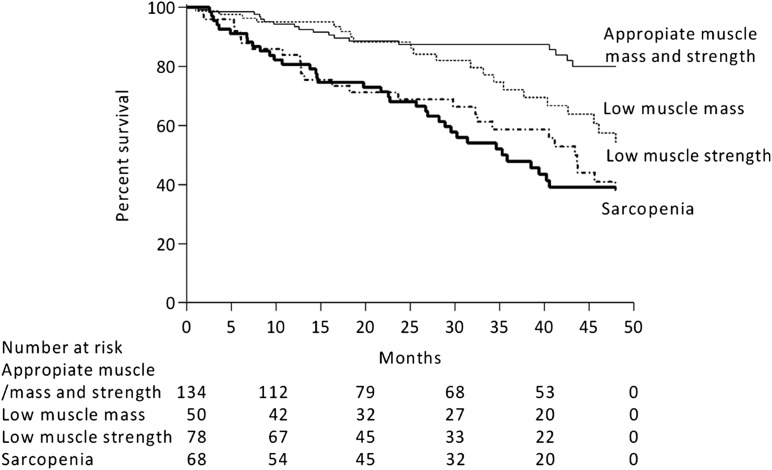
During a median follow-up of 29 months (10th percentile–90th percentile, 1–48), 95 patients (29%) died. Cox proportional hazard models using low muscle mass or low muscle strength as separate entities are shown in Table 4. When considered as continuous variables, muscle mass and strength inversely associated with mortality. When considered as categories against young reference populations, both low muscle mass and strength associated with higher mortality risk in crude analysis, but minimal adjustment rendered these associations nonstatistically significant for the case of low muscle mass. On the other hand, the association between low muscle strength and higher mortality risk remained strong in fully adjusted models. The mortality association of these muscle dysfunction entities combined is presented in Figure 2 and Table 5. When addressing the continuous associations between muscle strength and mass with mortality in the same model (Table 5), only the risk-associated muscle strength remained statistically significant. When categorizing patients according to young reference population cutoffs (Table 5), individuals with low muscle mass alone did not exhibit a higher risk of mortality compared with those with appropriate levels of both muscle mass and strength. Individuals with low muscle strength were at higher risk of death, irrespective of their muscle mass stores being appropriate or not. Interaction tests showed no departure from additivity. For model 2, the synergy index was 0.39 (−0.07, 2.1), the relative excess risk due to interaction was −0.69 (−2.18, 0.80), and the attributable proportion due to interaction was 47% (−0.47; −1.47, 0.52).
Table 4.
HRs and 95% CIs for all-cause mortality in relation to appendicular muscle mass or strength, without taking into consideration both entities together
| Exposures | Model | HR (95% CI) | P Value |
|---|---|---|---|
| As continuous variables in separate models | |||
| Muscle mass, per SD increase (N=330) | 1 | 0.25 (0.08 to 0.78) | 0.02 |
| 2 | 0.21 (0.06 to 0.73) | 0.01 | |
| Muscle strength, per SD increase (N=330) | 1 | 0.25 (0.08 to 0.74) | 0.01 |
| 2 | 0.32 (0.18 to 0.57) | 0.001 | |
| As categories, in separate models, according to young reference populations | |||
| Low muscle mass (n=146) versus appropriate (n=184) | 1 | 1.46 (0.99 to 2.15) | 0.05 |
| 2 | 1.17 (0.73 to 1.87) | 0.51 | |
| Low muscle strength (n=118) versus appropriate (n=212) | 1 | 2.54 (1.64 to 3.94) | <0.001 |
| 2 | 1.79 (1.09 to 2.94) | 0.02 |
Model 1 includes minimal adjustment for age and sex. Model 2 includes adjustment for age, sex, diabetes, CVD, cholesterol, hemoglobin, GFR, and hsCRP. HR, hazard ratio, 95% CI, 95% confidence interval.
Figure 2.
Survival rate of incident dialysis patients stratified according to the presence of appropriate muscle mass and strength, low muscle mass alone, low muscle strength alone, or both (sarcopenia).
Table 5.
HRs and 95% CIs associated with muscle strength and mass together as continuous variables and after categorization regarding the presence of low muscle mass alone, low muscle strength alone, or both in incident dialysis patients
| Exposures | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Together as continuous variables in the same model | ||||
| Muscle mass, per SD increase | 0.58 (0.20 to 1.70) | 0.32 | 0.43 (0.12 to 1.52) | 0.19 |
| Muscle strength, per SD increase | 0.26 (0.16 to 0.43) | <0.001 | 0.34 (0.18 to 0.61) | <0.001 |
| Crosscombined as categories according to young reference populations | ||||
| Group 1: Appropriate muscle mass and strength (n=134) | 1.00 | 1.00 | ||
| Group 2: Low muscle strength alone (n=50) | 2.82 (1.57 to 5.21) | 0.001 | 1.98 (1.01 to 3.87) | 0.04 |
| Group 3: Low muscle mass alone (n=78) | 1.35 (0.67 to 2.68) | 0.39 | 1.23 (0.56 to 2.67) | 0.59 |
| Group 4: Low muscle mass and strength (sarcopenia) (n=68) | 2.94 (1.64 to 5.27) | <0.001 | 1.93 (1.01 to 3.71) | 0.04 |
Model 1 includes minimal adjustment for age and sex. Model 2 includes adjustment for age, sex, diabetes, CVD, cholesterol, hemoglobin, GFR, and hsCRP. HR, hazard ratio; 95% CI, 95% confidence interval; hsCRP, high-sensitivity C-reactive protein.
Discussion
Sarcopenia, as defined by recent consensus (5–7), refers to the progressive and generalized loss of muscle mass and strength/function that occurs with aging. Sarcopenia is considered an important geriatric condition and a key precursor to the development of frailty, being a powerful predictor of late-life disability, worse quality of life, and higher mortality rate in elderly individuals (5–8,29). In our study, sarcopenia was present in 20% of incident dialysis patients. This prevalence is in line with a previous report in CKD stage≥3 (16) and prevalent hemodialysis patients (17). It has been proposed that advanced CKD is a condition associated with a process of “accelerated aging” (11,12) and that the effect of various prevalent CKD-associated catabolic alterations may explain why sarcopenia is such a prominent typical feature in CKD (14). Although a lack of control group in our study does not allow us to make solid conclusions, the observed prevalence is within the range reported in geriatric populations (8–10).
The evidence that both muscle mass and strength are important predictors of outcome in dialysis patients is solid (2,3). Likewise, in our study, both low muscle mass and strength were strong mortality predictors when considered alone. We here attempted to disentangle the concurrent contributions of these two entities. In our study, muscle strength showed a stronger association with mortality than muscle mass, and patients with low muscle strength were more likely to die, irrespective of their concomitant appendicular muscle stores. This should not be understood as a lack of importance for muscle mass over function. Indeed, the magnitude of risk associated with low muscle mass was still large, albeit not significant. Clark and Manini (18) classically argue that muscle strength and stores are two different entities to target. Data suggest that loss of muscle mass only marginally contributes to explain the loss of muscle strength (30). In addition, gradual muscle strength loss is longitudinally associated with both mortality and physical disability even when adjusting for muscle mass (31–34), altogether suggesting that low muscle mass may be secondary to the effects of low muscle strength (35). Such findings are consistent with the physiologic underpinnings of muscle strength, explained not only by alterations in muscle quantity but also by contractile quality, neural activation, and systemic inflammation (36–38). Supporting this, important differences in our patients’ phenotype (e.g., differences in age, comorbidities, presence of PEW, inflammation, and self-reported physical activity) were associated with low muscle strength rather than with low muscle mass (Figure 2, Table 3). It is noteworthy that inflammation was a prominent feature of reduced muscle strength but was little affected by muscle mass (Tables 1 and 3). Our observations agree with the findings of Storer et al. (39), who demonstrated that endurance exercise improves muscle strength, power, and physical performance in hemodialysis patients, while systemic inflammatory markers normalize in parallel.
Although muscle mass and strength are strongly related to each other, we speculate that these entities may be affected by different risk factors and that the association between muscle mass and mortality may be partly explained by poor muscle function. Increasing muscle mass stores with anabolic agents and/or amino acid supplements has been the option considered most in CKD-associated PEW to date (40,41). Our results, however, provide support for the hypothesis that therapeutic measures specifically targeting muscle strength may also be of importance, and both approaches combined are likely to bring the best benefit for the patient. This was shown already by Johansen et al. (42), who tested the sole or combined approach of anabolic agents and endurance exercise training in dialysis patients for 12 weeks. The investigators reported that although exercise alone could marginally improve muscle mass (only quadriceps muscle area was increased) and anabolic agents alone could not improve muscle strength, the combined therapeutic approach elicited a stronger effect on nutritional status and muscle mass than each treatment separately.
This study has strengths and limitations. Strengths include the use of incident consecutive dialysis patients, the adoption of robust methods like DEXA, and the use of consensus definitions and cutoffs based on reference populations. Limitations include the observational study design, a relatively low sample size, and the use of measurements at single time points. We thus consider our findings as hypothesis-generating. Because the underlying mechanisms cannot be elucidated from here, we hope that these results stimulate further research on the topic. Bias could have been introduced because DEXA estimations of fat-free mass can be influenced by hydration status (43), likely underestimating the reported associations. Several factors such as dietary intake, adipokines, and the atherosclerosis burden can influence muscle stores and muscle function, but such data were not readily available in our study. Because of the study inclusion criteria (incident, clinically stable, and aged <75 years), our results do not extrapolate to the general dialysis population. Finally, because the study spans a long time period, we cannot exclude the possibility that changes in practice patterns may have influenced the results.
In conclusion, sarcopenia was observed in 20% of adult incident dialysis patients and associated with old age and PEW. In cross-sectional analyses, low muscle strength, rather than low muscle mass, was associated with aging, comorbidities, PEW, physical inactivity, and inflammation. During follow-up, low muscle strength was more strongly associated with the risk of mortality than low muscle mass. Assessment of muscle functionality may provide additional diagnostic and prognostic information to muscle-mass evaluation. On the basis of these findings, it seems prudent to suggest that treatment options in dialysis patients should target not only maintenance or increase of muscle mass but also muscle functionality. Achieving better muscle functionality may in fact be a prodromal step toward improved physical functioning and the reduction of frailty (44).
Disclosures
B.L. is affiliated with Baxter Healthcare.
Acknowledgments
We acknowledge research grants from the Swedish Research Council. Baxter Novum is the result of a grant from Baxter Healthcare Corporation to the Karolinska Institute.
Footnotes
P.S. and J.J.C. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, Suliman ME, Lindholm B, Stenvinkel P, Qureshi AR: Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 27: 557–564, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Heimbürger O, Qureshi AR, Blaner WS, Berglund L, Stenvinkel P: Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis 36: 1213–1225, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, ter Wee P, Franch HA: Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 23: 77–90, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M: Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD, Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop : Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc 12: 403–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC: Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29: 154–159, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, Edwards M, Dennison E, Cooper C, Aihie Sayer A: Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 42: 378–384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, Cameron ID, Westendorp RG, Maier AB: Defining sarcopenia: The impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 35: 871–881, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, Pérez-Zepeda MU, Cesari M: Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 17: 259–262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenvinkel P, Larsson TE: Chronic kidney disease: A clinical model of premature aging. Am J Kidney Dis 62: 339–351, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Anand S, Johansen KL, Kurella Tamura M: Aging and chronic kidney disease: The impact on physical function and cognition. J Gerontol A Biol Sci Med Sci 69: 315–322, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JC, Kalantar-Zadeh K, Kopple JD: Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 24: 337–351, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Fahal IH: Uraemic sarcopenia: Aetiology and implications [published online ahead of print April 25, 2013]. Nephrol Dial Transplant doi:10.1093/ndt/gft070 [DOI] [PubMed] [Google Scholar]
- 15.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Foley RN, Wang C, Ishani A, Collins AJ, Murray AM: Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 27: 279–286, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW, Kim HJ, Song YR: Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr 33: 64–68, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Clark BC, Manini TM: Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci 63: 829–834, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB: The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA: Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56: B209–B217, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Clark BC, Manini TM: What is dynapenia? Nutrition 28: 495–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kyle UG, Schutz Y, Dupertuis YM, Pichard C: Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition 19: 597–604, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN, Jr: Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am J Clin Nutr 52: 214–218, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, Bergström J: Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int 53: 773–782, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD: Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L: Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol (1985) 95: 1851–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A: Calculating measures of biological interaction. Eur J Epidemiol 20: 575–579, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL: Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH, Health, Aging, and Body : Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB: Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61: 72–77, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Metter EJ, Talbot LA, Schrager M, Conwit R: Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB: Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90: 2157–2165, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Bruce SA, Newton D, Woledge RC: Effect of age on voluntary force and cross-sectional area of human adductor pollicis muscle. Q J Exp Physiol 74: 359–362, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Clark BC, Manini TM: Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care 13: 271–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark BC, Taylor JL: Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci 4: 192–199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL: Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Delbono O: Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci 4: 248–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storer TW, Casaburi R, Sawelson S, Kopple JD: Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant 20: 1429–1437, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Feldt-Rasmussen B, Lange M, Sulowicz W, Gafter U, Lai KN, Wiedemann J, Christiansen JS, El Nahas M, APCD Study Group : Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 18: 2161–2171, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Supasyndh O, Satirapoj B, Aramwit P, Viroonudomphol D, Chaiprasert A, Thanachatwej V, Vanichakarn S, Kopple JD: Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin J Am Soc Nephrol 8: 271–279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T: Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Konings CJ, Kooman JP, Schonck M, van Kreel B, Heidendal GA, Cheriex EC, van der Sande FM, Leunissen KM: Influence of fluid status on techniques used to assess body composition in peritoneal dialysis patients. Perit Dial Int 23: 184–190, 2003 [PubMed] [Google Scholar]
- 44.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]