Abstract
Background and objectives
The relative influence of facilities and regions on the timing of dialysis initiation remains unknown. The purpose of the study is to determine the variation in eGFR at dialysis initiation across dialysis facilities and geographic regions in Canada after accounting for patient-level factors (case mix).
Design, setting, participants, & measurements
In total, 33,263 dialysis patients with an eGFR measure at dialysis initiation between January of 2001 and December of 2010 representing 63 dialysis facilities and 14 geographic regions were included in the study. Multilevel models and intraclass correlation coefficients were used to evaluate the variation in timing of dialysis initiation by eGFR at the patient, facility, and geographic levels.
Results
The proportion initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 was 35.3%, varying from 20.1% to 57.2% across geographic regions and from 10% to 67% across facilities. In an unadjusted, intercept-only linear model, 90.7%, 6.6%, and 2.7% of the explained variability were attributable to patient, facility, and geography, respectively. After adjustment for patient and facility factors, 96.9% of the explained variability was attributable to patient case mix, 3.1% was attributable to the facility, and 0.0% was attributable to the geographic region. These findings were consistent when the eGFR was categorized as a binary variable (≥10.5 ml/min per 1.73 m2) or in an analysis limited to patients with >3 months of predialysis care.
Conclusions
Patient characteristics accounted for the majority of the explained variation regarding the eGFR at the initiation of dialysis. There was a small amount of variation at the facility level and no variation among geographic regions that was independent of patient- and facility-level factors.
Keywords: clinical epidemiology, epidemiology and outcomes, ESRD, GFR, dialysis
Introduction
It has been nearly four decades since the widespread availability of dialysis treatment for ESRD. However, the fundamental question of when to start dialysis to optimize survival and minimize morbidity remains unanswered. A growing body of evidence suggests that early initiation of dialysis therapy is associated with no measurable benefit, higher cost, and a potential increase in subsequent mortality (1–8).
Despite these concerns, it is clear that patients with CKD have consistently been starting dialysis at progressively higher levels of eGFR over the last two decades (9,10). These trends are concerning, and many studies have suggested that patient-related factors, such as advancing age, comorbid illness, and/or increasing fragility (case mix), may be driving earlier initiation. However, numerous regional- and facility-level factors have previously been shown to exert strong influences on dialysis-related practices, such as vascular access, dialysis adequacy, and modality selection (11–13). Local expertise, physicians opinion and knowledge, use of eGFR reporting, and reimbursement policies may all influence the initiation of dialysis at the facility or regional level. Two recent survey studies have provided considerable insight (14,15). First, a study of attitudes and opinions of physicians regarding the timing of dialysis initiation suggested that reimbursement may play a role, because physicians practicing at for-profit dialysis facilities were more likely to initiate dialysis earlier (14). Although Canada does not have for-profit dialysis facilities, physician remuneration does increase on the basis of dialysis caseload, thus possibly financially incentivizing early dialysis initiation. Second, a study presenting clinical vignettes shows that physicians were more likely to initiate dialysis earlier if the case was presented with an eGFR as opposed to a serum creatinine value (15). In Canada, eGFR reporting varies considerably by facility and geographic region. These studies suggest that nonpatient-related factors may be contributing to decision-making regarding the initiation of dialysis.
Understanding the relative contributions of patient, facility, and regional factors to the timing of dialysis initiation will aid in the development of appropriate health policy. Measures of facility variation are particularly important in this emerging era of quality improvement programs and pay-for-performance initiatives. To date, no studies have quantitated the relative variation in the timing of dialysis initiation because of regional- and facility-related factors. The purpose of this study is to determine the variation in eGFR at dialysis initiation across dialysis facilities and geographic regions in Canada after accounting for patient-level factors (case mix). We hypothesize that there will be considerable facility- and geographic-level variations across Canada.
Materials and Methods
Population and Data Sources
We studied incident dialysis patients from January of 2001 to December of 2010 captured in the Canadian Organ Replacement Registry (CORR; n=33,263). CORR is a validated registry that includes demographics, comorbidities, dialysis modality, vascular access, transplantation, and mortality on all patients with ESRD in Canada (excluding Quebec) (12,16). Data are collected by dialysis facilities and housed centrally with the Canadian Institute for Health Information. All adult patients (>18 years of age) with a serum creatinine at dialysis initiation were included in our analysis (>93%). Regional ethics board approval was obtained.
Cohort Definitions
Renal function was estimated using the Modification of Diet in Renal Disease equation on the basis of a patient’s blood-sample serum creatinine collected immediately before their first dialysis session. The eGFR was examined as a continuous variable. First visit date with a nephrologist was used to estimate the length of predialysis nephrologist care. Comorbidities (angina, chronic obstructive pulmonary disease, diabetes, malignancy, serious illness, hypertension, lung disease, coronary artery bypass grafting, pulmonary edema, peripheral vascular disease, stroke, cigarette smoking, and acute coronary syndrome) were recorded at the initiation of dialysis. Race was self-reported. Distance to facility was calculated as the direct linear distance in kilometers between a patient's primary residence (estimated from postal code at the time of dialysis initiation) to the nearest dialysis provider using the Vincenty Equation (12). Individual patients and dialysis facilities were de-identified for analytic purposes. Additional laboratory values (hemoglobin, albumin, and phosphate) were recorded in cross-section at dialysis initiation.
Facility-level variables were created to account for differences in care across centers and selected on the basis of clinical relevance, known association with outcomes, and quality of care indicators (13–15,17). The majority of centers (87.3%) provided data for the entire 10-year study period. Variables included the proportion of patients who received dialysis through a central venous catheter, whether the facility offered renal transplantation or peritoneal dialysis, the mean hemoglobin and phosphate of patients at dialysis initiation, the average distance in kilometers between a patient’s primary residence and the nearest dialysis facility, and the center size. Information on patients’ geographic regions was included and categorized as Atlantic (Newfoundland, New Brunswick, Prince Edward Island, and Nova Scotia), Ontario (North, Greater Toronto, East, and Western regions), Prairies (Manitoba, Saskatchewan, and Alberta [North and South regions]), and Pacific (Eastern British Columbia, Vancouver, and Other British Columbia regions).
Statistical Analyses
Patient, facility, and geographic characteristics were compared between patients who were started on dialysis with an eGFR>10.5 or <10.5 ml/min per 1.73 m2. This cutoff was on the basis of data from the Canada USA study, which helped to establish a recommended peritoneal dialysis target for weekly Kt/V urea of 2, translating roughly to an eGFR of 10.5 ml/min per 1.73 m2 (18). Continuous variables of interest were summarized as means or medians with SDs or interquartile ranges as appropriate. Differences in characteristics were determined by the t or the Mann–Whitney test for continuous variables and the chi-squared or the Fisher exact test for dichotomous variables.
Facility and geographic variations were examined using multilevel modeling with patients nested within facilities and facilities nested within geographic regions. A three-level linear regression model was used to assess variables associated with the eGFR at dialysis initiation. Models were adjusted for factors thought to potentially influence decisions to initiate dialysis, including facility-level factors (percentage catheter use, transplantation facility, peritoneal dialysis facility, average hemoglobin and phosphate, average distance that a patient resided from the nearest dialysis center, and number of patients), patient case mix (age, sex, body mass index, race, comorbidities, distance to facility, length of predialysis care, serum phosphate, albumin, and hemoglobin), and calendar year. Unadjusted, fully adjusted, and reduced models were created. Covariates for inclusion in our models were selected a priori, and covariates selected to be included in the reduced models were on the basis of (1) a P value <0.01 in the full model and (2) a P value <0.05 in the reduced model. Patient, facility, and geographic variations were determined by the intraclass correlation coefficient (ICC) (19). The ICCs were calculated by dividing the variance estimate at each level by the total model variance. In our study, the ICC determines the proportion of explained variation in the eGFR at dialysis initiation that is caused by being a member of a particular group, such as patient, facility, and geographic region, and is reported as a percentage. Facility-level variables were centered for the facility averages (20). R2 to determine the percentage of variation explained at each level for the full and reduced models was determined by the method by Raudenbush and Bryk (21), whereas the R2 for the total model was determined by the method by Snijders and Bosker (22). Sensitivity analyses were performed to ensure the validity of our findings. A three-level logistic regression model was created examining the eGFR≥10.5 ml/min per 1.73 m2. In a separate sensitivity analysis, an eGFR cutoff of >12 ml/min per 1.73 m2 was used. We used the SAS GLIMMIX procedure (SAS 9.2) using a logit link and the latent variable approach at the patient level (20). In this model, the ICCs were calculated for the facility and geographic regions by assuming a patient-level variance of π2/3 (23,24). Because emergent dialysis starts are not categorized separately in CORR, we also repeated our analyses by limiting our cohort to individuals who had received nephrology predialysis care for >90 days before dialysis initiation. To interrogate the patient-level variance assumption of π2/3, the ICC was further calculated using a probit link assuming a patient level variance=1. Because patients initiating peritoneal dialysis may start at higher levels of eGFR, we repeated our analyses limited to patients initiating hemodialysis only (25). Furthermore, to examine the effect of reducing the eGFR level at dialysis initiation, we excluded the top 25th percentile of facilities with the highest mean eGFR level at dialysis initiation and repeated our models.
Multiple imputation was used for missing values, with a random draw from the predictive distribution of an imputation model repeated 10 times (26). Analyses were performed using SAS, version 9.2. All hypothesis tests were two-sided, with statistical significance determined at a P value <0.05.
Results
Patient/Regional Characteristics and eGFR
The baseline patient-level (n=33,263), facility-level (n=63), and regional-level (n=14) characteristics are presented in Table 1 in relation to eGFR>10.5 or <10.5 ml/min per 1.73 m2. Patients who initiated dialysis at an eGFR≥10.5 ml/min per 1.73 m2, on average, were older, were women, were on longer predialysis care, and had more comorbid illness. They were more likely to have higher hemoglobin, have lower serum phosphate, and receive peritoneal dialysis as their initial modality. Facilities that started more patients at eGFR>10.5 ml/min per 1.73 m2 were more likely to offer peritoneal dialysis, had higher average hemoglobin, had lower serum phosphate, and had fewer total numbers of patients. Pacific regions of the country had a higher proportion of patients initiating with an eGFR≥10.5 ml/min per 1.73 m2, whereas the central region (Prairies) had the lowest.
Table 1.
Baseline patient, facility, and geographic characteristics of the study cohort according to individuals who initiated dialysis with an eGFR≥10.5 or <10.5 ml/min per 1.73 m2
| Characteristic | eGFR<10.5 | eGFR≥10.5 | P Value |
|---|---|---|---|
| N | 21,533 (64.7%) | 11,730 (35.3%) | |
| Median eGFR (intraquartile range) | 7.4 (5.8–8.8) | 13.2 (11.7–15.9) | <0.001 |
| Patient characteristics | |||
| Age | 62.9±15.2 | 66.6±14.5 | <0.001 |
| Sex (% men) | 60.4 | 39.6 | <0.001 |
| Body mass index | 27.6±6.4 | 27.5±6.6 | 0.02 |
| Race | <0.001 | ||
| Caucasian | 69.0 | 75.3 | |
| East Asian | 7.5 | 4.8 | |
| Aboriginal | 7.3 | 4.4 | |
| South Asian | 4.2 | 4.0 | |
| Black | 3.2 | 3.0 | |
| Other | 3.9 | 2.4 | |
| Unknown | 4.8 | 6.2 | |
| Median number of days with predialysis care (intraquartile range) | 386 (41–1159) | 458 (101–1205) | <0.001 |
| Median number of days with >90 d of predialysis care (intraquartile range) | 779 (345–1567) | 752 (335–1525) | 0.09 |
| Predialysis care>30 d (%) | 76.9 | 83.5 | <0.001 |
| Comorbidities (%) | |||
| Angina | 20.2 | 28.8 | <0.001 |
| Acute coronary syndrome | 19.7 | 29.7 | <0.001 |
| Pulmonary edema | 23.9 | 32.3 | <0.001 |
| Diabetes mellitus | 45.2 | 52.5 | <0.001 |
| Stroke | 13.6 | 18.3 | <0.001 |
| Peripheral vascular disease | 17.6 | 26.4 | <0.001 |
| Malignancy | 13.1 | 14.3 | 0.002 |
| Lung disease | 10.7 | 14.8 | <0.001 |
| Hypertension medications | 86.4 | 86.4 | 0.92 |
| Current smoker | 15.3 | 15.3 | 0.87 |
| Coronary artery bypass graft | 11.8 | 20.6 | <0.001 |
| Serious illness | 12.1 | 16.4 | <0.001 |
| Number of comorbidities | 2.9±1.8 | 3.5±2.0 | <0.001 |
| Cause of ESRD (%) | <0.001 | ||
| Hypertension | 19.0 | 23.4 | |
| Diabetes mellitus | 35.7 | 40.3 | |
| GN | 17.0 | 10.2 | |
| Obstruction | 2.7 | 2.2 | |
| Interstitial | 2.8 | 2.5 | |
| Polycystic kidney disease | 5.1 | 2.8 | |
| Other | 9.6 | 10.2 | |
| Unknown | 8.1 | 8.3 | |
| Hemoglobin, g/L | 100.2±17.5 | 105.5±16.8 | <0.001 |
| Phosphate, mmol/L | 2.10±0.69 | 1.61±0.46 | <0.001 |
| Albumin, g/L | 29.7±8.66 | 27.0±8.1 | <0.001 |
| Distance from facility (km) | <0.001 | ||
| <50 | 73.4 | 78.1 | |
| 50–150 | 16.8 | 14.3 | |
| >150 | 9.8 | 7.6 | |
| Peritoneal dialysis | 20.1 | 24.2 | <0.001 |
| Facility characteristicsa (n=63) | |||
| Arteriovenous fistula at initiation of dialysis (%) | 16.9±7.4 | 16.9±7.1 | 0.89 |
| Transplant facility | 39.7 | 32.8 | <0.001 |
| Peritoneal dialysis facility | 93.8 | 95.0 | <0.001 |
| Average hemoglobin (g/L) | 101.7±3.4 | 102.9±3.5 | <0.001 |
| Average phosphate (mmol/L) | 1.95±0.13 | 1.89±0.13 | <0.001 |
| Geographic regions (%) | <0.001 | ||
| Atlantic | 10.7 | 9.0 | |
| Ontario | 49.2 | 49.8 | |
| Prairies | 25.7 | 19.3 | |
| Pacific | 14.4 | 21.9 |
Atlantic provinces include Prince Edward Island, Nova Scotia, New Brunswick, and Newfoundland. Prairies include Manitoba, Saskatchewan, and Alberta. Pacific includes British Columbia. Continuous variables are presented as the mean ± SD.
Facilities were categorized in <10.5 or ≥10.5 ml/min per 1.73 m2 on the basis of the mean eGFR at dialysis initiation of their total incident patient population.
eGFR at Dialysis Initiation across Dialysis Facilities and Geographic Regions
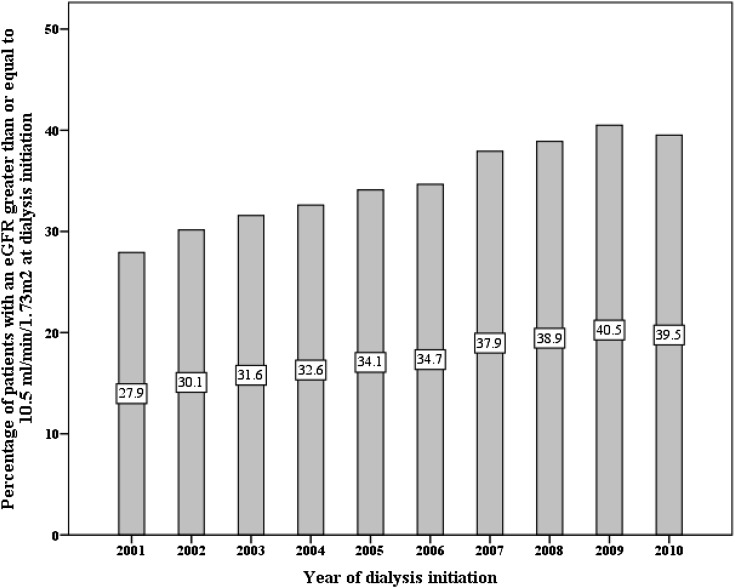
Overall, the mean eGFR at dialysis initiation was 9.7±4.4 ml/min per 1.73 m2, and this value increased annually from 9.1±4.1 ml/min per 1.73 m2 in 2001 to 10.1±4.5 ml/min per 1.73 m2 in 2010. The mean eGFR across geographic regions ranged from a low in Manitoba (8.2±3.8 ml/min per 1.73 m2) to a high in Other British Columbia (11.9±4.6 ml/min per 1.73 m2). Overall, the proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 was 35.3%, and this value increased annually from 27.9% in 2001 to 39.5% in 2010. There was a large difference among geographic regions in the proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2, with the lowest proportion in Manitoba (20.1%) and the highest proportion in Other British Columbia (57.2%) (Figures 1 and 2).
Figure 1.
Crude proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 according to geographic region. N values in each region are MB (2027), North ALB (2582), NFLD/NB/NS/PEI (3338), Toronto (3818), North ON (1630), West ON (8149), South AB (1538), East ON (2832), SK (1656), Vancouver (3559), East BC (960), and Other BC (1155). ALB, Alberta; BC, British Columbia; MB, Manitoba; NFLD/NB/NS/PEI, Newfoundland/New Brunswick/Nova Scotia/Prince Edward Island; North, Northern; ON, Ontario; SK, Saskatchewan; South, Southern; West, Western.
Figure 2.
Crude proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 according to incident year (P<0.01 for trend).
Association between Patient, Dialysis Facilities, and Geographic Regions with eGFR at Initiation
The unadjusted ICCs for patient, facility, and geographic region were 90.7%, 6.6%, and 2.7%, respectively. On adjustment for case mix, facility-level factors, and calendar year, the facility and geographic ICCs reduced to 3.1% and 0.0%, respectively. The fully adjusted model had level-specific R2 values of 26.8% and 67.6% at the patient and facility levels, respectively. The total R2 for the fully adjusted model was 31%. Additional models examining eGFR≥10.5 ml/min per 1.73 m2 and limited to patients with >90 days of predialysis care yielded similar findings (Table 2). When the ICC was calculated using a probit link assuming a patient-level variance=1, the findings were similar (results not shown). In a sensitivity analysis examining eGFR>12 ml/min per 1.73 m2, the results were consistent with ICCs for patient, facility, and geographic region of 88.9%, 8.1%, and 3.0% (unadjusted) and 95.2%, 4.6%, and 0.2% (adjusted), respectively. When our cohort was limited to incident hemodialysis patients only (n=25,201), the ICCs for patient, facility, and geographic region were 89.6%, 8.1%, and 2.3% (unadjusted) and 95.9%, 4.1%, and 0.0% (adjusted), respectively. After exclusion of the top 25th percentile of facilities with the highest mean eGFR level at dialysis initiation, there remained n=25, 158 individuals, and 48 facilities. Among the reduced cohort, the proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 was 29.8% (versus 35.3% with all facilities included), and the adjusted ICCs for patient, facility, and geographic region were 97.9%, 2.1%, and 0.0%, respectively.
Table 2.
Multilevel model analysis of the unadjusted and adjusted variations at the patient, facility, and geographic levels for the level of eGFR at dialysis initiation
| Model | Intraclass Correlation (%) | Variance Estimate | SEM | P Value |
|---|---|---|---|---|
| eGFR as a continuous variablea | ||||
| Unadjusted | ||||
| Patient | 90.7 | 17.6 | 0.14 | <0.001 |
| Facility | 6.6 | 1.3 | 0.28 | <0.001 |
| Geography | 2.7 | 0.5 | 0.42 | 0.11 |
| Fully adjusted | ||||
| Patient | 96.9 | 12.9 | 0.10 | <0.001 |
| Facility | 3.1 | 0.4 | 0.09 | <0.001 |
| Geography | 0.0 | 0.0 | 0.00 | >0.99 |
| eGFR≥10.5 ml/min per 1.73 m2a | ||||
| Unadjusted | ||||
| Patient | — | — | — | — |
| Facility | 8.2 | 0.3 | 0.07 | <0.001 |
| Geography | 2.6 | 0.1 | 0.09 | 0.26 |
| Fully adjusted | ||||
| Patient | — | — | — | — |
| Facility | 4.5 | 0.2 | 0.04 | <0.001 |
| Geography | 0.1 | 0.0 | 0.02 | 0.40 |
| eGFR as a continuous variable and predialysis care>90 db | ||||
| Unadjusted | ||||
| Patient | 88.2 | 16.0 | 0.15 | <0.001 |
| Facility | 8.7 | 1.6 | 0.34 | <0.001 |
| Geography | 3.1 | 0.6 | 0.48 | 0.12 |
| Fully adjusted | ||||
| Patient | 95.7 | 11.9 | 0.11 | <0.001 |
| Facility | 4.2 | 0.5 | 0.12 | <0.001 |
| Geography | 0.2 | 0.0 | 0.07 | 0.38 |
The patient-level intraclass correlation was not calculated for the binary outcome to avoid a fixed value assumption. The fully adjusted model included the following covariates: facility-level factors (percentage catheter use, transplantation facility, peritoneal dialysis facility, average hemoglobin and phosphate, average distance that a patient resided from the nearest dialysis center, and number of patients), patient case mix (age, sex, body mass index, race, comorbidities, distance to facility, length of predialysis care, peritoneal dialysis, serum phosphate, albumin, and hemoglobin), and year.
Geographic regions, 12; facilities, 63; patients, 33,263. Median number of patients per facility=795 (interquartile range=532–1053).
Geographic regions, 14; facilities, 63; patients, 23,902. Median number of patients per facility=795 (interquartile range=532–963).
Factors associated with initiation of dialysis at a higher eGFR from the reduced model are presented in Table 3. On average, women with comorbid illness (with the exception of hypertension) started dialysis earlier. Similarly, patients initiated on peritoneal dialysis and those with higher hemoglobin were more likely to initiate with an eGFR≥10.5 ml/min per 1.73 m2. In addition, lower body mass index, lower serum phosphate, lower average facility–serum phosphate, and decreased distance from a dialysis facility were associated with starting at a higher eGFR.
Table 3.
Variables associated with the initiation of dialysis with an eGFR≥10.5 ml/min per 1.73 m2
| Variable | Reduced Model | ||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | P Value | |
| Facility level | |||
| Phosphate average (per 0.5-mmol/L increase) | 0.26 | 0.17 to 0.39 | <0.001 |
| Patient level | |||
| Women | 1.78 | 1.68 to 1.87 | <0.001 |
| Body mass index | 0.99 | 0.99 to 1.00 | <0.001 |
| Hemoglobin (per 1-g/L increase) | 1.01 | 1.01 to 1.01 | <0.001 |
| Phosphate (per 0.5-mmol/L increase) | 0.45 | 0.43 to 0.46 | <0.001 |
| Peripheral vascular disease | 1.24 | 1.16 to 1.33 | <0.001 |
| Acute coronary syndrome | 1.16 | 1.08 to 1.24 | <0.001 |
| Pulmonary edema | 1.51 | 1.41 to 1.61 | <0.001 |
| Chronic obstructive pulmonary disease | 1.16 | 1.07 to 1.25 | <0.001 |
| Diabetes mellitus | 1.43 | 1.31 to 1.56 | <0.001 |
| Hypertension | 0.84 | 0.78 to 0.91 | <0.001 |
| Serious illness | 1.31 | 1.21 to 1.41 | <0.001 |
| Coronary artery bypass graft | 1.27 | 1.17 to 1.37 | <0.001 |
| Peritoneal dialysis | 1.15 | 1.07 to 1.23 | <0.001 |
| Predialysis care>30 d | 1.28 | 1.19 to 1.38 | <0.001 |
| Distance from facility (km) | |||
| <50 (referent) | |||
| 50–150 | 0.90 | 0.84 to 0.98 | 0.02 |
| >150 | 0.89 | 0.80 to 0.99 | 0.05 |
| Cause of ESRD | |||
| Hypertension (referent) | |||
| Diabetes | 0.96 | 0.87 to 1.06 | 0.39 |
| GN | 0.76 | 0.69 to 0.84 | <0.001 |
| Obstruction | 0.78 | 0.65 to 0.93 | 0.01 |
| Interstitial | 0.86 | 0.73 to 1.02 | 0.09 |
| PCKD | 0.50 | 0.43 to 0.58 | <0.001 |
| Other | 0.94 | 0.85 to 1.04 | 0.24 |
| Unknown | 0.95 | 0.85 to 1.06 | 0.37 |
| Race | |||
| Caucasian (referent) | |||
| Asian | 0.61 | 0.54 to 0.69 | <0.001 |
| Black | 0.97 | 0.83 to 1.13 | 0.71 |
| Indian Subcontinent | 0.87 | 0.76 to 1.00 | 0.05 |
| Aboriginal | 0.86 | 0.75 to 0.98 | 0.02 |
| Unknown | 1.12 | 1.00 to 1.26 | 0.06 |
| Other | 0.62 | 0.53 to 0.72 | <0.001 |
| Year of first treatment | 1.07 | 1.06 to 1.08 | <0.001 |
The reduced model included the following covariates: facility-level factors (average phosphate), patient case mix (sex, body mass index, race, comorbidities, distance to facility, length of predialysis care, serum phosphate, and hemoglobin), and year. Geographic regions, 14; facilities, 63; patients, 23,902. Median number of patients per facility=795 (interquartile range=532–963). PCKD, polycystic kidney disease.
Discussion
In this Canadian cohort study of 33,263 patients initiating dialysis between 2001 and 2010, we observed significant variability in the eGFR at dialysis initiation across facility and geographic regions, which was largely explained by patient case mix. Patient-related factors, such as demographics, comorbid conditions, laboratory variables, and length of predialysis care, accounted for over 95% of the explained variability in eGFR at dialysis start. The treatment facility contributed 3.1% variability, whereas the influence of geographic region was minimal. These results suggest that patient factors explain much of the variability in eGFR at dialysis initiation, with a small but significant contribution on the basis of the treatment facility.
This study is the first examining facility- and geographic-level variations for the timing of dialysis initiation. Other studies have examined variability across facilities and geographic regions regarding dialysis practices (27–36). Two recent studies of 173 United States dialysis facilities reported significant facility variations for arteriovenous fistula use and dialysis adequacy (urea reduction ratio >65%) of 7.6% and 11.5%, respectively (28,29). These findings and similar findings have led to the implementation of quality improvement programs targeting these metrics and serve as a baseline measure for improvement. Our findings suggest that patient factors are integrally important regarding the decision to initiate dialysis, with nonclinical factors contributing a smaller role. The small measured degree of regional- and facility-level variation may be indicative that physicians and facilities find early dialysis initiation beneficial and practice accordingly. A recently published survey study of Canadian nephrologists found that the majority, in general, did not agree with early dialysis initiation for asymptomatic patients (37). Furthermore, it should be noted that, although we examined eGFR categorically (>10.5 or <10.5 ml/min per 1.73 m2), this finding does not imply an acceptable absolute eGFR for dialysis initiation. The most recent Kidney Disease Improving Global Outcomes guidelines suggest that an acceptable threshold for the initiation of dialysis would be the presence of more clinical criteria, such as serositis, acid-base or electrolyte abnormalities, and pruritus; inability to control volume status or BP; a progressive deterioration in nutritional status refractory to dietary intervention; and cognitive impairment (38).
We found an increase in facility-level variation when the study cohort was limited to patients on hemodialysis only. This result shows that the variability in dialysis initiation is modality-dependent and higher among patients on intermittent hemodialysis compared with patients on peritoneal dialysis. Although the variability at the facility level increases in patients on hemodialysis, a recent Canadian study reported that patients on peritoneal dialysis are more likely to initiate dialysis at higher levels of eGFR (25). Combining that finding with our observation suggests that the practice of starting patients on peritoneal dialysis at higher levels of eGFR is occurring more uniformly (or with less variability) across Canada.
Patient-level factors associated with initiating dialysis at higher eGFR levels included being a woman, residing closer to a dialysis facility, and having a lower serum phosphate. The low serum phosphate is a novel finding and may be a surrogate marker of malnutrition and decreased protein catabolic rate, which are recognized features of advancing uremia and themselves indications for elective initiation of dialysis. In this regard, other correlates of malnutrition, such as a lower body mass index, were also associated with early initiation in this study. Similar to patients with a higher burden of comorbid conditions, patients with low phosphate are also likely to have diminished protein intake and reduced muscle mass. As such, creatinine-based eGFR may be biased in this population and lead to false conclusions about the timing of dialysis initiation.
Despite the low level of facility-level variation, quality improvement interventions targeting a decrease in the eGFR level at dialysis initiation at the facility level may be warranted (39,40). Previous interventions targeted at further reducing low levels of variation at the facility or physician level have been successful. For example, a multifaceted quality improvement intervention led to improved dialysis adequacy across 196 United States dialysis centers, despite a low initial variability between facilities (33). Similar improvements have been shown in a study examining four primary care indicators (lipids, BP, patient experiences, and mammograms) all with <4% initial facility-level variation (41). On the basis of these studies, it seems that, when total patient-level variability is high but facility- or hospital-level variation is low, interventions that show only minimal improvements at the facility-level can lead to large overall reductions in variation (41,42). This finding may represent the case with our data, because explained adjusted facility variation was 3.1%. However, the unadjusted variation was high at 36.1% (Other British Columbia versus Manitoba in Figure 2). To examine the effect of a quality intervention in our population, we removed the top quartile of facilities with the highest mean eGFR at dialysis initiation; a decline in the proportion of patients initiating dialysis with an eGFR≥10.5 ml/min per 1.73 m2 by 5.5% was seen, and the adjusted variation reduced to 2.1%. The development of evidence-based guidelines, facility-level feedback with national rankings, directed education sessions, and site visits by opinion leaders may represent knowledge translation activities geared toward decreasing variations and improving care. Conversely, it still remains uncertain whether the timing of dialysis initiation is a truly modifiable quality measure, because even in the setting of a stringent randomized control trial (Initiating Dialysis Early and Late, IDEAL), it was very difficult to start patients at set, predetermined ranges of eGFR (8). Because it remains uncertain whether the timing of dialysis initiation is a modifiable metric, it would be premature to include it as a possible pay-for-performance measure.
The strengths of our study include a large nationally representative cohort capturing incident patients in Canada (excluding Quebec) during the study period. We used a multilevel model that accounted for within-facility and geographic regional clustering. We reported the ICC, a well reported method for quantitating the relative variability at differing levels in health care systems (43). Facility-level variables were created to serve as surrogates of a facility's quality of dialysis care. We examined facility-level variation as opposed to individual physicians, because many nephrology facilities in Canada use shared-care models. Our models were adjusted for a large number of covariates, and our findings were consistent across numerous sensitivity analyses.
Limitations of the study include the use of registry data, which lacks information regarding the indication for dialysis initiation or measures of frailty. Our study would not detect variability in facility and regional practices if all physicians were practicing consistently and starting dialysis at higher levels of eGFR in a uniform manner. From 2001 to 2010, the mean eGFR at dialysis initiation increased from 9.1±4.1 to 10.1±4.5 ml/min per 1.73 m2. During this period, the increasing average age and number of comorbidities make interpretation difficult. We used surrogate measures for facility quality, and they may not necessarily reflect factors at the individual physician level. We were unable to detect variation between individual clinicians, because individual physician-level data were not available. Because Canada does not have for-profit dialysis facilities, our study was unable to examine the question of whether initiation of dialysis varies on the basis of the profit status of the dialysis unit. Finally, many provinces began regional eGFR reporting during the study period, and on the basis of recent survey results, eGFR reporting may influence the timing of dialysis initiation (44).
In conclusion, we showed, in a national study of >30,000 patients with ESRD over 10 years, substantial variation in dialysis initiation in Canada that is largely attributable to patient factors, with a small but significant component at the facility level. Whether facility-level variation can be further reduced or modified requires additional investigation.
Disclosures
M.M.S. has been on advisory boards for Roche and Amgen and received speaking honoraria from Roche, Amgen, and Sanofi. S.N. has been on advisory boards for Baxter Healthcare Canada and received speaking honoraria from Baxter Healthcare Canada and Merck Frost. B.M., A.D., B.H., J.K., P.K., A.M., D.N., S.N., O.R., S.S., and M.Z. have no conflicts of interest.
Acknowledgments
We thank Bob Williams, Frank Ivis, Dolores Friesen, the Canadian Institutes of Health Research (CIHR), and the Kidney Foundation of Canada (KFoC).
M.M.S. is provided salary support by the Jindal Research Chair for the Prevention of Kidney Disease at the University of Ottawa. N.T. is supported by the Kidney Research Scientist Core Education and National Training Program New Investigator Award, a joint initiative of the KFoC, the CIHR, and the Canadian Society of Nephrology.
This work was presented as an abstract at the 50th European Renal Association-European Dialysis and Transplant Association Congress in Istanbul, Turkey on May 19, 2013.
M.M.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “What Drives Early Dialysis Initiation and How Do We Optimize Timing of RRT?,” on pages 1671–1673.
References
- 1.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A: Survival and dialysis initiation: Comparing British Columbia and Scotland registries. Nephrol Dial Transplant 24: 3186–3192, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL: GFR at initiation of dialysis and mortality in CKD: A meta-analysis. Am J Kidney Dis 59: 829–840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS: Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 5: 1828–1835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF: Early start of hemodialysis may be harmful. Arch Intern Med 171: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK: Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 14: 2305–2312, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hwang S-J, Yang W-C, Lin M-Y, Mau L-W, Chen H-C, Taiwan Society of Nephrology : Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: A national cohort study in Taiwan. Nephrol Dial Transplant 25: 2616–2624, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ: Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 24: 3175–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA, IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Clark WF, Na Y, Rosansky SJ, Sontrop JM, Macnab JJ, Glassock RJ, Eggers PW, Jackson K, Moist L: Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ 183: 47–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Renal Data System : USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 11.Canadian Institute for Health Information : Dialysis and Renal Transplantation, Canadian Institute for Health Information, Ottawa, Ontario, Canada, 2009 [Google Scholar]
- 12.Vincenty T: Direct and Inverse Solutions of Geodesics on the Ellipsoid with Application of Nested Equations. Survey Review XXIII 176: 88–93, 1975 [Google Scholar]
- 13.Mendelssohn DC, Pisoni RL, Arrington CJ, Yeates KE, Leblanc M, Deziel C, Akiba T, Krishnan M, Fukuhara S, Lameire N, Port FK, Wolfe RA: A practice-related risk score (PRS): A DOPPS-derived aggregate quality index for haemodialysis facilities. Nephrol Dial Transplant 23: 3227–3233, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Benner D, Hollister D, McAllister C,Thiry K: The DaVita Quality Index (DQI): A measure of clinical performance. Dial Transplant 32: 269–273, 2003 [Google Scholar]
- 15.Schaubel DE, Blake PG, Fenton SSA: Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 60: 1517–1524, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Moist LM, Richards HA, Miskulin D, Lok CE, Yeates K, Garg AX, Trpeski L, Chapman A, Amuah J, Hemmelgarn BR: A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol 6: 813–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocco MV, Frankenfield DL, Hopson SD, McClellan WM: Relationship between clinical performance measures and outcomes among patients receiving long-term hemodialysis. Ann Intern Med 145: 512–519, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Churchill DTW, Keshaviah PR, Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Heck R, Thomas S, Tabata LN: Multilevel and Longitudinal Modeling with IBM SPSS, New York, Routledge, 2010 [Google Scholar]
- 20.Greenland S: Principles of multilevel modelling. Int J Epidemiol 29: 158–167, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Raudenbush SW, Bryk AS: Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd Ed., Thousand Oaks, CA, Sage Publications Inc., 2002 [Google Scholar]
- 22.Snijders T, Bosker R: Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling, London, Sage Publications Ltd., 1990 [Google Scholar]
- 23.Rodriguez G, Elo I: Intra-class correlation in Random-Effects Models for Binary Data. Stata J 1: 32–46, 2003 [Google Scholar]
- 24.Heo M, Leon AC: Performance of a mixed effects logistic regression model for binary outcomes with unequal cluster size. J Biopharm Stat 15: 513–526, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jain AK, Sontrop JM, Perl J, Blake PG, Clark WF, Moist LM: Timing of peritoneal dialysis initiation and mortality: analysis of the Canadian Organ Replacement Registry. Am J Kidney Dis 63: 798–805, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Schafer JL: Multiple imputation: A primer. Stat Methods Med Res 8: 3–15, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 44[Suppl 2]: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tangri N, Moorthi R, Tighiouhart H, Meyer KB, Miskulin DC: Variation in fistula use across dialysis facilities: Is it explained by case-mix? Clin J Am Soc Nephrol 5: 307–313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangri N, Tighiouart H, Meyer KB, Miskulin DC: Both patient and facility contribute to achieving the Centers for Medicare and Medicaid Services’ pay-for-performance target for dialysis adequacy. J Am Soc Nephrol 22: 2296–2302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink JC, Hsu VD, Zhan M, Walker LD, Mullins CD, Jones-Burton C, Langenberg P, Seliger SL: Center effects in anemia management of dialysis patients. J Am Soc Nephrol 18: 646–653, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI: Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis 58: 73–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fink JC, Blahut SA, Briglia AE, Gardner JF, Light PD: Effect of center- versus patient-specific factors on variations in dialysis adequacy. J Am Soc Nephrol 12: 164–169, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Fink JC, Zhan M, Blahut SA, Soucie M, McClellan WM: Measuring the efficacy of a quality improvement program in dialysis adequacy with changes in center effects. J Am Soc Nephrol 13: 2338–2344, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, Gillespie BW, Young EW: Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 50: 602–611, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK: Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 82: 570–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann B, Manns B, Dart QA, Kappel J, Molzahn A, Naimark D, et al. : An assessment of dialysis provider's attitudes towards timing of dialysis initiation in Canada. Can J Kidney Health Dis 1: 3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KDIGO Working Group : Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3: 116–118, 2012 [Google Scholar]
- 39.Sood MM, Manns B, Nesrallah G, Canadian Kidney Knowledge Translation, Generation Network : Using the knowledge-to-action framework to guide the timing of dialysis initiation. Curr Opin Nephrol Hypertens 23: 321–327, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Manns B, Barrett B, Evans M, Garg A, Hemmelgarn B, Kappel J, et al. : Establishing a national knowledge translation and generation network in kidney disease: The CAnadian KidNey KNowledge TraNslation and GEneration NeTwork. Can J Kidney Health Dis 1: 2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selby JV, Schmittdiel JA, Lee J, Fung V, Thomas S, Smider N, Crosson FJ, Hsu J, Fireman B: Meaningful variation in performance: What does variation in quality tell us about improving quality? Med Care 48: 133–139, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Fung V, Schmittdiel JA, Fireman B, Meer A, Thomas S, Smider N, Hsu J, Selby JV: Meaningful variation in performance: A systematic literature review. Med Care 48: 140–148, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Howley PP, Gibberd R: Using hierarchical models to analyse clinical indicators: A comparison of the gamma-Poisson and beta-binomial models. Int J Qual Health Care 15: 319–329, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Brimble KS, Mehrotra R, Tonelli M, Hawley CM, Castledine C, McDonald SP, Levidiotis V, Gangji AS, Treleaven DJ, Margetts PJ, Walsh M: Estimated GFR reporting influences recommendations for dialysis initiation. J Am Soc Nephrol 24: 1737–1742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]