Key Points
Human IgM memory B cells possess immunoregulatory properties analogous to transitional B cells.
IL-10–producing B cells are deficient in cGVHD.
Abstract
A subset of regulatory B cells (Bregs) in mice negatively regulate T-cell immune responses through the secretion of regulatory cytokines such as IL-10 and direct cell-cell contact and have been linked to experimental models of autoimmunity, inflammation, and cancer. However, the regulatory function of Bregs in human disease is much less clear. Here we demonstrate that B cells with immunoregulatory properties are enriched within both the CD19+IgM+CD27+ memory and CD19+CD24hiCD38hi transitional B-cell subsets in healthy human donors. Both subsets suppressed the proliferation and interferon-γ production of CD3/CD28-stimulated autologous CD4+ T cells in a dose-dependent manner, and both relied on IL-10 secretion as well as cell-cell contact, likely mediated through CD80 and CD86, to support their full suppressive function. Moreover, after allogeneic stem cell transplantation, Bregs from patients with chronic graft-versus-host disease (cGVHD) were less frequent and less likely to produce IL-10 than were Bregs from healthy donors and patients without cGVHD. These findings suggest that Bregs may be involved in the pathogenesis of cGVHD and support future investigation of regulatory B cell–based therapy in the treatment of this disease.
Introduction
Interleukin-10 (IL-10)–producing B cells are defined by an important set of regulatory functions that could be harnessed for therapeutic purposes.1 Designated regulatory B cells (Bregs) by Mizoguchi et al,2,3 these cells can suppress inflammatory responses in experimental autoimmune encephalomyelitis,4 collagen-induced arthritis,5 and colitis,3 and were recently implicated in the generation and maintenance of T-regulatory (Treg) cells in the periphery.6 A number of Breg phenotypic markers have been identified in murine models,7,8 but exclusive reliance on phenotypic markers to distinguish between pathogenic and regulatory B cells can produce conflicting results, so that assays for functional properties such as IL-10 production are required to identify Bregs in a definitive, reproducible manner.1,9 Given the large gaps in understanding Breg phenotypic markers as they relate to immunosuppressive function, it is clear that more detailed investigation of the Breg “signature” is needed to permit meaningful exploration of therapies based on B cells with regulatory potential.
The study of human Bregs, which share many functional characteristics with murine Bregs,10,11 has been largely limited to IL-10–producing immature/transitional B cells in a small group of autoimmune diseases, including systemic lupus erythematous,10 immune thrombocytopenia,12 active chronic sarcoidosis,13 and multiple sclerosis.14 CD27+CD24hi B cells have also been shown to modulate the monocyte innate immune response by suppressing their ability to produce tumor necrosis factor (TNF)-α in vitro,10,11 although evidence for their direct suppression of T-cell proliferation is lacking. Moreover, despite compelling evidence that human Breg cells can function as modulators of autoimmune disorders,10,12 very little is known about their activities in chronic graft-versus-host disease (cGVHD), where CD4+CD25+ Tregs have attracted the most attention.15-17
Chronic GVHD remains a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT), and the prognosis for patients who fail to respond to corticosteroids is historically poor; hence, new therapies for this disorder are urgently needed. The pathogenesis of cGVHD is poorly understood, although it clearly resembles an autoimmune disease both clinically and pathologically. Multiple autoantibodies are often detected in patients with cGVHD,18 suggesting a critical breakdown in peripheral B-cell tolerance and insufficient immune regulation after allogeneic HSCT. Indeed, B cells isolated from patients with cGVHD are typically activated with increased signaling through the AKT and ERK pathways.19 Thus, using B cells from both normal healthy donors and patients undergoing allogeneic HSCT, we sought to identify IL-10–producing cells with immunosuppressive properties within discrete B-cell subpopulations in peripheral blood (PB).
Our results demonstrate the presence of IL-10–producing Bregs with Treg-independent immunosuppressive functions in both the IgM memory (CD19+IgM+CD27+) and transitional (CD19+CD24hiCD38hi) B-cell subsets in healthy donors. Moreover, the regulatory function of these human Bregs against T cells required cell-cell contact as well as IL-10 production. Unlike Breg cells from healthy donors, those from cGVHD patients showed impaired IL-10 production when activated by CD40L, suggesting that infusion of donor-derived regulatory B cells may be used to minimize damage caused by active cGVHD and to reduce the risk of cGVHD development.
Material and methods
Patients and controls
All samples were collected patients gave written informed consent according to the local ethics policy guidelines and the Declaration of Helsinki.
Human cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient-separation (Lymphoprep) and cryopreserved in 20% dimethyl sulfoxide. B-cell subsets were sorted by FACSAria (Becton Dickinson) using CD19-PC7 (Immunotech), CD27-FITC (DakoCytomation), IgM-PerCP-Cy5.5, CD24-FITC (Biolegend), and CD38-PECy7 (ebioscience) antibodies. CD4+ T cells and CD19+ B cells were isolated by magnetic-bead purification (Miltenyi Biotic Ltd.).
Characterization of IL10+CD19+ B cells
For IL10+ B-cell characterization, PBMCs were stimulated with irradiated L cells for 12 to 15 hours. Phorbol myristate acetate (PMA) (50 ng/mL), ionomycin (250 ng/mL) (Sigma-Aldrich), and Brefeldin A (5 µg/mL, GolgiPlug; Sigma-Aldrich) were added for the last 6 to 8 hours of the culture. Cells were harvested, washed in staining buffer, and incubated for 20 minutes at room temperature with a cocktail of CD19-PE, CD3-PerCP, CD22-FITC, CD24-FITC, CD5-FITC, BAFFR-FITC, CD1d-PE, CD27-PE, IgD-PE, CD40-PE, and IgM-PerCPCy5.5, all from BD Pharmingen; CD21-FITC and FCRL4-PE (BioLegend), and CD38-PECy7 (eBioscience). Cells were then washed in staining buffer and fixed/permeabilized for 30 to 45 minutes at 4°C with an intracellular staining kit (eBioscience). At this stage, 20 µL of FcR blocking reagent (Miltenyi Biotec) was added. Cells were incubated for 30 minutes at 4°C with 10 µL of FITC-conjugated interferon (IFN)-γ and 0.5 µL of either allophycocyanin (APC)-conjugated IL-10 or IgG2aК isotype antibodies. All data were acquired using BD-FACSCalibur (Becton Dickinson) and analyzed with FlowJo software.
Cell culture
CD40L-transfected L cells and nontransfected control fibroblasts (kindly provided by Dr Wagner, Imperial College, London, United Kingdom) were grown in RPMI 1640/10% fetal calf serum.
Cytokine detection
CD19+-selected B cells or total PBMCs were stimulated with irradiated L cells at a ratio of 1:10, and IL-10 production was assessed on gated CD19+ B cells by intracellular cytokine assay. Data were acquired on BD-FACSCalibur (Becton Dickinson) and analyzed with FlowJo software. Cytokine secretion was assayed in supernatants by luminex (eBiosciences) according to the manufacturer’s instructions.
Proliferation assay
Magnetically selected carboxyfluorescein succinimidyl ester (CFSE)-labeled (eBioscience) CD4+ T cells were activated with anti-CD3/CD28 Dynabeads (InvitroGen) and cultured with IgM+CD27+ memory, CD24hiCD38hi transitional, IgM-CD27+ switched-memory, and IgM+CD27– naïve B cells at a 1:1 ratio for 96 hours. No stimulation (negative control) and stimulation with anti-CD3/anti-CD28 beads alone (positive proliferation control) were included in every experiment. Magnetically purified Tregs were cocultured with CD3/anti-CD28-stimulated CD4+ T cells as a suppression control.
Cytokine suppression
Magnetically selected CD4+ T cells were cultured with fluorescence-activated cell-sorted B-cell subsets at a B- to T-cell ratio of 1:1 for 48 hours. Unstimulated CD4+ T cells (negative control) and stimulated CD4+ T cells (positive control) were included with each experiment. Brefeldin A (10 μg/mL) was added for the last 6 hours of the culture. Cells were stained with CD19-PE and CD4-PerCP, fixed/permeabilized, and followed by IFN-γ-FITC and TNF-α-APC antibodies (all BD Biosciences).
Transwell cultures
CFSE+CD4+ T cells were either directly added to sort-purified B-cell subsets at a ratio of 1:1 or placed in transwell chambers (Millicell, 1.0 μm; Millipore). After 96 hours in the presence of anti-CD3/CD28 Dynabeads (Life Technologies), cultured cells were harvested and analyzed by flow cytometry.
Blocking experiments
Purified B cells and T cells were cocultured and stimulated with anti-CD3/anti-CD23 Dynabeads in the presence or absence of 5 µg/mL of anti-IL-10 (JEs#-9D7), anti-IL-10 receptor (3F9), anti-CD80 (10 μg/mL), anti-CD86 (10 μg/mL) (IT2.2), or antitransforming growth factor (TGF)-β (2 μg/mL) (TB21).
Statistics
All values are expressed as medians and ranges. Statistical significance was assessed with Prism software (GraphPad) by unpaired or paired 2-tailed Student t test analysis and by nonparametric 2-way analysis of variance (ANOVA), as appropriate. P ≤ .05 was considered statistically significant.
Results
IL-10–producing B cells are enriched within both the CD19+CD24hiCD38hi transitional and CD19+IgM+CD27+ memory B-cell subsets of healthy donors
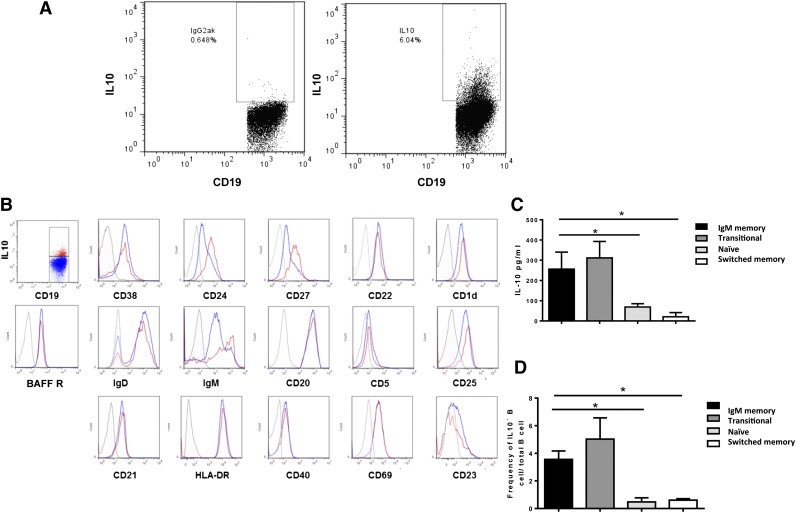
IL-10 production has long been considered a defining trait of Bregs.9 Thus we applied intracellular staining for IL-10 together with a panel of surface antibodies to PBMCs from 10 healthy donors (Figure 1A). The PBMCs had been cultured with irradiated fibroblasts transfected with CD40-ligand (L cells) for 15 hours, because this duration of stimulation was found to be optimal for IL-10 production without the risk of undue changes in the overall B-cell phenotype (data not shown). IL-10+CD19+ B cells (IL-10+ B cells) expressed CD1d, CD20, CD21, CD22, CD40, CD268 (BAFFR), and HLA-DR. The majority also expressed CD27, CD24, IgD, and IgM (Figure 1A-B). Compared with IL-10– B cells, IL-10+ B cells expressed higher levels of CD24, CD27, and IgM. Nearly all IL-10+ B cells expressed a high level of CD25, in keeping with B-cell activation, as shown by concomitant upregulation of CD69 (Figure 1B), whereas IL-10– and IL-10+CD19+ B cells expressed similar levels of CD5.
Figure 1.
IL-10–producing B cells from healthy donors are enriched within the IgM memory and transitional B-cell subsets. PBMCs were stimulated with irradiated CD40L-transfected fibroblasts (L cells) for 12 to 15 hours. PMA (50 ng/mL), ionomycin (250 ng/mL) (Sigma Aldrich), and Brefeldin A (5 µg/mL) (Sigma Aldrich) were added for the last 6 to 8 hours of the culture. Cells were harvested, washed in staining buffer (1× phosphate-buffered saline, 2% heat-inactivated fetal calf serum, 0.1% sodium azide), and incubated for 20 minutes at room temperature with a cocktail of CD19-PE, CD3 PerCP (BD Biosciences); CD22 FITC, CD24 FITC, CD5 FITC or BAFFR FITC, CD1d PE, CD27 PE, IgD PE or CD40 PE, and IgM PerCP Cy5.5 (all from BD Pharmingen); CD21 FITC (BioLegend); and CD38 PE Cy7 (eBioscience). Cells were fixed/permeabilized (eBioscience) and stained with APC-conjugated IL-10 or IgG2a-К isotype antibodies. All data were analyzed with FlowJo software. (A) Representative flow cytometry plots are shown for IL-10+CD19+ B cells vs IL-10-CD19+ B cells. (B) Extended phenotyping of IL-10+ vs IL-10– B cells vs isotype control. (C) IL-10 production by sort-purified B-cell subsets cultured with irradiated CD40L-expressing fibroblasts (no Brefeldin A was added). (D) IL-10–producing B cells are enriched within the transitional and IgM memory B-cell compartments. The data in (C) and (D) are from 3 independent experiments using PB samples from 5 healthy controls. Bars represent medians, and whiskers indicate the upper range. P < .001 by nonparametric ANOVA.
To discover whether IL-10–producing B cells are enriched within any of the commonly recognized B-cell subsets in PB,20 we measured IL-10 concentrations in the supernatants derived from sort-purified CD19+CD24hiCD38hiCD27-IgMhi transitional, CD19+IgM+CD27+CD24hiCD38–/lo IgM memory, CD19+CD24intCD38intCD27-IgM+ naïve, and CD19+IgM-CD27+CD24hiCD38–/lo switched memory B-cell subsets (supplemental Figure 1A-D, available on the Blood Web site) that had been cultured with irradiated, CD40L-transfected fibroblasts. The majority of IL-10+ cells were enriched within either the transitional or the IgM memory B-cell compartment, compared with findings for the naïve and switched memory B-cell subsets (Figure 1C). Median-fold increases in the frequency of IL-10–producing cells were 3.6 (range 2.5-4.6) and 4.42 (range 3.78-6.59) for the first 2 subsets, and 0.48 (range 0.33-0.78) and 0.60 (range 0.43-0.80) for the latter 2 (Figure 1D). These results underscore the regulatory capacity of immature transitional B cells (CD19+CD24hiCD38hi), first described by Blair et al,10 and support IgM memory B cells (CD19+IgM+CD27+) as a new candidate Breg subset.
IgM memory and transitional B cells inhibit proliferation and proinflammatory cytokine production by CD4+ T cells
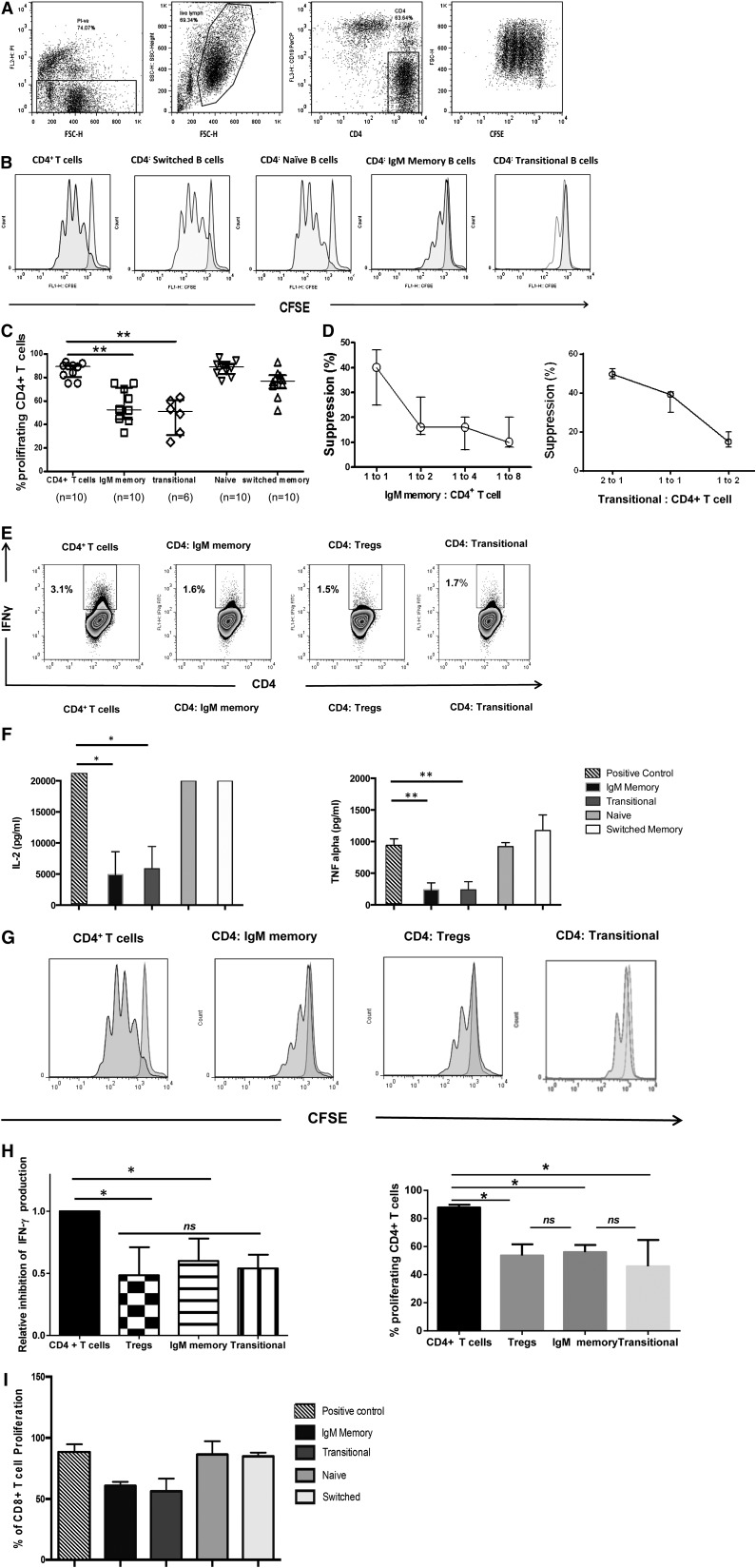
To gain further insight into the biology of IL-10–enriched transitional and IgM memory B cells, we sort-purified these subsets as well as naïve and switched memory B cells from 10 healthy donors and evaluated their effects on CD4+ T-cell proliferation and cytokine production (Figure 2). The gating strategies and postsort purity checks are outlined in supplemental Figure 1A-C. In 96-hour cultures with CD4+ T cells (stimulated with anti-CD3/anti-CD28 beads) at a ratio of 1:1, only the IgM memory and transitional B cells significantly suppressed CD4+ T-cell proliferation (P = .0001 vs positive control, both comparisons) (Figure 2A-C), and these effects were cell dose–dependent (Figure 2D). They were also consistent with the ability of the transitional and IgM memory subsets to significantly suppress IFN-γ, TNF-α, and IL-2 production by CD4+ T cells in culture, whereas neither the naïve nor the switched memory subset showed this capacity (Figure 2E-F). We further compared the suppressive capacity of IgM memory and transitional B cells with that of Tregs, defined as CD4+CD25hiCD127lo. In experiments in which magnetically purified Tregs from 7 healthy volunteers were cocultured with autologous CD4+ T cells at a 1:1 ratio, and the cultured cells were stimulated with anti-CD3/anti-CD28 beads, the inhibition of T-cell proliferation and IFN-γ production by transitional and IgM memory B cells was comparable with that by Tregs (Figure 2G-H). Together, these results show that transitional and IgM memory B cells share with Tregs a robust capacity to suppress the expansion of CD4+ T cells. Transitional and IgM memory B cells also suppressed the proliferation of sort-purified anti-CD3/anti-CD28–stimulated autologous CD8+ T cells (Figure 2I).
Figure 2.
IL-10–producing IgM memory and transitional B cells suppress CD4+ T-cell proliferation in a robust and dose-dependent manner. Magnetically selected CD4+ T cells were labeled with CFSE (eBioscience) and plated in 96-well flat-bottom tissue culture plates. IgM+CD27+ memory, CD24hiCD38hi transitional, IgM-CD27+ switched memory, and IgM+CD27– naïve cells were added to separate wells at a B-cell to T-cell ratio of 1:1 for 96 hours. T cells were activated with anti-CD3/CD28 Dynabeads (Invitrogen) at a concentration of 2 µL/ 8 × 105 cells. CFSE-stained T cells cultured with no stimulation (negative control) and CFSE-stained T cells cultured with anti-CD3/anti-CD28 beads (positive proliferation control) were included in each experiment. Cells were stained with CD4-APC, CD19-PE, and PI (all from BD Biosciences) for 20 minutes and acquired on a BD FACSCalibur instrument. (A) Representative flow cytometry plots of results of CD4+ T-cell suppression assay with gating strategy. (B) Proliferation of CD4+ T cells cultured alone, with switched-memory B cells, naïve B cells, IgM memory B cells, and transitional B cells at a ratio of 1:1. (C) In vitro suppressive effects of different CD19+ B-cell subsets (1:1 ratio) were assayed with anti-CD3/anti-CD28–stimulated CD4+ T cells. Bars represent medians and interquartile ranges for the indicated numbers of donors. P < .001 for suppression by both IgM memory and transitional B cells vs positive control by nonparametric ANOVA. (D) Dose-dependent suppression of CD4+ T-cell proliferation in the presence of IgM memory (left) and transitional B cells (right) incubated at the indicated B-cell to T-cell ratios. Data are representative of 3 independent experiments with cells from 3 healthy donors. Each data point represents the median and range. (E) Sort-purified IgM memory and transitional B cells and Tregs from healthy individuals were cultured 1:1 with anti-CD3/anti-CD28–stimulated CD4+ T cells. Frequencies of IFN-γ+ CD4+ were assessed relative to the corresponding unstimulated control. (F) Bar graphs show the suppression of TNF-α and IL-2 after coculture with IgM memory, transitional, naïve, and switched memory B cells. Data are representative of 3 independent experiments with cells from 4 healthy donors. Bars represent medians, and whiskers indicate the upper range. *P = .015 vs positive control, **P < .001 vs positive control, for comparisons with both IgM memory and transitional subsets (by nonparametric ANOVA). (G) Frequency of proliferating CD4+ T cells cultured with IgM memory, Tregs, or transitional B cells at a ratio of 1:1. (H) Bar graphs show equivalent inhibition of IFN-γ production and proliferation of CD4+ T cells by IgM memory, Tregs, and transitional B-cell subsets. Values are medians and upper ranges for 4 healthy donors. *P = .018 for individual comparisons with controls by nonparametric ANOVA; ns, not significant. (I) In vitro suppressive effects of different CD19+ B-cell subsets (1:1 ratio) were assayed with anti-CD3/anti-CD28–stimulated CD8+ T cells. P < .05 for suppression by both IgM memory and transitional B cells vs positive control by nonparametric ANOVA.
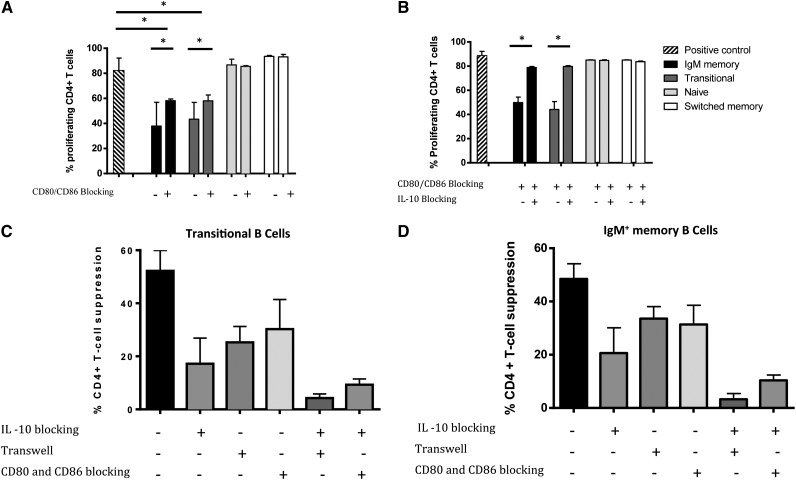
Suppressive activity of IgM memory and transitional B cells is IL-10–dependent
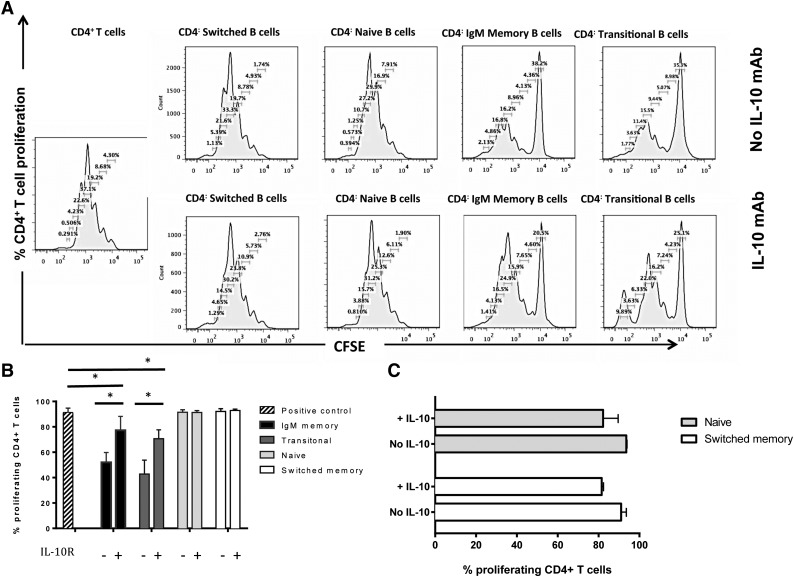
To clarify the mechanism(s) by which IgM memory B cells and transitional B cells suppress CD4+ T-cell proliferation and effector cytokine function, we cultured CD4+ T cells either alone or 1:1 with IgM memory, transitional, naïve, or switched memory B cells in the presence of mAbs against IL-10 or IL-10 receptor (IL-10R) in 4 independent experiments. This blockade restored the proliferation of CD4+ T cells cultured with the IgM memory or transitional B-cell subsets but lacked any impact on the proliferation of CD4+ T cells cultured with naïve or switched memory B cells (Figure 3A-B). These results indicate that the regulatory properties of transitional B cells are mediated by IL-10, consistent with previous reports,10 and that the same mechanism operates in IgM memory B cells. It should be stressed, however, that the IL-10 blockade did not fully reverse the suppressive capacity of either the IgM memory or transitional B-cell subsets, even at higher IL-10/IL-10R–blocking Ab concentration (supplemental Figure 2). This suggests that other mechanisms, most likely other soluble factors or costimulatory molecules, are involved in regulating Breg-mediated suppression of CD4+ T cells.
Figure 3.
Suppressive activity of IgM memory and transitional B cells depends on IL-10 secretion. (A) Suppressive effect of B-cell subsets on proliferation of CFSE-labeled CD4+ T cells in the presence or absence of IL-10 blockade. Flow cytometry histograms show CD4+ T cells cultured alone, with switched memory B cells, naïve B cells, IgM memory B cells, and transitional B cells in the presence or absence of IL-10 mAb. (B) Comparison of inhibitory effects by different B-cell subsets on proliferating CD4+ T cells, with or without IL-10 blockade. Bars indicate medians, and whiskers indicate the upper range for 3 healthy donors. *P < .05 vs positive control or IL-10R blockade by nonparametric ANOVA. (C) The addition of IL-10 to cocultures of CD4+T cells and sorted naïve and switched memory B cells has a minimal suppressive effect. Bars represent medians, and whiskers indicate the upper ranges for 3 healthy individuals.
Adding IL-10 to cocultures of CD4+ T cells with naïve or switched-memory B cells had a minimal suppressive effect (Figure 3C). This result strengthens the hypothesis that IL-10 does not by itself confer suppressive capacity to B cells. To pursue the notion that TGF-β might mediate at least part of the immunoregulatory capacity of Breg cells, we performed additional blocking experiments using TGF-β–specific mAbs. TGF-β blockade failed to alter the suppression of CD4+ T cells by either transitional or IgM memory B cells (P = .46 and P = .86, respectively, n = 3; data not shown), indicating that this cytokine lacks any appreciable role in human Breg-mediated inhibition of CD4+ effector T cells.
Mechanism for the suppressive activity of IL-10+ IgM memory and transitional B cells also depends on cell-cell contact, mediated through CD80/CD86
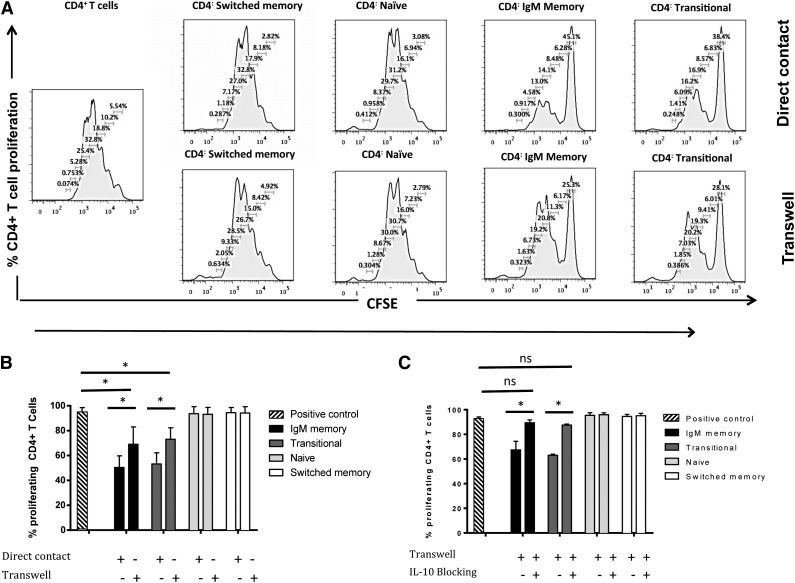
The suppressive capability of murine Bregs appears to be mediated by both the secretion of IL-10 and direct contact with CD4+ T cells.2,3,7,10 It is still unclear, however, whether similar direct cell-cell contact contributes to human Breg-mediated T-cell suppression. We therefore performed transwell experiments, in which transitional and IgM memory B cells were either in direct contact or separated from anti-CD3/anti-CD28–stimulated and CFSE-stained CD4+ T cells by a permeable membrane. The proliferation of CD4+ T cells was measured at 96 hours after the culture was initiated. Direct co-culture of CFSE-stained CD4+ T cells with transitional B cells or IgM memory B cells resulted in marked suppression of CD4+ T-cell proliferation (Figure 4A-B). By contrast, separation of transitional B cells and IgM memory B cells from anti-CD3/anti-CD28–activated CD4+ T cells by a transwell membrane partially reversed the suppressive effect of these B-cell subsets (median percentages of proliferating CD4+ T cells 74% [range 59.7-85.5] [P = .01] and 67.7% [52.5-88.7] [P = .03], respectively, n = 4), although this effect was not complete compared with the positive control (Figure 4A-B).
Figure 4.
Direct cell-cell contact contributes to the T cell–suppressive activity of IL-10+ IgM memory and transitional B-cell subsets. (A) Representative flow cytometry histograms showing proliferation of CD4+ T cells cultured either in separate transwell chambers or directly with CD19+ B-cell subsets: CD4+ T cells cultured alone or with switched memory B cells, naïve B cells, IgM memory B cells, or transitional B cells at a ratio of 1:1. (B) Impact of direct contact with CD4+ T cells in suppressive activity of IL-10+ B-cell subsets. (C) IgM memory and transitional B cell–mediated suppression of CD4+ T cells depends on both IL-10 and cell-cell contact. In both panels, the data shown are medians and upper ranges from 3 independent experiments with 5 samples per group. *P < .05 by nonparametric ANOVA; ns, not significant.
Thus, we next asked whether a combination of IL-10 blockade and abrogation of direct cell-cell contact could entirely eliminate the suppressive effect of candidate Bregs on CD4+ T-cell proliferation. In experiments in which IL-10 and IL-10 receptor (IL-10R) mAbs were added to the sort-purified transitional and IgM memory B cells in a transwell setting, IL-10 blockade alone did not fully reverse the suppressive effect of either sort-purified transitional or IgM memory B cells on the proliferation of CD4+ T cells. Subsequent addition of IL-10 and IL-10R Abs in a transwell setting essentially abolished the suppression of CD4+ T-cell proliferation (Figure 4C).
Finally, we tested the contribution of CD80 and CD86 co-stimulatory signaling to the suppressive capacity of sort-purified transitional or IgM memory B-cell subsets, prompted by evidence from both murine and human B-cell experimental systems.7,10 The addition of blocking antibodies against the CD80 or CD86 molecules individually did not reverse the suppressive activity of IgM memory and transitional B cells (data not shown); however, blocking antibodies against the 2 molecules together partially inhibited the ability of both transitional and IgM memory B cells to suppress the proliferation of CD4+ T cells (Figure 5A). Even though blocking mAbs against IL-10/IL-10R or CD80/CD86 individually failed to completely reverse the suppressive capacity of transitional and IgM memory B cells (Figure 5B), this end point was achieved with a combination of cell-cell contact blocking (with either a transwell block or CD80/CD86 antibodies) and antibodies to IL-10/IL-10R (Figure 5C). Thus, the suppressive effect of human Bregs depends on IL-10 production and cell-cell contact involving CD80 and CD86, consistent with findings in murine experimental models.7 However, the suppressive effect of human transitional and IgM memory B cells was independent of CD80 cointeraction with the inhibitory receptor PD-1 expressed on T cells (data not shown).
Figure 5.
CD80 and CD86 costimulatory signaling contributes to the suppressive effect of IgM memory and transitional B cells. (A) Purified CD4+ T cells cultured with sorted CD19+ B-cell subsets from healthy volunteers and stimulated with anti-CD3/anti-CD28 beads in the presence or absence of CD80- and CD86-blocking antibodies. Stimulated CD4+ T cells labeled with CFSE and cultured alone served as a positive control. (B) Purified CD4+ T cells cultured with sorted CD19+ B-cell subsets from healthy volunteers and stimulated with anti-CD3/anti-CD28 beads in the presence of CD80- and CD86-blocking antibodies and in the presence or absence of IL-10 blocking. Stimulated CD4+ T cells labeled with CFSE and cultured alone served as a positive control. (C-D) A combination of cell-cell contact blocking (with either a transwell block or CD80/CD86 antibodies) and antibodies to IL-10/IL-10R fully reverses the suppressive capacity of IgM memory and transitional B-cell subsets. In (A-D), bars represent the medians, and whiskers indicate the upper ranges of values from 3 independent experiments with 5 samples per group. *P < .05 by nonparametric ANOVA; ns, not significant.
To determine whether soluble CD40L is the trigger for IL-10 production in the transwell setting, we measured the levels of soluble CD40L in the supernatant by enzyme-linked immunosorbent assay. Soluble CD40L is naturally secreted by activated T cells21 and can induce IL-10 production by B cells.22 As is shown in supplemental Figure 4, soluble CD40L was present in the cocultures with CD3/CD28-activated CD4+ T cells. We propose that soluble CD40L can cross the membrane and induce IL-10 production by transitional and IgM memory B cells to mediate T-cell suppression.
Suppressive effect of IgM memory and transitional B cells in vitro is independent of Treg activity
To test whether the suppressive effects of IL-10+ transitional and IgM memory B cells are partly mediated by Tregs, we depleted the CD4+ T cells of CD25hiCD127lo Tregs by using magnetic cell purification. Treg depletion was highly efficient, with <0.1% residual CD25hiCD127loCD4+ T cells in the final T-cell product. The IgM memory and transitional B-cell subsets were then cultured with anti-CD3/anti-CD28–stimulated and CFSE-stained Treg-depleted CD4+ T cells at a ratio of 1:1. Both subsets, but not naïve or switched memory B cells, significantly suppressed the proliferation of Treg-depleted CD4+ T cells (supplemental Figure 3).
Bregs are deficient in patients with cGVHD
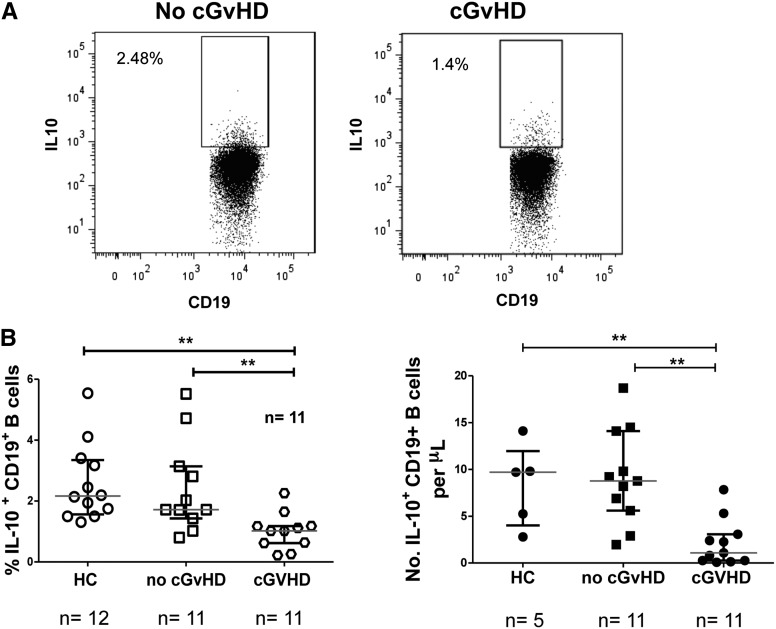
Results presented in Figure 2, showing that IgM memory and transitional B-cell subsets can suppress CD4+ T-cell proliferation and effector function, suggest that cGVHD might arise partly from a deficiency in either B-cell subsets or both. To assess this possibility, we examined 11 patients with cGVHD (supplemental Table 1), 11 without cGVHD and 12 healthy controls; the 2 patient groups had similar clinical characteristics (Table 1). Those receiving high-dose steroids (>0.75 mg/kg prednisone) at the time of sampling or those who had received rituximab for any reason were excluded. Chronic GVHD status at the time of sample collection was classified according to published criteria.23,24 In keeping with previous reports,25-27 cGVHD patients had fewer circulating CD19+ B cells than those without cGVHD (median 216 vs 378 cells/µL; P = .02), and transitional B cells were also significantly lower in patients with cGVHD compared with those without this complication or healthy controls (median 0.5 vs 22.3 vs 4.8 cells/µL, P = .0003 and P = .0001, respectively). We then compared the absolute number of IgM memory B cells per μL in cGVHD patients (median 14 [range 0.4-30.5]) with findings in patients without cGVHD (median 21.6 [range 2.4-69.9], P = .29) or in healthy controls (median 22 [5.4-73.8], P = .6); the differences were not significant. Repeated findings in patients with autoimmune disease10 suggest that regulatory B-cell responses may also be defective in patients with cGVHD. We therefore stimulated PBMCs from the same subjects reported in Table 1 and 12 healthy controls with irradiated L cells at a ratio of 1:10 for 48 hours, asking whether B cells from cGVHD patients had impaired IL-10 production when activated through CD40L followed by PMA+ionomycin stimulation. As is shown in Figure 6A-B, significantly fewer B cells from cGVHD patients secreted IL-10 when stimulated with CD40L compared with results in patients without cGVHD (median 1.02% [range 0.22-2.26] vs1.72% [range 0.8-5.52], P = .001) or healthy controls (1.02% [range 0.22-2.26] vs 2.16 [range 1.3- 5.6], P = .001). Furthermore, patients with moderate or severe cGVHD had lower absolute numbers of IL10+CD19+ B cells (0.5 vs 5 cells/μl P = .07). These findings suggest a fundamental defect in the ability of B cells in transplant recipients with cGVHD to respond to CD40L stimulation, thus restricting their production of IL-10.
Table 1.
Clinical characteristics of patients
| No cGVHD (n = 11) | cGVHD (n = 11) | P value | |
|---|---|---|---|
| Median age (y), n (range) | 56 (26-65) | 42 (37-64) | .74 |
| Median days post-SCT, n (range) | 436 (349-1112) | 342 (100-2947) | .24 |
| Sex* | |||
| Male | 6 (55) | 9 (82) | .23 |
| Female | 5 (45) | 2 (18) | |
| Disease status at transplantation* | |||
| Standard risk | 7 (64) | 6 (55) | .29 |
| High risk | 4 (36) | 5 (45) | |
| Conditional regimen* | |||
| Myeloablative conditioning | 4 (36) | 5 (45) | .5 |
| Reduced intensity conditioning | 7 (64) | 6 (55) | |
| Stem cell donors* | |||
| Related | 2 (18) | 8 (73) | .7 |
| Unrelated | 9 (82) | 3 (27) | |
| Stem cell source* | |||
| Bone marrow | 3 (27) | 1 (9) | .6 |
| Peripheral blood stem cells | 8 (73) | 10 (91) | |
| Median CD34+ cells transplanted ×106/kg, n (range) | 5.8 (0.8-12.2) | 8.7 (2.8-12.77) | |
| Posttransplant immunosuppressive prophylaxis* | |||
| Cyclosporine-methotrexate | 4 (36) | 5 (46) | .26 |
| Cyclosporine-methotrexate- T-cell antibodies | 7 (64) | 4 (36) | |
| Other | 0 (0) | 2 (18) | |
| Acute GVHD* | |||
| Grade 0 | 7 (64) | 3 (27) | .37 |
| Grades I-II | 3 (27) | 5 (46) | |
| Grades III-IV | 1 (9) | 3 (27) | |
| Donor lymphocyte infusions* | |||
| Yes | 3 (27) | 8 (73) | .14 |
| No | 8 (73) | 3 (27) |
The cohort includes patients who had received reduced intensity or myeloablative conditioning, followed by bone marrow or mobilized PB stem cell grafts. Those receiving high-dose steroids (>0.75 mg/kg prednisone) at the time of sampling or those who had received rituximab for any reason were excluded. cGVHD status at the time of sample collection was classified according to the National Institutes of Health cGVHD Consensus Criteria and modified Seattle Criteria for limited-vs-extensive disease. Eleven patients who were at a median of 14 months (range 11-37) posttransplant had active cGvHD (limited or extensive), whereas 11 patients who were 11 months (range 3-96) posttransplant lacked any history of cGVHD after HSCT. None of the no-cGVHD patients was on immunosuppressive therapy at the time of PB collection. All clinical characteristics, including age, sex, type of transplant, and underlying hematologic malignancy, were similar in patients with or without active cGVHD.
n (%).
Figure 6.
B cells from cGVHD patients show deficient IL-10 production. PBMCs from cGVHD (n = 11) or no-cGVHD (n = 11) patients and controls (n = 12) were incubated with L cells alone for 48 hours. PMA, ionomycin, and BFA were added for the last 6 hours of culture, and the cells were subsequently stained for the surface expression of CD19, CD3, and PI, and then intracellularly for IL-10. (A) Representative flow cytometry plots of IL-10 staining of gated CD19+ B cells from patients with or without cGVHD. Frequencies of IL10+ CD19+ B cells were assessed relative to the isotype controls. (B) cGVHD patients had lower frequencies and absolute numbers of IL-10+ B cells after stimulation with L cells and were assessed in relation to either the no-cGVHD patients or healthy controls (HC). The absolute number of CD19+ B-cell subsets was calculated by multiplying their frequencies as determined by flow cytometry by the absolute lymphocyte number (cells/µL) obtained from a diagnostic complete blood count (CBC) panel performed on the same day. CBC numbers were available for absolute CD19+IL-10+ B-cell calculation for 5 healthy controls. All values are reported as medians and interquartile ranges indicated by brackets. **P = .001 for cGVHD vs HC or no GVHD by nonparametric ANOVA.
Active cGVHD is characterized by a reduced IL-10+ B-cell to effector T-cell ratio
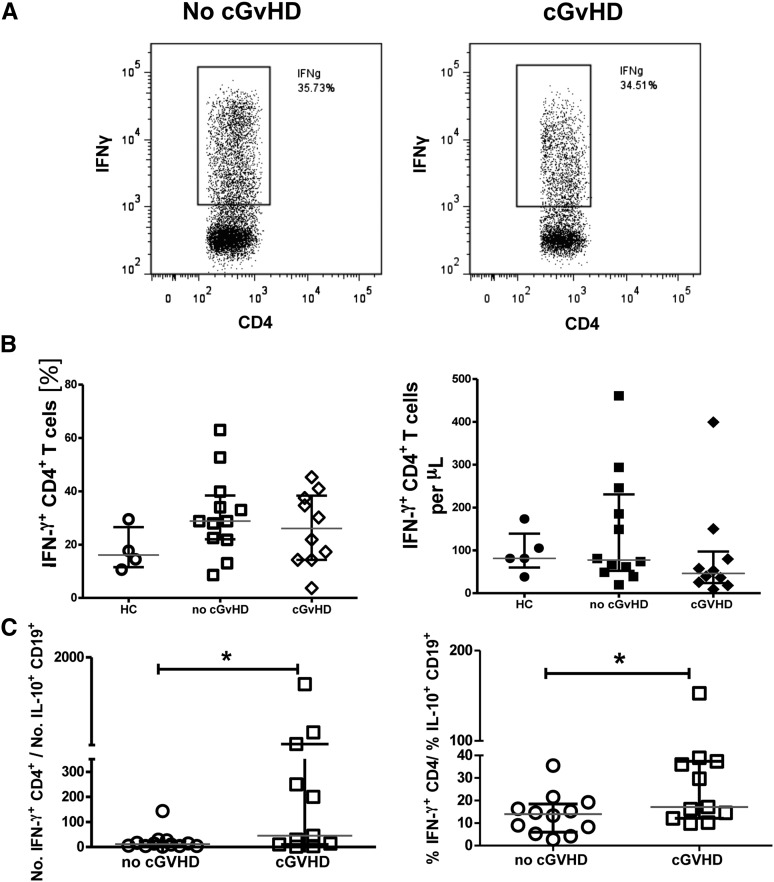
An imbalance between regulatory and effector T cells has been reported in patients with GVHD.15-17 This raises the possibility that the functional deficit noted before in the overall population of IL-10+CD19+ B cells may contribute to an altered ratio of regulatory-to-effector cells in transplant recipients with cGVHD. Thus, we investigated IFN-γ production by CD4+ T cells in patients with or without cGVHD and in healthy controls. CD4+ T cells were stimulated with PMA and inomycin in vitro for 6 hours, and the frequency of IFN-γ+CD4+ T cells assessed. Neither the frequency nor the number of IFN-γ+CD4+ T cells differed significantly between patients with or without cGVHD posttransplantation (Figure 7A-B). Nonetheless, patients with cGVHD had a significantly higher IFN-γ+CD4+/IL-10+CD19+ B-cell ratio, whether in terms of relative frequency or absolute counts, compared with that for the no-GVHD group (Figure 7C). Interestingly, patients with moderate or severe cGVHD had higher Th1/IL10 ratios (91.5 vs 5.6, P = .04) than did those with mild cGVHD. These results clearly suggest an imbalance between the regulatory and proinflammatory networks in patients with cGVHD, one that may stem from inadequate regulation of effector T cells by IL-10+ B cells.
Figure 7.
Patients with cGVHD have a reduced ratio of IL-10+ B-cell to IFN-γ+ CD4+ T cells. PBMCs from patients with cGVHD or no-cGVHD and healthy controls (HC) were stimulated with PMA (50 ng/mL) and ionomycin (250 ng/mL) (Sigma-Aldrich), and Brefeldin A (5 µg/mL) (Sigma-Aldrich) was added for the last 6 hours of the culture. Cells were then stained for the surface expression of CD4 and PI and intracellularly for IFN-γ. (A) Representative flow cytometry plots of IFN-γ staining of gated CD4+ T cells from cGVHD and no-cGVHD patients. Frequencies of IFN-γ+ CD4+ T cells were assessed relative to unstimulated controls. (B) Patients with cGVHD had comparable frequencies (left) and absolute numbers (right) of IFN-γ+ CD4+ T cells compared with no-cGVHD patients and healthy controls. (C) Dot plots comparing the ratios of IFN-γ+CD4+ T cells/IL10+ CD19+ B cells between patients with or without cGVHD. Values are medians, with interquartile ranges indicated by brackets (n = 11 per group); *P < .05 by 2-tailed nonparametric Student t test analysis.
Discussion
Regulation of the delicate balance between effector T-cell activities against invading pathogens and the tolerance of self- and environmental antigens has been largely ascribed to regulatory T cells.15,28,29 Recent research indicates that at least one functional group of B cells in human PB, CD19+CD24hiCD38hi transitional B cells, appears to possess robust immune regulatory capacity.10 CD40L-stimulated CD24hiCD27+ memory B cells have also been shown to release IL-10 and to suppress monocyte activation and cytokine production in vitro; however, they did not differentially reduce TNF-α expression by activated CD4+ T cells when compared with CD24lowCD27− B cells.22,30 Our results support the immunoregulatory function of CD19+CD24hiCD38hi transitional B cells and are the first to identify CD19+IgM+CD27+ memory B cells as a new candidate Breg subset in healthy individuals. These IgM memory B cells secreted IL-10 and suppressed both the proliferation and IFN-γ production of CD4+ T cells in a manner that was comparable with that of Tregs and transitional B cells. Immunoregulatory functions have also been attributed in mice to CD19+CD1dhiCD5+ B cells,3,31,32 marginal-zone B cells,33 marginal-zone precursors (T2-MZP), and transitional B cells,5,34,35 but evidence to formally link these cells to suppressive function in humans is lacking. Our discovery broadens the proportion of B cells with suppressive capacity among all circulating B cells in PB from <5% (CD19+CD24hiCD38hi transitional cells only) to 20% to 30% (transitional plus CD19+IgM+CD27+ memory B cells), suggesting a prominent role for this functional B-cell subset in the maintenance of immune tolerance.
To investigate whether IL-10 mediates Breg function, we used IL-10 blockade, which led to partial reversal of CD4+ T-cell proliferation in the presence of either IgM memory or transitional B cells. This supports the hypothesis that the regulatory function of these subsets relies mainly on IL-10. Recombinant IL-10 has been tried unsuccessfully as a therapeutic measure against some autoimmune diseases such as Crohn disease, rheumatoid arthritis, and psoriasis.36 Similarly, in our studies, exogenous addition of IL-10 to cocultures of naïve or switched memory B cells failed to induce CD4+ T-cell suppression to the same extent as is seen with transitional and IgM memory B cells, indicating that cell-cell contact may be required for IL-10 to deliver optimal immune-regulatory signals. Indeed, transwell experiments and C80/86 blockade confirmed that such contact is needed for full exertion of IgM memory and transitional B-cell suppressive activity against CD4+ T cells, although we found that the suppressive effect of these 2 subsets was independent of CD80 interaction with the inhibitory receptor PD-1 expressed on T cells (data not shown).
The B cells in patients with cGVHD display a spectrum of abnormalities, including aberrant expression or function of key signaling molecules, costimulatory pathways, cytokines, and perturbations in developmental B-cell subsets.37 These defects, together with the numerical and functional alterations of Tregs in cGVHD,17 led us to propose that human Bregs might be similarly affected in this disease. Indeed, a recent study showed an important role for Bregs in protection against a murine model of cGVHD.38,39 In addition, previously published work supports a role for IL-10 deficiency in this disease.40-42 We report that unlike healthy B cells, those from cGVHD patients display impaired IL-10 production when activated with CD40. These findings are consistent with the observation that in SLE, Breg cells are refractory to CD40 engagement.10 Such defects carry a number of implications, because the CD40L–CD40 axis is crucial for B-cell proliferation, immunoglobulin class switching, germinal center development, and the survival of memory B cells.43 However, because of the profound lymphopenia that characterizes the majority of patients with cGVHD, and the small volumes of PB available from patients with active cGVHD, it will still be necessary to directly test the suppressive capacity of purified IgM memory and transitional B cells derived from cGVHD patients (using coculture assays with CD4+ T cells).
As a result of the impaired production of IL-10, the ratio of IL-10+ B cells to IFN-γ+CD4+ T cells was significantly lower in cGVHD patients compared with the no-cGVHD group, which implies an imbalance between the regulatory B-cell and effector T-cell compartments analogous to that recognized with Tregs during the development of cGVHD.28 Our findings therefore support a broader than expected deficit, involving both Breg and Treg cells, in the immune-regulated network that is involved in the control of cGVHD, and may partly explain the observations of persistent B-cell activation19,44 and autoimmune phenomena45 that have been described in treatment-resistant cGVHD patients.
That some cGVHD patients,46 but not others, respond to rituximab suggests that B cells may have variable roles in this disease. CD20 is expressed equally on both IL-10–producing and IL-10–negative B cells. Hence, B-cell depletion by rituximab is likely to deplete both Bregs and pathogenic B cells involved in cGVHD. One could therefore speculate that B-cell depletion might improve cGVHD in cases in which effector B cells play a major role in the complex development of the disease, but not where Breg deficiency is the predominant component of the disease process. Thus, our data support future investigation of regulatory B cell–based therapy to tip the scales in favor of immune regulation so that cGVHD is either prevented or attenuated.
Acknowledgments
This work was funded in part by the Chronic Lymphocytic Leukemia Global Research Foundation. The flow studies were performed in the Flow Cytometry & Cellular Imaging Facility, which is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support grant CA016672.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.K. and A.S. performed experiments and designed, interpreted, analyzed, and wrote the manuscript; A.Alsuliman provided advice and performed experiments; C.C., T.S., N.C., S.M., H.d.L., M.M., I.F.C., H.S., E.L., P.A.M., A.Alousi, K.S., S.P., N.S., H.S., E.Y., J.M., R.R., R.C., I.M., C.M., and E.J.S. provided advice on experiments and commented on the manuscript; and K.R. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katayoun Rezvani, Stem Cell Transplantation and Cellular Therapy, MD Anderson Cancer Centre, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: krezvani@mdanderson.org.
References
- 1.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 3.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 4.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 5.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei B, Velazquez P, Turovskaya O, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci USA. 2005;102(6):2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 8.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6(11):636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 10.Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood. 2012;120(16):3318–3325. doi: 10.1182/blood-2012-05-432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saussine A, Tazi A, Feuillet S, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS ONE. 2012;7(8):e43588. doi: 10.1371/journal.pone.0043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64(2):187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielke S, Rezvani K, Savani BN, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110(5):1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93(11):1702–1711. doi: 10.3324/haematol.13311. [DOI] [PubMed] [Google Scholar]
- 19.Allen JL, Fore MS, Wooten J, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120(12):2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20(1):67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 22.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in man that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson K. Clinical Bone Marrow and Blood Stem Cell Transplantation. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 25.Corre E, Carmagnat M, Busson M, et al. Long-term immune deficiency after allogeneic stem cell transplantation: B-cell deficiency is associated with late infections. Haematologica. 2010;95(6):1025–1029. doi: 10.3324/haematol.2009.018853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgendorf I, Mueller-Hilke B, Kundt G, et al. The lack of memory B cells including T cell independent IgM+ IgD+ memory B cells in chronic graft-versus host disease is associated with susceptibility to infection. Transpl Int. 2012;25(1):87–96. doi: 10.1111/j.1432-2277.2011.01388.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita K, Choi U, Woltz PC, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4(+) effector memory cells relative to central memory cells. Blood. 2004;103(10):3986–3988. doi: 10.1182/blood-2003-09-3286. [DOI] [PubMed] [Google Scholar]
- 29.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 30.Bouaziz JD, Calbo S, Maho-Vaillant M, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40(10):2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 31.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182(12):7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104(35):14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 35.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55(2):241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 37.Goerner M, Gooley T, Flowers ME, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8(1):47–56. doi: 10.1053/bbmt.2002.v8.pm11858190. [DOI] [PubMed] [Google Scholar]
- 38.Le Huu D, Matsushita T, Jin G, et al. Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood. 2013;121(16):3274–3283. doi: 10.1182/blood-2012-11-465658. [DOI] [PubMed] [Google Scholar]
- 39.Weber M, Stein P, Prüfer S, et al. Donor and host B cell-derived IL-10 contributes to suppression of graft-versus-host disease. Eur J Immunol. 2014;44(6):1857–1865. doi: 10.1002/eji.201344081. [DOI] [PubMed] [Google Scholar]
- 40.Barak V, Levi-Schaffer F, Nisman B, Nagler A. Cytokine dysregulation in chronic graft versus host disease. Leuk Lymphoma. 1995;17(1-2):169–173. doi: 10.3109/10428199509051718. [DOI] [PubMed] [Google Scholar]
- 41.Liem LM, Fibbe WE, van Houwelingen HC, Goulmy E. Serum transforming growth factor-beta1 levels in bone marrow transplant recipients correlate with blood cell counts and chronic graft-versus-host disease. Transplantation. 1999;67(1):59–65. doi: 10.1097/00007890-199901150-00009. [DOI] [PubMed] [Google Scholar]
- 42.Körholz D, Kunst D, Hempel L, et al. Decreased interleukin 10 and increased interferon-gamma production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19(7):691–695. doi: 10.1038/sj.bmt.1700718. [DOI] [PubMed] [Google Scholar]
- 43.Wykes M. Why do B cells produce CD40 ligand? Immunol Cell Biol. 2003;81(4):328–331. doi: 10.1046/j.1440-1711.2003.01171.x. [DOI] [PubMed] [Google Scholar]
- 44.Sarantopoulos S, Stevenson KE, Kim HT, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117(7):2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117(7):2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 46.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]