Abstract
Annexin A9 (ANXA9) is involved with the interaction with membrane phospholipids in a Ca2+-dependent manner. A previous study has shown that ANXA9 expression is associated with bone metastasis in breast cancer, whereas its significance in colorectal cancer (CRC) is unknown. The present study was comprised of 100 patients who underwent surgery for CRC. The correlation between gene expression and the clinical parameters of the patients was assessed. Patients with high ANXA9 expression were statistically susceptible to a relatively worse prognosis, and those with low ANXA9 expression showed improved overall survival compared with those with high expression. In conclusion, the present data suggests that ANXA9 expression is a prognostic factor in CRC patients.
Keywords: ANXA9, prognosis, colorectal cancer
Introduction
In developed countries where the aging population is increasing, cancer is one of the most prominent illnesses in terms of public welfare and health. One in four mortalities in the United States, for example, is due to cancer (1). In the United States, the incidence of CRC has increased significantly in recent years, based on changing lifestyles. CRC is one of the most prominent causes of mortality from neoplastic disease in Japan. Distant metastases, such as liver or lung metastases, are the major cause of mortality in CRC (2). The ability to identify the genes responsible for CRC development and progression and the ability to understand the associated clinical significance are vital for diagnosing and treating CRC sufficiently. The characterization of key molecules has great potential with regard to the generation of novel approaches for the treatment of CRC.
The annexins are a family of well-conserved proteins, characterized by the ability to interact with membrane phospholipids in a Ca2+-dependent manner (3). The structures of all annexins contain type II Ca2+ sites that are located in the protein core domains, built of four or eight homologous segments known as annexin repeats (4,5). In breast cancer, ANXA9 has been reported as a gene that is associated with the relapse in bone (6). However, the association between ANXA9 expression and CRC remains unknown.
The aim of the present study was to analyze the correlation between ANXA9 expression levels in the CRC tissues of patients and the clinicopathological factors, and to investigate the possible functions of the gene in the tumorigenesis and metastasis of CRC.
Materials and methods
Clinical tissue samples
In total, 100 patients (61 males and 39 females) with CRC were registered and underwent curative surgery for resection of CRC and distant metastases, if present, at the Medical Institute of Bioregulation at Kyushu University (Beppu, Ohita, Japan) and the Department of Gastroenterological Surgery, Osaka University Graduate School of Medicine (Suita, Osaka, Japan) between 1994 and 2003. None of the patients received chemotherapy or radiotherapy prior to surgery. Primary CRC specimens and adjacent normal colorectal mucosa samples were obtained from the patients following the receipt of written informed consent. This study was approved by the ethics committee of Osaka University Graduate School of Medicine (Osaka, Japan). The surgical specimens were fixed in formalin, processed through graded ethanol and embedded in paraffin. The sections were stained with hematoxylin and eosin, and Elastica van Gieson (Merck Millipore, Billerica, MA, USA) stains, and the degree of histological differentiation, lymphatic invasion and venous invasion was examined. Additionally, sections from all specimens were frozen in liquid nitrogen immediately after resection and kept at −80°C until RNA extraction. Subsequent to surgery, the patients underwent follow-up blood examinations to assess the tumor markers, serum carcinoembryonic antigen (CEA) and cancer antigen (CA19-9), and imaging modalities, such as abdominal ultrasonography, computed tomography and chest X-rays, were performed every 3 to 6 months. Post-operatively, the stage III and IV patients received 5-fluorouracil-based chemotherapy for six months [mFOLFOX6; (oxaliplatin, 85 mg/m2, 5-fluorouracil 2800 mg/m2, for 2 weeks, for 12 courses), UFT (300 mg/m2/day × 28 days/5 weeks, for 5 courses), capecitabine (2500 mg/m2/day × 14 days/3 weeks, for 8 courses), or TS-1 (80 mg/m2/day × 28 days/6 weeks, for 4 courses)], whereas the stage I and II patients principally received no chemotherapy. All therapies were performed according to the Japanese Society for Cancer of the Colon and Rectum guidelines (7). Clinicopathological factors were assessed according to the tumor node metastasis (TNM) classification of the International Union Against Cancer (8).
RNA preparation and expression analysis
Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) or with DNase using a modified acid guanidium-thiocyanate-phenol-choroform procedure (9). Reverse transcription was performed with SuperScriptII (Life Technologies, Carlsbad, CA, USA) or by the methods reported previously (10). A 242-bp ANXA9 fragment was amplified. Two human ANXA9 oligonucleotide primers for the polymerase chain reaction (PCR) were designed as follows: Forward, 5′-TGAGCCCAATTACCAAGTCC-3′ and reverse, 5′-GTTCAGCCAAACACGGAAAT-3′. The forward primer is located in exon 13 and the reverse primer in exon 14. A PCR kit (TaKaRa Ex Taq; Takara, Kyoto, Japan) on GeneAMP PCR System 9600 (PE Applied Biosystems, Foster City, CA, USA) was used to perform 35 cycles of PCR with the following parameters: 95°C for 40 sec, 45°C for 40 sec and 72°C for 60 sec. An 8-μl aliquot of each reaction mixture was size-fractionated in a 1.5% agarose gel and visualized with ethidium bromide staining. To ensure that the RNA was not degraded, a PCR assay with primers specific for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was performed for 1 min at 95°C, 1 min at 56°C and 1 min at 72°C for 30 cycles. The GAPDH primers were as follows: Forward, 5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse, 5′-TGTCATCATATTGGCAGGTT-3′, and produced a 270-bp amplicon. Complementary DNA from the Human Reference Total RNA (Clontech, Palo Alto, CA, USA) was studied concurrently as a source of positive controls. For quantitative assessment, reverse transcription-quantitative PCR (RT-qPCR) was performed using a LightCycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Tokyo, Japan) for cDNA amplification of ANXA9 and GAPDH. The amplification protocol consisted of 35 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec and elongation at 72°C for 10 sec. The products were then subjected to a temperature gradient from 55°C to 95°C with continuous fluorescence monitoring to produce a melting curve of the products. The expression ratios of the ANXA9 mRNA copies in the tumor and normal tissues were calculated following normalization against GAPDH mRNA expression.
Statistical analysis
The ANXA9 expression levels between the CRC and normal colorectal mucosa (normal tissue) samples, and the association between ANXA9 expression and the clinicopathological factors were analyzed with the χ2 test. Kaplan-Meier survival curves were plotted and compared with the generalized log-rank test. Univariate and multivariate analyses to identify prognostic factors were performed using Cox’s proportional hazard regression model. The values in the in vitro assays were analysed with Wilcoxon’s rank test. All tests were analyzed with JMP software (SAS Institute, Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of ANXA9 in clinical tissue specimens
RT-qPCR analysis was performed with primary CRC tissues and samples from adjacent normal colorectal regions. ANXA9 expression was calculated as ANXA9/GAPDH expression for each tumor or normal tissue sample (Fig. 1). The mean expression level in the tumor tissues was found to be larger than that of the normal tissues, and there was a significant difference between the tumor and normal tissues subsequent to dividing the samples into two groups according to the mean expression value of the tumor and normal tissues (P=0.047) (Table I). In the following analyses, ANXA9 expression normalized by GAPDH expression in the tumor tissue was calculated following division by ANXA9 expression level in the normal tissue.
Figure 1.
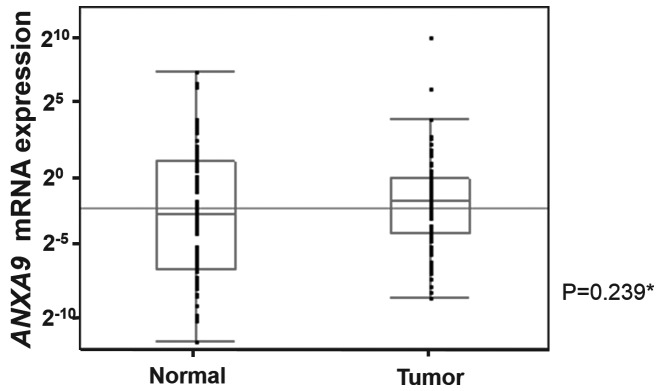
ANXA9 mRNA expression in clinical tissue specimens. Reverse transcription-quantitative polymerase chain reaction on 100 paired clinical samples showed that 56 of these cases (56.0%) exhibited higher levels of ANXA9 mRNA in the tumor samples compared with the paired normal tissues. The mean ANXA9 mRNA expression level in the tumor tissues (normalized by GAPDH gene expression) was not significantly different compared with that of the corresponding normal tissues (P=0.239, Wilcoxon’s rank test). ANXA9, annexin A9; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Table I.
ANXA9 mRNA expression in primary CRC specimens and normal colorectal mucosa samples.
| ANXA9 expression | Primary CRC | Normal mucosa | P-value |
|---|---|---|---|
| <Mean | 41 | 55 | 0.047* |
| ≥Mean | 59 | 45 |
Statistically significant, P<0.05.
ANXA9, annexin A9; CRC, colorectal cancer.
Expression of ANXA9 and clinicopathological characteristics
For the clinicopathological evaluation, experimental samples were divided into two groups according to the expression status. Patients with values >1 (ANXA9 expression level in the tumor tissue was larger than that of the corresponding normal tissue) were assigned to the high expression group and the others were assigned to the low expression group. Clinicopathological factors associated with the ANXA9 expression status of the 100 patients are summarized in Table II. The data indicated that ANXA9 expression was not significantly correlated with these clinicopathological factors.
Table II.
Clinicopathological factors of 100 colorectal cancer patients with high (n=56) and low (n=44) levels of ANXA9 mRNA expression.
| Factors | Low expression, n (%) | High expression, n (%) | P-value |
|---|---|---|---|
| Age, years | |||
| <68 | 25 (56.8) | 27 (48.2) | 0.392 |
| ≥68 | 19 (43.2) | 29 (51.8) | |
| Gender | |||
| Male | 24 (54.5) | 37 (66.1) | 0.244 |
| Female | 20 (45.5) | 19 (33.9) | |
| Histological grade | |||
| Well-moderate | 41 (93.2) | 55 (98.2) | 0.202 |
| Poor | 3 (6.8) | 1 (1.8) | |
| Tumor size, mm | |||
| <30 | 8 (18.2) | 9 (16.1) | 0.780 |
| ≥30 | 36 (81.8) | 47 (83.9) | |
| Tumor invasion | |||
| Tis | 4 (9.1) | 3 (5.4) | 0.316 |
| T1 | 7 (15.9) | 6 (10.7) | |
| T2 | 9 (20.4) | 6 (10.7) | |
| T3 | 19 (43.2) | 28 (50.0) | |
| T4 | 5 (11.4) | 13 (23.2) | |
| Lymph node metastasis | |||
| N0 | 31 (70.5) | 33 (58.9) | 0.233 |
| N1–2 | 13 (29.5) | 23 (41.1) | |
| Lymphatic invasion | |||
| Absent | 20 (45.5) | 26 (46.4) | 0.824 |
| Present | 24 (54.5) | 30 (53.6) | |
| Venous invasion | |||
| Absent | 33 (75.0) | 42 (75.0) | 1.000 |
| Present | 11 (25.0) | 14 (25.0) | |
| Metastasis | |||
| M0 | 38 (86.4) | 50 (89.3) | 0.655 |
| M1 | 6 (13.6) | 6 (10.7) | |
| UICC stage | |||
| 0-I | 17 (38.6) | 13 (23.2) | 0.221 |
| II | 13 (29.5) | 18 (32.1) | |
| IIIA | 4 (9.1) | 14 (25.0) | |
| IIIB | 4 (9.1) | 5 (8.9) | |
| IV | 6 (13.6) | 6 (10.7) | |
ANXA9, annexin A9; UICC, International Union Against Cancer.
Association between ANXA9 expression and prognosis
The data shows that the post-operative overall survival rate was significantly lower in the patients in the high expression group compared with that in the low expression group (P=0.010) (Fig. 2). The median follow-up time was 3.83 years. Table III shows the results of the univariate and multivariate analyses for factors associated with overall survival. The univariate analysis showed that age (P=0.010), tumor invasion (P=0.002), lymph node metastasis (P=0.001), lymphatic invasion (P=0.012), venous invasion (P=0.026), metastasis (P<0.001) and ANXA9 expression (P=0.004) were significantly correlated with overall survival. The multivariate regression analysis indicated that the ANXA9 high expression group (hazard ratio, 3.13; 95% confidence interval, 1.32–12.86; P=0.007), tumor invasion (hazard ratio, 2.21; 95% confidence interval, 1.09–5.02; P=0.026) and distant metastasis (hazard ratio, 10.82; 95% confidence interval, 3.08–35.36; P=0.001) were independent predictors of overall survival.
Figure 2.
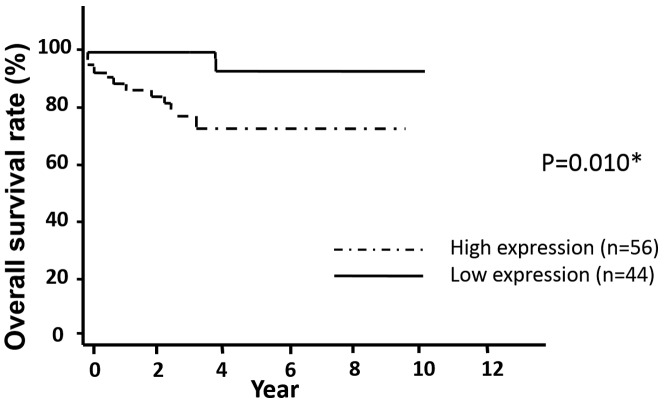
Overall survival curves based on ANXA9 mRNA expression status of colorectal cancer patients. The post-operative overall survival rate was significantly lower in the patients in the high expression group compared with the low expression group (P=0.010, log-rank test). ANXA9, annexin A9.
Table III.
Univariate and multivariate analyses for overall survival (Cox’s proportional hazards regression model).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age, years (<68/≥68) | 5.43 | 1.42–35.40 | 0.010* | 2.58 | 0.52–18.92 | 0.252 |
| Gender (male/female) | 1.96 | 0.58–8.84 | 0.288 | |||
| Histological grade (poor/well-moderate) | 0.01 | 0.00–3.02 | 0.495 | |||
| Tumor size, mm (≥30/<30) | 1.13 | 0.58–2.90 | 0.731 | |||
| Tumor invasion (T4/Tis-3) | 2.47 | 1.39–4.54 | 0.002* | 2.21 | 1.09–5.02 | 0.026* |
| Lymph node metastasis (N1–2/N0) | 6.88 | 2.04–31.12 | 0.001* | 2.07 | 0.45–10.76 | 0.342 |
| Lymphatic invasion (present/absent) | 5.31 | 1.39–34.63 | 0.012* | 1.49 | 0.29–10.93 | 0.640 |
| Venous invasion (present/absent) | 3.78 | 1.17–12.14 | 0.026* | 2.77 | 0.77–10.59 | 0.116 |
| Distant metastasis (M1/M0) | 10.82 | 3.08–35.36 | <0.001* | 16.64 | 2.92–132.64 | 0.001* |
| ANXA9 expression (high/low) | 3.00 | 1.32–12.86 | 0.004* | 3.13 | 1.30–13.69 | 0.007* |
Statistically significant.
HR, hazard ratio; CI, confidence interval; ANXA9, annexin A9.
Discussion
The annexin gene family was discovered in 1984 and the members were isolated in the presence of calcium, which served as a substrate for the epidermal growth factor receptor/kinase (11,12). ANXA9, initially termed annexin 31, is a protein that is believed to function in the organization and regulation of membrane/cytoskeleton linkage (13). The gastrointestinal cancer cell line, HepG2, expresses ANXA9 protein, which can be detected by A9-specific antibodies (3). The expression of annexin has been reported in Xenopus, Drosophilia, Dictyostelium, Caenorhabditis elegans, Neurospora, Giardia and all plants (14–16). However, the role of annexin in cancer has not been clearly defined and the biological analysis remains incomplete.
The present study showed that ANXA9 expression is an independent prognostic factor for CRC. This suggests that tumor malignancy correlates with ANXA9 expression and that this may also affect the values of other prognostic factors in multivariate analysis, such as distant metastasis, which was significant in the univariate analysis. ANXA9 expression was a significant prognostic factor reflecting overall survival and distant metastasis. To the best of our knowledge, the present study is the first to show ANXA9 as a statistically significant predictor for CRC prognosis following curative resection, as well as other reported factors (17). The present results suggest that an ANXA9-dependent pathway may be involved in the progression of CRC.
The prediction of recurrence and metastases following curative surgical resection aid the determination of the necessity for intensive follow-up and adjuvant CRC therapy (18–20). While certain patients respond well to CRC treatment, others do not, therefore individualized predictions and strategies with higher precision are required for treating metastasis (21). In the present study, the clinicopathological analysis revealed that CRC patients with low levels of ANXA9 expression showed an improved prognosis for overall survival compared with those patients with high levels of expression. The data indicates that ANXA9 is a presumptive novel predictor of CRC prognosis.
Several adjuvant chemotherapies are valuable in specific stages of CRC and indicate the usefulness of less invasive surgery for the disease (17–20,22–28). For these cases, an informative prognostic marker, which is independent from the traditional TNM classification and contributes to diagnoses and treatments, is extremely important. The present data indicate the candidacy of ANXA9. While improved pre-operative and post-operative treatments, such as chemotherapy and radiotherapy combined with surgery, have contributed to the reduction of recurrences of CRC, half of all cases eventually metastasize despite systemic chemotherapy followed by surgery (29). Adjuvant chemotherapy for CRC is preferred in highly suspicious cases of recurrence. In these cases, ANXA9 analysis may aid in the predictions and treatment of patients with a poor prognosis.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Stein U, Schlag PM. Clinical, biological, and molecular aspects of metastasis in colorectal cancer. Recent Results Cancer Res. 2007;176:61–80. doi: 10.1007/978-3-540-46091-6_7. [DOI] [PubMed] [Google Scholar]
- 3.Goebeler V, Ruhe D, Gerke V, Rescher U. Atypical properties displayed by annexin A9, a novel member of the annexin family of Ca(2+) and lipid binding proteins. FEBS Lett. 2003;546:359–364. doi: 10.1016/s0014-5793(03)00634-3. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins TE, Merrifield CJ, Moss SE. Calcium signaling and annexins. Cell Biochem Biophys. 2000;33:275–296. doi: 10.1385/cbb:33:3:275. [DOI] [PubMed] [Google Scholar]
- 5.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 6.Smid M, Wang Y, Klijn JG, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24:2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum: Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 8.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Mimori K, Mori M, Shiraishi T, et al. Clinical significance of tissue inhibitor of metalloproteinase expression in gastric carcinoma. Br J Cancer. 1997;76:531–536. doi: 10.1038/bjc.1997.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori M, Staniunas RJ, Barnard GF, et al. The significance of carbonic anhydrase expression in human colorectal cancer. Gastroenterology. 1993;105:820–826. doi: 10.1016/0016-5085(93)90900-w. [DOI] [PubMed] [Google Scholar]
- 11.Fava RA, Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984;259:2636–2645. [PubMed] [Google Scholar]
- 12.Huang KS, Wallner BP, Mattaliano RJ, et al. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986;46:191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- 13.Morgan RO, Bell DW, Testa JR, Fernandez MP. Human annexin 31 genetic mapping and origin. Gene. 1999;227:33–38. doi: 10.1016/s0378-1119(98)00597-6. [DOI] [PubMed] [Google Scholar]
- 14.Morgan RO, Fernández MP. Molecular phylogeny of annexins and identification of a primitive homologue in Giardia lamblia. Mol Biol Evol. 1995;12:967–979. doi: 10.1093/oxfordjournals.molbev.a040290. [DOI] [PubMed] [Google Scholar]
- 15.Morgan RO, Fernandez MP. Annexin gene structures and molecular evolutionary genetics. Cell Mol Life Sci. 1997;53:508–515. doi: 10.1007/s000180050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun EL, Kang S, Nelson MA, Natvig DO. Identification of the first fungal annexin: analysis of annexin gene duplications and implications for eukaryotic evolution. J Mol Evol. 1998;47:531–543. doi: 10.1007/pl00006409. [DOI] [PubMed] [Google Scholar]
- 17.André T, Quinaux E, Louvet C, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: final results of GERCOR C96.1. J Clin Oncol. 2007;25:3732–3738. doi: 10.1200/JCO.2007.12.2234. [DOI] [PubMed] [Google Scholar]
- 18.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornmann M, Formentini A, Ette C, et al. Prognostic factors influencing the survival of patients with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol. 2008;34:1316–1321. doi: 10.1016/j.ejso.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Bathe OF, Dowden S, Sutherland F, et al. Phase II study of neoadjuvant 5-FU + leucovorin + CPT-11 in patients with resectable liver metastases from colorectal adenocarcinoma. BMC Cancer. 2004;4:32. doi: 10.1186/1471-2407-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadanandam A, Lyssiotis CA, Homicsko K, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19:619–625. doi: 10.1038/nm.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iijima M, Kano Y, Nohno T, Namba M. Cloning of cDNA with possible transcription factor activity at the G1-S phase transition in human fibroblast cell lines. Acta Med Okayama. 1996;50:73–77. doi: 10.18926/AMO/30489. [DOI] [PubMed] [Google Scholar]
- 23.Hansen WJ, Cowan NJ, Welch WJ. Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins. J Cell Biol. 1999;145:265–277. doi: 10.1083/jcb.145.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgson G, Hager JH, Volik S, et al. Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet. 2001;29:459–464. doi: 10.1038/ng771. [DOI] [PubMed] [Google Scholar]
- 25.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 26.Weeks JC, Nelson H, Gelber S, et al. Clinical Outcomes of Surgical Therapy (COST) Study Group: Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 28.Jayne DG, Guillou PJ, Thorpe H, et al. UK MRC CLASICC Trial Group: Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 29.Koshariya M, Jagad RB, Kawamoto J, et al. An update and our experience with metastatic liver disease. Hepatogastroenterology. 2007;54:2232–2239. [PubMed] [Google Scholar]


