Abstract
TROP-2 is a pancarcinoma marker that is expressed at high levels in many epithelial cancers, including prostate cancer (PC). The trivalent bispecific antibody TF12 (anti-TROP2×anti-HSG [histamine-succinyl-glycine]) has shown to effectively target PC. In this study, the efficacy of pretargeted radioimmunotherapy (PRIT) with multiple cycles of TF12 and 177Lu-labeled diHSG-peptide (IMP288) in mice with s.c. PC3 tumors was investigated and compared with that of conventional RIT with 177Lu-labeled anti-TROP-2 mAb hRS7.
Methods: The potential of one, two, and three cycles of PRIT using the TF12 pretargeted 177Lu-IMP288 (41 MBq per cycle) was determined in mice with s.c. PC3 tumors, and compared with the efficacy and toxicity of RIT with 177Lu-hRS7 dosed at the maximum tolerated dose (11 MBq).
Results: PRIT of two and three cycles showed significantly higher median survival (>150 days) compared with PRIT of one cycle of TF12 and 177Lu-IMP288 (111 days, p<0.001) or the controls (76 days, p<0.0001). All mice treated with the mAb 177Lu-hRS7 survived at the end of the experiment (150 days), compared with 80% in the mice that were treated with three cycles of PRIT and 70% in the group that received two cycles of PRIT. Clinically significant hematologic toxicity was found only in the groups that received either three cycles of PRIT (p<0.0009) or RIT (p<0.0001).
Conclusions: TROP-2-expressing PC can be targeted efficiently with TF12 and radiolabeled IMP288. 177Lu-IMP288 accumulated rapidly in the tumors. PRIT of multiple cycles inhibited the growth of s.c. PC3 tumors. Clinically relevant hematological toxicity was observed in the group that received three cycles of PRIT; however, conventional RIT with the parent mAb 177Lu-hRS7 was at least as effective with similar toxicity.
Key words: : bispecific monoclonal antibody, pretargeted radioimmunotherapy, prostate cancer, TROP-2
Introduction
Treatment options for metastatic castrate resistant prostate cancer (CRPC) are increasing. As current therapeutic agents have only limited efficacy, there remains a great need to develop effective treatments of prostate cancer (PC), once it has advanced to the hormone-independent stage. Radioimmunotherapy (RIT) with the use of radiolabeled monoclonal antibodies in PC patients has been reported with the 177Lu-labeled anti-PSMA antibody J591.1,2 However, due to the long circulatory half-life of agents based on intact antibody molecules, clinically relevant myelotoxicity limits the activity dose that can be safely administered. To avoid toxicity related to slow clearance of radiolabeled antibodies from the circulation, a pretargeting approach can be applied. In pretargeting, tumors are targeted by a nonradiolabeled bispecific antibody, allowing the unbound antibody to clear from the circulation, followed by injection of a radiolabeled small molecule that is recognized by the bispecific antibody. The unbound radiolabeled compound then rapidly accumulates in the tumor or clears quickly from the circulation.3 A new and potent pretargeting strategy consists of administration of a trivalent bispecific monoclonal antibody (bsmAb) followed by administration of a radiolabeled diHSG hapten peptide. In a phase I/II clinical trial Schoffelen et al. have demonstrated the potential of such an approach to target colorectal carcinoma in patients.4
For pretargeting of PC, the bsmAb TF12 was developed, based on the monoclonal antibody hRS7.5 hRS7 is a humanized IgG1 monoclonal antibody directed against TROP-2, also known as EGP-1 (epithelial glycoprotein-1), GA733-1, gp50/T16, and TACSTD2 (tumor-associated calcium signal transducer 2). TROP-2 is a 46-kDa transmembrane glycoprotein overexpressed in carcinomas of the lung, bladder, breast, cervix, ovary, stomach, and prostate.6 Most normal human tissues do not express TROP-2, but low levels are present in several normal glandular cells, including glands in the bronchus, breast, prostate and skin, and ducts and acini of the pancreas.7 Given its overexpression in PC, we studied the potential targeting ability of hRS7 IgG in a nude mouse–human PC model,8 showing excellent in vivo targeting of PC3 xenografts with 89Zr- and 111In-hRS7 IgG within 3 days. The slow clearance from the circulation results in low tumor-to-background ratios, especially at earlier time points after i.v. injection.
The bsmAb TF12 is a trivalent bsmAb that consists of two anti-TROP-2 Fab fragments and one antihistamine-succinyl-glycine (HSG) Fab fragment.9 In this approach, unlabeled TF12 is injected intravenously, and when it has localized in the tumor and cleared from the blood, a diHSG-substituted radiolabeled hapten-peptide is injected. This hapten-peptide will be trapped in the tumor by the anti-HSG arm of the bsmAb or is rapidly cleared from the body. Previous feasibility studies have shown the potential of pretargeted radioimmunotherapy (PRIT) using TF12 and 177Lu-IMP288.5 Due to the unavailability of carrier-free 177LuCl3, studies were performed with a 177Lu dose that was below maximum tolerated dose (MTD). Since then, 177LuCl3 with high specific activity (>3000 GBq/mg) has become available, enabling labeling of the low peptide dose of IMP288 with a higher activity dose.
In this study, the potential of different regimens of PRIT with TF12 and the radiolabeled di-HSG peptide IMP288 in mice with human PC xenografts was investigated.
Materials and Methods
The anti-TROP-2×anti-HSG bsmAb TF12 was produced using the Dock-and-Lock technology (DNL®) as described by Rossi et al.,10 and made available from IBC Pharmaceuticals, Inc., a subsidiary of Immunomedics, Inc. The production and characterization of the anti-TROP-2 mAb hRS7 have been described previously.6 The DOTA-conjugated hapten-peptide IMP288 [DOTA-D-Tyr-D-Lys(HSG)-D-Glu-D-Lys(HSG)-NH2] was prepared as described by McBride et al.11
Cell culture
The human PC cell line PC3 is an androgen-independent cell line, originally derived from a PC bone metastasis. Cells were obtained from ATCC (CRL 1435) and were grown in RPMI 1640 medium (GIBCO, Life Technologies Corporation), supplemented with 10% fetal calf serum (Life Technologies). For subcutaneous inoculation, PC3 cells were washed with 0.9% NaCl, disaggregated with trypsin, and resuspended in 67% complete RPMI 1640 medium and 33% Matrigel (BD Biosciences) to the appropriate concentration (3×106 cells/200 μL).
Tumor model
All experiments were approved by the institutional Animal Welfare Committee of the Radboud University Nijmegen Medical Centre, and were conducted in accordance with the principles set forth by the Revised Dutch Act on Animal Experimentation.
Male BALB/c nude mice (Janvier SAS), 8–9-weeks old, were adapted to laboratory conditions for at least 1 week before experimental use. They were housed under nonsterile standard conditions in individually ventilated cages (five mice per cage; Tecniplast), with free access to animal chow (Sniff Voer®) and water.
The mice were inoculated s.c. in the flank with 200 μL of PC3 cell suspension (3×106 cells in 67% complete RPMI 1640 medium and 33% Matrigel; BD Biosciences). The s.c. PC3 tumors grew to ∼0.1 g in 10 days after tumor cell inoculation, as determined by caliper measurements in three dimensions using the formula V=4/3π (length/2×width/2×height/2), assuming that the density of tumor tissue is 1 g/cm3. The radiolabeled preparations (0.2 mL) were injected intravenously via the tail vein.
Radiolabeling of IMP288 and hRS7
The DOTA-conjugated IMP288 was radiolabeled with 177Lu (177LuCl3, noncarrier added; ITG Isotopen Technologien Garching AG). Radiolabeling was performed essentially as described previously by Schoffelen et al.12 Prior to i.v. injection, the preparation was diluted at least 2.5 times with 0.5% bovine serum albumin (BSA; Sigma-Aldrich) in phosphate-buffered saline (PBS).
DTPA conjugation and subsequent labeling of hRS7 IgG with 177Lu were performed essentially as described previously.6,8,13 The radiochemical purity (RCP) of the labeled IMP288 and hRS7 preparations was determined using instant thin-layer chromatography (ITLC), and reversed-phase high-performance liquid chromatography for IMP288 as described previously.12 ITLC using silicagel strips (Biodex) was performed to determine the fraction of unbound 177Lu (mobile phase: 0.1 M citrate, pH 6.0; Merck).
For RP-HPLC, the C18 column (Zorbax Rx-C18; 5 μm, 4.6×250 mm2; Agilent Technologies) was eluted with a mixture of 97% of a 0.1% trifluoroacetic acid in H2O solution (TFA; Sigma-Aldrich) with 3% of a 0.1% TFA in acetonitrile solution (Lab-scan, Analytical Sciences) with a linear gradient to 100% of the latter solution over 10 minutes at a flow rate of 1 mL/min. RCP of all labeled IMP288 preparations always exceeded 97%; the RCP of 177Lu-hRS7 exceeded 98%.
Immunoreactivity
The anti-TROP2 reactivity of radiolabeled TF12 and hRS7 was determined using freshly trypsinized PC3 cells, as described by Lindmo et al.14 with minor modifications. The bispecific immunoreactivity of TF12 was demonstrated by incubating PC3 cells with TF12 (10 μg/mL) during 30 minutes at 37°C and washing and subsequent incubation with 111In-IMP288 (40,000 cpm). After 1 hour at 37°C, the total activity and activity in the cell pellet were determined in a γ-counter. About 88% of the added hapten-peptide specifically bound to the pretargeted PC3 cells.
Pretargeted RIT
The therapeutic effect of a single or multiple cycles of PRIT with TF12 and 177Lu-IMP288 was assessed in mice with PC3 xenografts (10 mice/group) and compared with the efficacy of conventional RIT using the radiolabeled parent mAb 177Lu-hRS7. Mice were treated with the first cycle of TF12/177Lu-IMP288 or 177Lu-hRS7, 10 days after PC3 cell inoculation, when tumors weighed ∼0.1 g.
In the mice that were treated with PRIT, tumors were pretargeted with 2.5 nmol TF12 (462 μg) 16 hours before administration of the 177Lu-IMP288.5 The next day, mice received the maximum activity dose of 177Lu activity that could be labeled onto 0.1 nmol (160 ng) IMP288 (41 MBq, specific activity=410 MBq/nmol). Two additional groups of 10 mice received a second cycle or second and third cycles of TF12/177Lu-IMP288, respectively. Time interval between cycles was 48 hours, as determined in earlier experiments.5 Another group was treated with one cycle of 177Lu-hRS7 at the MTD (11 MBq, 15 μg [0.1 nmol], specific activity=110 MBq/nmol).
One control group of PC3-tumor-bearing mice was injected i.v. with vehicle (200 μL PBS and 0.5% BSA) twice instead of TF12 and IMP288. The second control group received vehicle instead of TF12 and was injected 16 hours later with 41 MBq of 177Lu-IMP288. A third control group was neither inoculated with PC3 cells, nor received any RIT. The latter group was included to determine whether effects seen in the kidneys were attributable to the RIT, or to regular aging processes.
Tumor size was measured in three dimensions with a caliper and body weight was determined twice weekly, starting 7 days after tumor cell inoculation. Blood samples of 0.1 mL of all mice were collected via submandibular bleeding before therapy, and 26, 33, 47, and 60 days after administration of the agents to determine hemoglobin levels, and leukocyte and platelet counts. When the humane endpoint was reached (tumor size >2 cm3 or animal discomfort level >3 as judged by a blinded biotechnician), mice were euthanized by O2/CO2-asphyxiation and dissected. At dissection, the tumor and kidneys were excised, weighed, fixed in 4% formalin, and processed for paraffin sectioning. The experiment was terminated 150 days after injection of the radiolabeled compounds and the remaining mice were euthanized and dissected. Sections of the kidneys were hematoxylin and eosin and periodic acid Schiff stained and evaluated by a nephropathologist to determine potential (chronic) kidney damage caused by the PRIT.
Biodistribution studies
The effect of the multiple cycles of TF12 and 177Lu-labeled IMP288 on tumor uptake in mice with s.c. PC3 tumors was determined by administering one, two, or three cycles of TF12/177Lu-IMP288 (0.4 MBq/cycle) to groups of five mice, as described previously. Two hours later, mice were dissected and tissue uptake was determined in a γ-counter. Biodistribution of the 177Lu-hRS7 (0.4 MBq) 3 days after injection of the radiolabeled antibody was also determined in this mouse model. Tumor uptake, blood levels, and uptake in relevant tissues (muscle tissue, lung, spleen, kidney, liver, intestine, prostate, femur, and bone marrow) were determined. The uptake as percentage of injected dose per gram tissue (% ID/g) in each tissue sample was calculated.
SPECT/CT imaging
To visualize the in vivo distribution of the radiolabeled compounds, SPECT/CT scans were acquired 7 hours (TF12/177Lu-IMP288) or 3 days (177Lu-hRS7) after injection of the 177Lu-labeled agent, respectively. Mice were scanned using a U-SPECT II microSPECT/CT scanner (MILabs). Mice were anesthetized using isoflurane/O2 (5% induction and 2.5% maintenance) and scanned for 30 minutes using the 1.0-mm-diameter pinhole collimator tube. Scans were reconstructed with MILabs reconstruction software, which uses an ordered-subset expectation maximization algorithm, with a voxel size of 0.375 mm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0 for windows. Survival curves were compared using the log-rank test. The level of significance was set at a p-value of <0.05. Differences in uptake were tested for significance using the nonparametric Mann–Whitney test; differences in survival were calculated using the log-rank test.
Results
Biodistribution studies
The in vivo distribution of 177Lu-IMP288 (0.1 nmol, 0.4 MBq) in mice that were pretargeted 16 hours earlier with TF12 was determined 2 hours after injection of the radiolabeled diHSG peptide. Uptake of 177Lu-IMP288 in the PC3 tumors after one (8.5%±1.4% ID/g), two (9.0%±2.2% ID/g), or three (8.2%±1.4% ID/g) cycles of PRIT showed no significant differences (p>0.9, Fig. 1A). Two hours after injection, blood levels (<1% ID/g), and uptake in normal tissues were low in all groups. Organ distribution of the radiolabel did not show any significant differences among the three groups, nor obvious trends. 177Lu-labeled parental mAb hRS7 showed high tumor uptake at 3 days after injection (62.0%±11.0% ID/g, Fig. 1B), along with high blood levels (10.0%±1.6% ID/g).
FIG. 1.
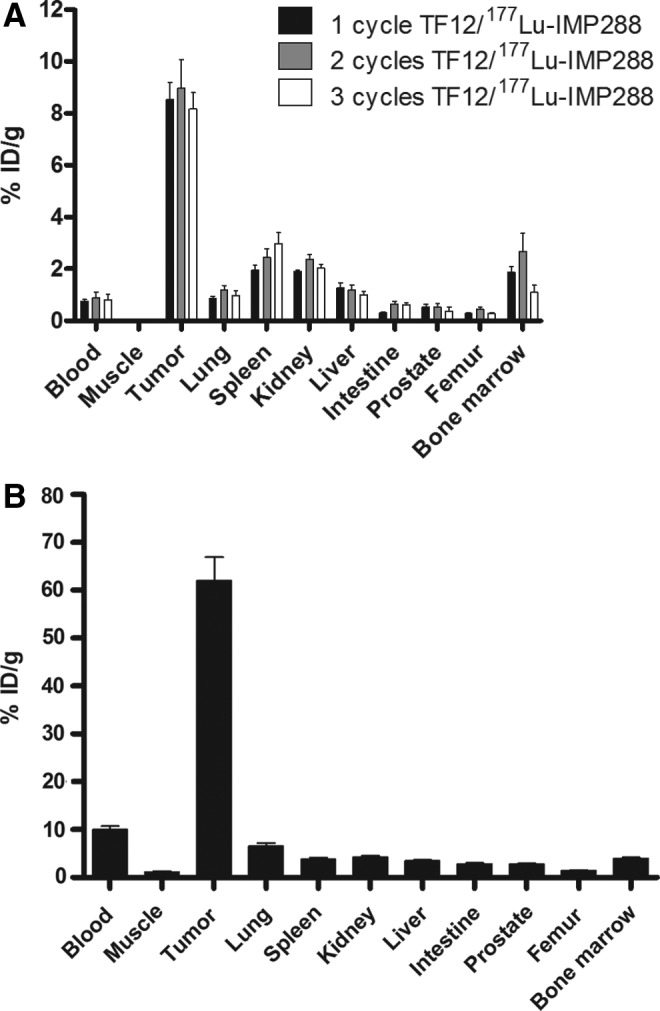
(A) Biodistribution of 177Lu-IMP288 after one, two, or three cycles of 177Lu-IMP288 (0.1 nmol, 0.4 MBq) injected i.v. 16 hours after the bsmAb TF12 (2.5 nmol) in BALB/c nude mice with a subcutaneous PC3 xenograft at 2 hours after injection (n=5). (B) Biodistribution of 177Lu-hRS7 (15 μg, 0.4 MBq) in BALB/c nude mice with a subcutaneous PC3 xenograft measured 3 days after injection (n=5). bsmAb, bispecific monoclonal antibody; PC, prostate cancer.
Pretargeted RIT
The potential of multiple cycles of PRIT using the bsmAb TF12 and 177Lu-labeled IMP288 was determined in mice with s.c. PC3 tumors and was compared with that of RIT with 177Lu-hRS7 (11 MBq, 15 μg). PRIT with 2.5 nmol TF12 and 41 MBq 177Lu-IMP288 significantly improved the median survival of mice with s.c. PC3 tumors from 76 to 111 days (p<0.0001, Fig. 2). The median survival in the groups that received multiple cycles of PRIT or the group that was treated with 177Lu-hRS7 (>150 days) was significantly higher (p<0.0001). The control groups received either 177Lu-IMP288 without TF12 or vehicle. Median survival of mice in both control groups was similar (76 vs. 72 days), indicating that there was no effect of administration of a high-activity dose of 177Lu-IMP288 alone. At the end of the experiment (150 days), all mice treated with 177Lu-hRS7 IgG were still alive. Of the groups that received either two or three cycles of TF12/177Lu-IMP288, 70% and 80% of mice survived, respectively. The difference in survival between the groups that were treated with two or three cycles of TF12/177Lu-IMP288 and the group that was treated with 177Lu-hRS7 was not significant (p=0.067 and 0.146, respectively). At the end of the experiment, the average tumor size of the treated groups was significantly smaller than tumor size in the control groups (Fig. 3). In the control groups and the group that received one cycle of PRIT, tumor (re)growth was seen, while the surviving mice in the other groups showed stabilization of tumor growth or a reduced tumor size.
FIG. 2.
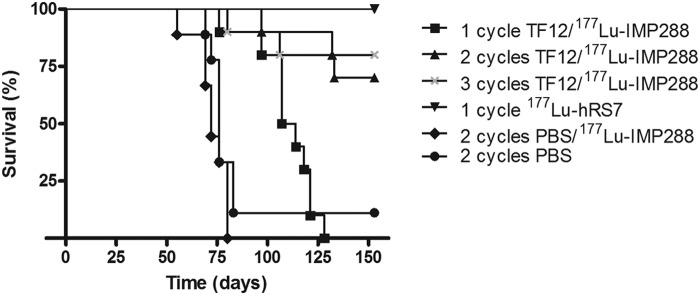
Survival of BALB/c nude mice with a subcutaneous PC3 xenograft treated with one, two, or three cycles of TF12/177Lu-IMP288 (2.5 nmol/0.1 nmol, 41 MBq per cycle), 177Lu-hRS7 (15 μg, 11 MBq), 177Lu-IMP288 without pretargeting (0.1 nmol, 41 MBq), or vehicle (PBS, n=10). PBS, phosphate-buffered saline.
FIG. 3.
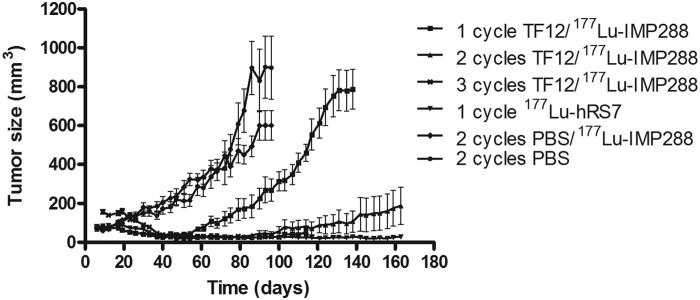
Tumor size of subcutaneous PC3 xenografts in BALB/c nude mice treated with one, two, or three cycles of TF12/177Lu-IMP288 (2.5 nmol/0.1 nmol, 41 MBq per cycle), 177Lu-hRS7 (15 μg, 11 MBq), 177Lu-IMP288 without pretargeting (0.1 nmol, 41 MBq), or vehicle (PBS, n=10).
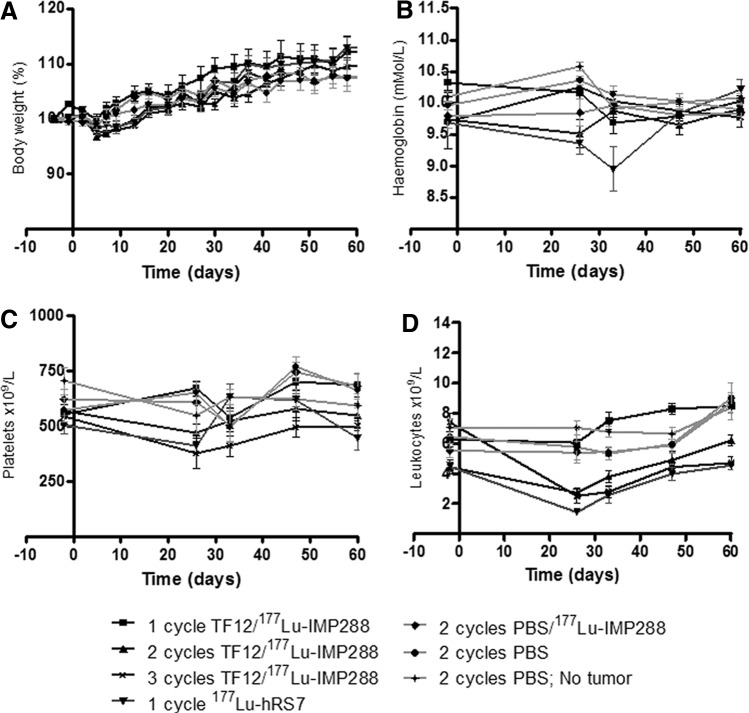
There was no significant decrease in hemoglobin levels, platelets, and body weight in either of the groups after treatment (Fig. 4A–C). However, leukocyte counts decreased significantly (Fig. 4D) in the groups that received either two (−39%, from 4.4±1.5×109/L at t=−2 days to 2.7±1.0×109/L at t=26 days, p=0.01) or three cycles of PRIT (−66%, from 7.4±2.0×109/L at t=−2 days to 2.5±1.3×109/L at t=26 days, p<0.0009) or RIT (−69%, from 4.5±0.9×109/L at t=−2 days to 1.4±0.6×109/L at t=26 days, p<0.0001). Thus, treatment with two or three cycles of TF12/177Lu-IMP288 or with 177Lu-hRS7 IgG was more effective, but also more toxic.
FIG. 4.
Body weight (A), hemoglobin levels (B), platelets (C), and leukocyte counts (D) of BALB/c nude mice with a subcutaneous PC3 xenograft treated with one, two, or three cycles of TF12/177Lu-IMP288 (2.5 nmol/0.1 nmol, 41 MBq per cycle), 177Lu-hRS7 (15 μg, 11 MBq), 177Lu-IMP288 without pretargeting (0.1 nmol, 41 MBq), or vehicle (PBS, n=10).
In PRIT, the kidney could also be the organ at risk. Therefore, the kidneys of mice that were treated with PRIT were examined histologically after completion of the experiment (150 days). The kidneys of the treated mice were compared with the kidneys of the age-matched control mice; at this analysis no indication of renal toxicity was observed.
SPECT/CT imaging
To visualize distribution of the radiolabel in the tumor-bearing mice, SPECT/CT images were acquired. In mice treated with TF12 and 177Lu-IMP288, images acquired 7 hours p.i. clearly showed uptake of 177Lu-IMP288 in the PC3 tumors (Fig. 5A), without retention of the radiolabeled hapten in other tissues. Due to its longer circulating half-life, images of 177Lu-hRS7 distribution were acquired at 3 days p.i. (Fig. 5B). The images of the mice that received 177Lu-hRS7 IgG showed the preferential uptake of the radiolabeled antibody in the tumor and low levels of the radiolabel in normal organs.
FIG. 5.
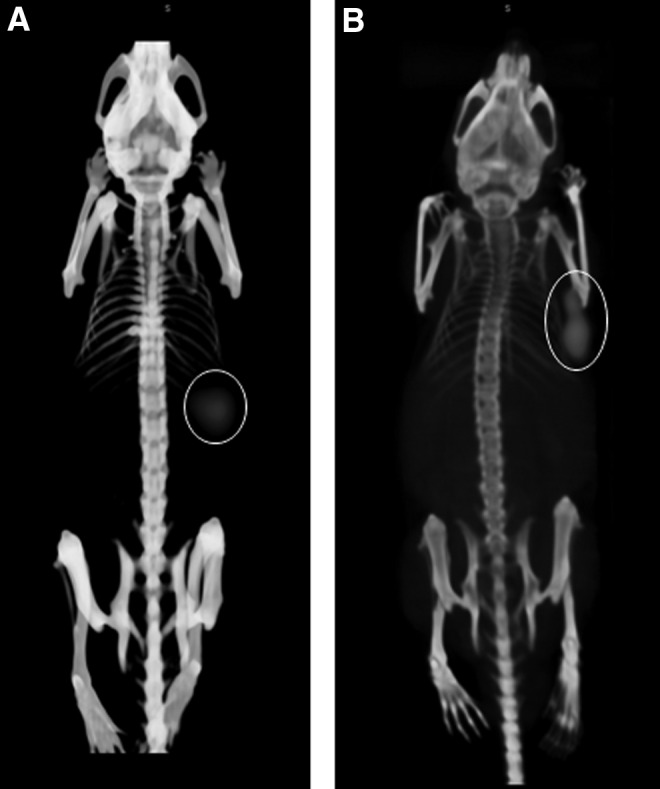
Anterior projections of SPECT/CT scans of BALB/c nude mice with an s.c. PC3 tumor in the right flank injected with (A) TF12 (2.5 nmol) and 177Lu-IMP288 (0.1 nmol, 41 MBq) or with (B) 177Lu-hRS7 (15 μg, 11 MBq). Circles indicate tumor. Images were acquired 7 hours (TF12/177Lu-IMP288) or 3 days (177Lu-hRS7) after injection of the radiolabel.
Discussion
In the present study the potential of multiple cycles of PRIT of PC with the bsmAb TF12 and the 177Lu-labeled hapten-peptide IMP288 was compared with that of conventional RIT with the parental mAb 177Lu-hRS7. Biodistribution studies showed that tumor uptake of radiolabeled IMP288 was similar in the groups that received one, two, or three cycles of PRIT (8.2%–9.0% ID/g), indicating tumors were targeted as efficiently with subsequent pretargeting cycles. Apparently, within 48 hours, sufficient TROP-2 epitopes in the tumor were available for another pretargeting cycle.
Two hours after injection of the radiolabeled hapten-peptide, almost all activity had cleared from the blood via the kidneys (<1% ID/g). Tumor uptake of the 177Lu-labeled parental mAb hRS7 was much higher (62.0%±11.0% ID/g), at the expense of higher blood levels (10.0%±1.6% ID/g), even at 3 days after injection of the radiolabel. Images of mice 7 hours after PRIT and 3 days after RIT clearly showed uptake of the radiolabel in the PC3 tumor, with very low background activity levels.
The pretargeted RIT experiment showed that one cycle of TF12/177Lu-IMP288 significantly improved the median survival of mice with s.c. PC3 tumors without significant hematological or renal toxicity. However, a marked improvement of the median survival was observed in the groups that received multiple cycles of PRIT, or RIT. The improved median survival especially in the groups of mice that received RIT or three cycles of PRIT was achieved at the expense of hematological toxicity, resulting in significant decrease of leukocyte levels after treatment. Two cycles of PRIT showed less hematological toxicity, but was also less effective. At the end of the experiment, 70% of animals in the group with two PRIT cycles were still alive, versus 80% in the three-cycle PRIT group and 100% of animals in the conventional RIT group, although these differences were not significant. This indicates that RIT with one dose of 177Lu-hRS7 is at least as effective as three cycles of TF12/177Lu-IMP288, with similar toxicity despite lower tumor/blood ratios of the 177Lu-hRS7 as compared with TF12/177Lu-IMP288. Further, tumor growth curves show regrowth of tumors after PRIT and stabilization of tumor size after RIT, underlining the long-term effectiveness of RIT compared with multiple cycles of PRIT. Most likely this is due to the much higher tumor uptake of the 177Lu-hRS7 (62.0% ID/g) compared with the tumor uptake of TF12/177Lu-IMP288 (8.0%–9.2% ID/g). These results are not in line with earlier studies that compared PRIT and RIT,15 and with an earlier study with comparable bsmAb-hapten combinations by Frampas et al.,16 which showed higher efficacy and similar toxicity of PRIT compared with RIT. However, in this study, the tumor uptake of the direct-labeled mAb was much lower (20.4% ID/g in s.c. tumors) than that of the radiolabeled hRS7, while tumor uptake of the bsmAb TF12 was comparable to that of TF12 (9.1% vs. 8.5% ID/g).
In the present study, it is shown that additional therapy cycles improve the therapeutic efficacy of PRIT, but also increases hematological toxicity. In future studies, other therapeutic regimens should be investigated. While a dose of three times 41 MBq is the MTD for three cycles of PRIT, a higher activity dose in one cycle might improve therapeutic efficacy of PRIT. However, higher radioactivity dose can only be achieved by increasing the amount of peptide (due to the maximum specific activity of 0.4 GBq/nmol that can be reached), resulting in decreased tumor uptake.5 Perhaps a hapten-peptide with multiple DOTA moieties could be developed to further enhance the specific activity of the hapten-peptide. Also, repetition of two cycles of PRIT 2 months after the initial therapy might increase survival, especially since tumor size has decreased dramatically, which could enable improved access of the therapeutic agent to the deeper layers of the tumor. PRIT could be particularly suited to treat PC in an adjuvant setting, either after prostatectomy or in case of biochemical recurrence as indicated by a rising PSA.
Conclusions
The pretargeting system consisting of the bsmAb TF12 and the radiolabeled hapten-peptide IMP288 showed efficient and very rapid targeting of TROP-2-expressing PC3 tumors in mice. PRIT of multiple cycles showed improved median survival, accompanied by hematological toxicity in the groups that received two or three cycles of PRIT. However, conventional RIT with the parent mAb 177Lu-hRS7 resulted in at least as effective treatment of the PC3 tumors, with similar toxicity.
Acknowledgments
The authors kindly thank Bianca Lemmers, Kitty Lemmens, Iris Lamers-Elemans, and Henk Arnts (Central Animal Facility, Radboud University Medical Center) for their excellent technical assistance in the animal experiments and Eric Steenbergen (Department of Pathology, Radboud University Medical Center) for reviewing the kidney samples. The reagents were generously provided by Drs. Chien-Hsing Chang and Edmund Rossi of IBC Pharmaceuticals, Inc. The work was supported by the Dutch Cancer Society (KWF Kankerbestrijding, Grant KUN-2010-0480).
Disclosure Statement
David M. Goldenberg, William J. McBride, Robert M. Sharkey, and Edmund Rossi have financial interest as employment and/or stock interest in Immunomedics, Inc.
References
- 1.Tagawa ST, Beltran H, Vallabhajosula S, et al. . Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer 2010;116(4 Suppl):1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagawa ST, Milowsky MI, Morris M, et al. . Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 2013;19:5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frampas E, Rousseau C, Bodet-Milin C, et al. . Improvement of radioimmunotherapy using pretargeting. Front Oncol 2013;3:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoffelen R, Boerman OC, Goldenberg DM, et al. . Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br J Cancer 2013;109:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rij CM, Lutje S, Frielink C, et al. . Pretargeted immuno-PET and radioimmunotherapy of prostate cancer with an anti-TROP-2×anti-HSG bispecific antibody. Eur J Nucl Med Mol Imaging 2013;40:1377. [DOI] [PubMed] [Google Scholar]
- 6.Basu A, Goldenberg DM, Stein R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer 1995;62:472. [DOI] [PubMed] [Google Scholar]
- 7.Stein R, Basu A, Chen S, et al. . Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer 1993;55:938. [DOI] [PubMed] [Google Scholar]
- 8.van Rij CM, Sharkey RM, Goldenberg DM, et al. . Imaging of prostate cancer with immuno-PET and immuno-SPECT using a radiolabeled anti-EGP-1 monoclonal antibody. J Nucl Med 2011;52:1601. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey RM, van Rij CM, Karacay H, et al. . A new tri-fab bispecific antibody for pretargeting trop-2-expressing epithelial cancers. J Nucl Med 2012;53:1625. [DOI] [PubMed] [Google Scholar]
- 10.Rossi EA, Goldenberg DM, Cardillo TM, et al. . Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A 2006;103:6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride WJ, Zanzonico P, Sharkey RM, et al. . Bispecific Antibody Pretargeting PET (ImmunoPET) with an 124I-Labeled Hapten-Peptide. J Nucl Med 2006;47:1678. [PubMed] [Google Scholar]
- 12.Schoffelen R, Sharkey RM, Goldenberg DM, et al. . Pretargeted immuno-positron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a 68Ga- and 18F-labeled hapten peptide in mice with human tumor xenografts. Mol Cancer Ther 2010;9:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers AH, van Eerd EF, Frielink C, et al. . Optimization of radioimmunotherapy of renal cancer: Labeling of monoclonal antibody with 131I, 90Y, 177Lu or 186Re. J Nucl Med 2004;45:327. [PubMed] [Google Scholar]
- 14.Lindmo T, Boven E, Cuttitta F, et al. . Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey RM, Chang CH, Rossi EA, et al. . Pretargeting: Taking an alternate route for localizing radionuclides. Tumour Biol 2012;33:591. [DOI] [PubMed] [Google Scholar]
- 16.Frampas E, Maurel C, Remaud-Le SP, et al. . Pretargeted radioimmunotherapy of colorectal cancer metastases: Models and pharmacokinetics predict influence of the physical and radiochemical properties of the radionuclide. Eur J Nucl Med Mol Imaging 2011;38:2153. [DOI] [PubMed] [Google Scholar]