Abstract
With the use of the International Working Group for Myelofibrosis Treatment and Research consensus criteria, we re-assessed the efficacy of thalidomide and lenalidomide in 125 patients with myelofibrosis treated in 3 consecutive phase 2 trials: 44 received single-agent thalidomide, 41 single-agent lenalidomide, and 40 a combination of lenalidomide plus prednisone. The thalidomide group included significantly more untreated patients and patients with performance status of 2. The Lenalidomide-based therapy produced higher efficacy (34%-38%) than thalidomide (16%; P = .06). Responses to thalidomide were seen within 3-15 weeks, whereas responses to the lenalidomide-based therapy were also seen after a prolonged course of therapy (range, 2-45 weeks). Lenalidomide plus prednisone therapy resulted in significantly longer response duration (median, 34 months) than single-agent lenalidomide or thalidomide (median, 7 and 13 months, respectively; P = .042). Fewer patients (P = .001) discontinued the lenalidomide plus prednisone therapy (13%) because of side effects then patients on single-agents therapy (32%-39%). In conclusion, the combination of lenalidomide plus prednisone appears to be more ef-fective and safer than single-agent thalidomide or lenalidomide.
Introduction
Myelofibrosis (MF) is a clonal stem-cell disorder characterized by intense BM stromal reaction that results in collagen fibrosis, osteosclerosis, and angiogenesis thought to be mediated in large part by high levels of proinflammatory, fibrogenic, and angiogenic cytokines.1 Thalidomide and lenalidomide are 2 immunomodulatory agents (IMiDs) with clinical activity in a subset of patients with MF, including improvements in anemia, thrombocytopenia, and splenomegaly, thought to be because of their effect on BM environment.2,3 In 2006, the International Working Group (IWG) for Myelofibrosis Treatment and Research proposed consensus criteria for evaluation of treatment response in patients with MF.4 Although now in widespread use, the IWG criteria have not been used in previous trials of single-agent thalidomide and lenalidomide in MF. Here, we report the efficacy and long-term outcome of patients with MF treated with thalidomide or lenalidomide with or without prednisone in 3 consecutive trials conducted at our institution between 2000 and 2007, as assessed by the IWG response criteria.
Patients and methods
A total of 125 patients with MF participated in 3 consecutive phase 2 trials with IMiDs after signing informed consent, in accordance with the Declaration of Helsinki, with approval from the Institution Review Board of M. D. Anderson Cancer Center. The study groups were as follows: (1) 44 patients received thalidomide at an initial dose of 200 mg daily with weekly escalation by 200 mg, as tolerated, up to 800 mg daily2; (2) 41 patients received lenalidomide 10 mg/d continuously (5 mg/d if pretherapy platelet count was < 100 × 109/L)3; and (3) 40 patients received lenalidomide 10 mg/d (5 mg/d if pretherapy platelet count was < 100 × 109/L) on days 1-21 of 28-day cycles, in combination with prednisone that was given for only 3 cycles as 30 mg/d during cycle 1, 15 mg/d during cycle 2, and 15 mg/d every other day during cycle 3.5 No prophylaxis agent for deep venous thrombosis was administered. Responders continued treatment until loss of the response or a development of intolerance. Responses were assessed with the IWG criteria, and response rates were calculated on the basis of intent-to-treat analysis. Eligibility criteria were similar for the 3 study groups. Differences among variables were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively. Toxicity was assessed by the National Cancer Institute Common Toxicity Criteria for Adverse Events, Version 3.
Results and discussion
Among patients' characteristics (Table 1), significant differences among the 3 groups included more treatment-naive patients and patients with a performance status of 2 in the thalidomide group than in lenalidomide groups.6 Patients receiving single-agent lenalidomid therapy had a higher rate of lower-risk disease (56%) than patients receiving the combination (32%) or single-agent thalidomide (32%; P = .016). All patients treated with single-agent thalidomide or lenalidomide have stopped their therapy by now. Seven of the 44 patients (16%) treated with thalidomide responded for a median duration of 13 months. Responses included 1 (2%) complete response (CR), 1 (2%) partial response (PR), and 5 (12%) clinical improvement (CI) in anemia (all becoming transfusion independent for a median duration of 11 months; range, 6-20 months). Of the 41 patients treated with single-agent lenalidomide, 14 (34%) responded: 2 (5%) with CR, 5 (12%) with PR, and 7 (17%) with CI. The median duration of response was 7 months. The median duration of the CI was 12 months (range, 2-36 months) with 4 patients becoming transfusion independent. After a median follow-up of 42 months, 15 of the 40 patients (38%) treated with the lenalidomide plus prednisone combination responded: 2 (5%) with CR, 4 (10%) with PR, and 9 (23%) with CI, with the median duration of a response of 34 months (range, 1 to > 49 months). One patient converted his response from PR to CR recently after > 10 cycles of therapy. The median duration of CI was 7 months (range, 1 to > 33 months) with 4 becoming transfusion independent. Six patients still remain on the study with 2 CRs, 3 PRs, and 1 stable disease.
Table 1.
Patient characteristics
| Characteristic | Thalidomide (N = 44) | Lenalidomide (N = 41) | Lenalidomide plus prednisone (N = 40) | P |
|---|---|---|---|---|
| Median age, y (range) | 65 (26-85) | 64 (42-83) | 64 (42-86) | .96 |
| Male, n (%) | 25 (57) | 22 (54) | 23 (58) | .93 |
| Previously untreated, n (%) | 25 (57) | 5 (12) | 10 (25) | .0001 |
| No. of prior treatments (range) | 0 (0-3) | 1 (0-6) | 1 (0-4) | .0009 |
| Median hemoglobin level (range), g/dL | 9.5 (5.8-15.1) | 9.8 (6.9-14.8) | 9.8 (6.6-15.4) | .12 |
| Median platelet count (range), × 109/L | 144 (14-572) | 203 (34-901) | 237 (8-1005) | .09 |
| Median WBC count (range), × 109/L | 10.2 (1.9-59.5) | 9 (2.4-45.4) | 8.6 (1.1-28.3) | .65 |
| Neutrophils, % (range) | 64 (28-90) | 66 (32-89) | 66 (10-89) | .55 |
| Splenomegaly, n (%) | 28 (64) | 20 (49) | 30 (75) | .25 |
| Median spleen size (range), cm | 15 (3-25) | 12 (3-30) | 11.5 (1-25) | .91 |
| Splenectomy, n (%) | 4 (9) | 7 (17) | 1 (2) | .08 |
| Cytogenetics | .75 | |||
| Diploid, n (%) | 24 (55) | 23 (56) | 20 (50) | |
| Abnormal, n (%) | 15 (34) | 15 (37) | 18 (45) | |
| 5 of 7 abnormalities, n (%) | 1 (2) | 2 (5) | 1 (2) | |
| ≥ 3 abnormalities, n (%) | 2 (5) | 2 (5) | 2 (5) | |
| IM + ND, n (%) | 5 (11) | 3 (7) | 2 (5) | |
| Myelofibrosis type | .96 | |||
| Primary, n (%) | 29 (71) | 29 (71) | 31 (78) | |
| After ET, n (%) | 7 (17) | 7 (17) | 5 (12) | |
| After PV, n (%) | 5 (12) | 5 (12) | 4 (10) | |
| ECOG Performance Status | .017 | |||
| 0, n (%) | 6 (14) | 16 (39) | 4 (10) | |
| 1, n (%) | 28 (64) | 22 (54) | 32 (80) | |
| 2, n (%) | 10 (22) | 3 (7) | 4 (10) | |
| DIPSS | .016 | |||
| Low, n (%) | 1 (2) | 2 (5) | 0 (0) | |
| Int-1, n (%) | 13 (30) | 21 (51) | 13 (32) | |
| Int-2, n (%) | 18 (41) | 15 (37) | 24 (60) | |
| High, n (%) | 12 (27) | 3 (7) | 3 (8) |
WBC indicates white blood cell; IM, insufficient metaphases; ND, not determined; ET, essential thrombocythemia; PV, polycythemia vera; ECOG, Eastern Cooperative Oncology Group; DIPSS, Dynamic International Prognostic Scoring System; Int-1, intermediate-1; and Int-2, intermediate-2.
A trend was observed for a higher efficacy in patients receiving lenalidomide-based therapy (P = .06; Table 2). Lenalidomide-based therapy had higher efficacy on reducing the spleen size than thalidomide. In a multivariate analysis conducted for response, the lenalidomide-based regimen was the only factor independently associated with a higher response rate. Response to thalidomide was seen after 8 weeks (range, 3-15 weeks) but not later, whereas responses to lenalidomide with or without prednisone were seen also after prolonged course of therapy (≤ 45 weeks), leading to significant differences in the median time to first and best response (Table 2; Figure 1A). Patients in the lenalidomide plus prednisone group had responses of significantly longer duration then patients in the single-agent groups, with ongoing median response duration of 34 months (Figure 1B). No survival difference was observed between the 3 groups of patients, partly because of sequential therapies administered when patients who failed thalidomide received salvage therapies (P = .129). Fourteen patients who failed thalidomide received subsequent lenalidomide-based therapy. Eight responded: 5 of 11 patients receiving lenalidomide single agent (2 PR and 3 CI) and 3 of 3 receiving lenalidomide plus prednisone (1 CR and 2 CI).
Table 2.
Efficacy of therapy
| Parameter | Thalidomide (N = 44) | Lenalidomide (N = 41) | Lenalidomide plus prednisone | P |
|---|---|---|---|---|
| Response | .06 | |||
| Overall, median (%) [95% CI] | 7 (16) [7-30] | 14 (34) [20-51] | 15 (38) [23-54] | |
| CR, median (%) [95% CI] | 1 (2) [0-12] | 2 (5) [1-17] | 2 (5) [1-7] | |
| PR, median (%) [95% CI] | 1 (2) [0-12] | 5 (12) [4-26] | 4 (10) [3-24] | |
| CI, median (%) [95% CI]* | 5 (12) [4-25] | 7 (17) [7-32] | 9 (23) [11-38] | |
| Hemoglobin level, median (%) [95% CI] | 5 | 4 | 4 | |
| Platelet count, median (%) [95% CI] | 1 | 1 | NA | |
| ANC, median (%) [95% CI] | NA | NA | 1 | |
| Spleen, median (%) [95% CI] | NA | 3 | 4 | |
| Median follow-up (range), mo | 116 (16-121) | 57 (5-67) | 42 (6 to ≥49) | < .0001 |
| Median response duration (range), mo | 13 (4-30) | 7 (1-41) | 34 (1 to ≥49) | .042 |
| Median time to first response (range), wk | 8 (3-15) | 17 (2-29) | 11 (2-45) | .0066 |
| Median time to best response (range), wk | 8 (3-32) | 18 (2-39) | 14 (2-79) | .0138 |
ANC indicates absolute neutrophil count; and CI, confidence interval.
Patients could have CI involving several lineages.
Figure 1.
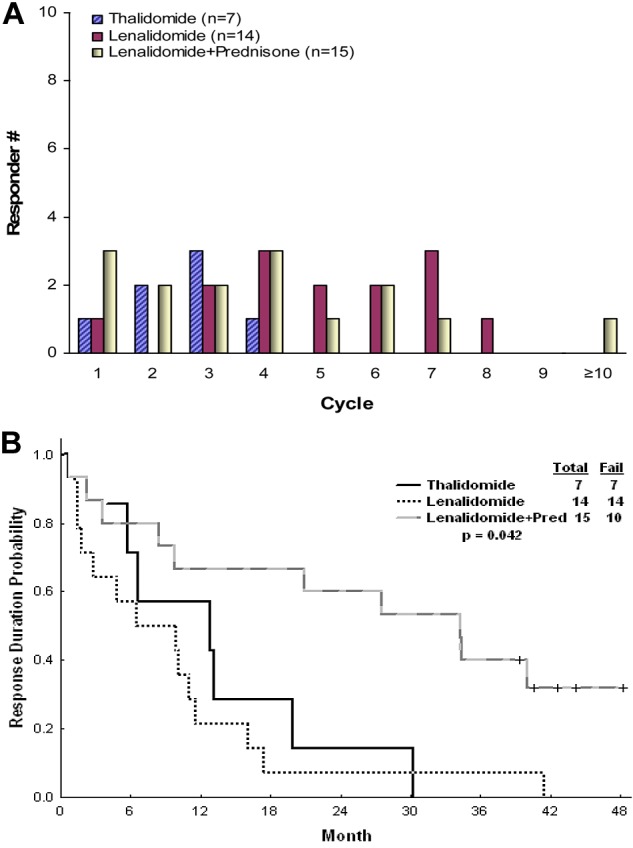
Time to first response and response durability. Time to first response (A) and response durability (B).
The most common thalidomide side effects were neuropathy, rash, and fatigue, as previously reported.2 In the lenalidomide single-agent group fatigue was also commonly reported but not in the lenalidomide plus prednisone group, probably because of prednisone use. Interestingly, rash was seen in the lenalidomide plus prednisone group later in the course of therapy when patients were off prednisone. Most nonhematologic side effects observed were of grade 1 and 2 (Table 3). The lenalidomide-based therapy caused more myelosuppression than thalidomide (Table 4), as expected from previous experience.3 Contrary to the experience in multiple myeloma,7 the combination of lenalidomide and prednisone did not induce a higher rate of thrombotic events. Overall, significantly fewer patients (P = .001) discontinued the lenalidomide plus prednisone therapy (13%) because of side effects than patients on single-agent thalidomide (39%) or lenalidomide (32%).
Table 3.
Nonhematologic side effects
| Toxicity | Thalidomide, n (%) (N = 44) |
Lenalidomide, n (%) (N = 41) |
Lenalidomide plus prednisone, n (%) (N = 40) |
P | |||
|---|---|---|---|---|---|---|---|
| All grades | Grades 3-4 | All grades | Grades 3-4 | All grades | Grades 3-4 | ||
| Thrombosis | 1 (2) | 1 (2) | 2 (5) | 1 (2) | 2 (5) | 2 (5) | .77 |
| Edema | 9 (20) | 1 (2) | 9 (22) | 1 (2) | 4 (10) | 2 (5) | .31 |
| Fatigue | 15 (34) | 1 (2) | 14 (35) | 5 (12) | 6 (15) | 4 (10) | .08 |
| Neuropathy | 23 (52) | 1 (2) | 2 (5) | 0 | 6 (15) | 0 | .001 |
| Increased liver enzymes | 0 | 0 | 4 (10) | 1 (2) | 3 (7) | 2 (5) | .12 |
| Rash | 16 (36) | 1 (2) | 7 (18) | 3 (7) | 15 (37) | 0 | .07 |
| Bleeding | 0 | 0 | 0 | 0 | 1 (3) | 1 (3) | .34 |
Table 4.
Hematologic parameters before therapy and grades 3-4 toxicity during therapy
| Toxicity grade at baseline | Neutropenia |
Anemia |
Thrombocytopenia |
|||
|---|---|---|---|---|---|---|
| No. of patients at baseline | Grade 3-4 during therapy, n | No. of patients at baseline | Grade 3-4 during therapy | No. of patients at baseline | Grade 3-4 during therapy, n | |
| Thalidomide | ||||||
| 0 | 39 | 4 | 22 | 1* | ||
| 1 | 0 | 14 | 1* | 9 | ||
| 2 | 5 | 21 | 1 | 4 | ||
| 3 | 0 | 5 | 6 | |||
| 4 | 0 | 0 | 3 | |||
| Overall | 44 | 44 | 44 | |||
| Lenalidomide | ||||||
| 0 | 39 | 7 | 6 | 28 | 5 | |
| 1 | 0 | 16 | 1* | 7 | 3 | |
| 2 | 1 | 1* | 17 | 3 | 1 | |
| 3 | 1 | 1* | 2 | 3 | 2* | |
| 4 | 0 | 0 | 0 | |||
| Overall | 41 | 41 | 41 | |||
| Lenalidomide plus prednisone | ||||||
| 0 | 35 | 18 | 4 | 28 | 1* | |
| 1 | 2 | 1 | 14 | 7 | 4 | |
| 2 | 0 | 21 | 6 | 3 | ||
| 3 | 1 | 1 | 1 | 1* | ||
| 4 | 2 | 0 | 4 | |||
| Overall | 40 | 40 | 40 | |||
| P | .001 | .001 | .001 | |||
Grade 4 only.
We and others have previously reported objective clinical responses in MF to thalidomide and lenalidomide single-agent therapy of ≤ 60%.2,3 However, different studies used different response criteria; therefore, IWG established a set of clinically relevant response criteria several years ago,4 now used in almost all MF studies. Reassessing our experience with IMiDs in MF we found the combination of lenalidomide plus prednisone to be possibly more effective (particularly important aspect is significantly longer response duration) and safer than single-agent thalidomide or lenalidomide. This could be explained in part by a better nonhematologic safety profile, leading to less treatment withdrawal because of toxicity in the lenalidomide plus prednisone group, but also by the potential therapeutic synergy of lenalidomide and prednisone.5,8 Corticosteroid therapy alone has shown a response rate in anemia (CI in anemia by IWG criteria) in 19% of patients with MF.9 The antiangiogenic, proapoptotic, and immunomodulatory effects of IMiD might be enhanced by the addition of prednisone to suppress inflammatory stimuli, reduce marrow fibrosis, and improve cytopenias.3,5
Three aspects of our results require discussion. First, low-dose (50 mg) thalidomide plus prednisone is an alternative regimen that may be better tolerated by patients with MF and may result in fewer patients discontinuing therapy because of toxicity, resulting in better efficacy (≤ 30% by IWG).10 However, the duration of response may not be longer than in our experience (median, 8.5 months).10 Second, responses to lenalidomide plus prednisone may occur after prolonged period of therapy and may improve over time.8 Continuous therapy is mandatory to achieve significant and durable responses. Recent multicenter study of lenalidomide plus prednisone in 42 patients with MF resulted in 23% IWG response rate; however, only 25 patients received ≤ 6 cycles of therapy, and therapy was then stopped.11 Third, despite that the inclusion criteria and patient characteristics were similar in our 3 consecutive trials, the design of the trials does not allow strict comparisons. However, our analysis with the use of the IWG-MRT consensus criteria of response identified the expected responses observed with IMiDs in MF; that may allow some degree of comparison with future novel MF therapies such as pegylated form of IFN and Jak-2 inhibitors.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. and S.V. analyzed the results and wrote the manuscript; D.T., H.K., and J.C. contributed patients; and S.P. and L.Z. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: sverstov@mdanderson.org.
References
- 1.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23(33):8520–8530. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DA, Giles FJ, Albitar M, et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer. 2006;106(9):1974–1984. doi: 10.1002/cncr.21827. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Cortes J, Verstovsek S, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108(4):1158–1164. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 5.Quintás-Cardama A, Kantarjian HM, Manshouri T, et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J Clin Oncol. 2009;27(28):4760–4766. doi: 10.1200/JCO.2009.22.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh J. Risk of thrombosis with lenalidomide and its prevention with aspirin. Chest. 2007;131(1):275–277. doi: 10.1378/chest.06-2360. [DOI] [PubMed] [Google Scholar]
- 8.Holle N, de Witte T, Mandigers C, Schaap N, Raymakers R. Thalidomide and lenalidomide in primary myelofibrosis. Neth J Med. 2010;68(1):293–298. [PubMed] [Google Scholar]
- 9.Tefferi A, Verstovsek S, Barosi G, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27(27):4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapaliya P, Tefferi A, Pardanani A, et al. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens. Am J Hematol. 2011;86(1):96–98. doi: 10.1002/ajh.21892. [DOI] [PubMed] [Google Scholar]
- 11.Mesa RA, Yao X, Cripe LD, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. 2010;116(22):4436–4438. doi: 10.1182/blood-2010-05-287417. [DOI] [PMC free article] [PubMed] [Google Scholar]


