Abstract

The proton-coupled oligopeptide transporter PEPT1 (SLC15A1) is abundantly expressed in the small intestine, but not colon, of mammals and found to mediate the uptake of di/tripeptides and peptide-like drugs from the intestinal lumen. However, species differences have been observed in both the expression (and localization) of PEPT1 and its substrate affinity. With this in mind, the objectives of this study were to develop a humanized PEPT1 mouse model (huPEPT1) and to characterize hPEPT1 expression and functional activity in the intestines. Thus, after generating huPEPT1 mice in animals previously nulled for mouse Pept1, phenotypic, PCR, and immunoblot analyses were performed, along with in situ single-pass intestinal perfusion and in vivo oral pharmacokinetic studies with a model dipeptide, glycylsarcosine (GlySar). Overall, the huPEPT1 mice had normal survival rates, fertility, litter size, gender distribution, and body weight. There was no obvious behavioral or pathological phenotype. The mRNA and protein profiles indicated that huPEPT1 mice had substantial PEPT1 expression in all regions of the small intestine (i.e., duodenum, jejunum, and ileum) along with low but measurable expression in both proximal and distal segments of the colon. In agreement with PEPT1 expression, the in situ permeability of GlySar in huPEPT1 mice was similar to but lower than wildtype animals in small intestine, and greater than wildtype mice in colon. However, a species difference existed in the in situ transport kinetics of jejunal PEPT1, in which the maximal flux and Michaelis constant of GlySar were reduced 7-fold and 2- to 4-fold, respectively, in huPEPT1 compared to wildtype mice. Still, the in vivo function of intestinal PEPT1 appeared fully restored (compared to Pept1 knockout mice) as indicated by the nearly identical pharmacokinetics and plasma concentration–time profiles following a 5.0 nmol/g oral dose of GlySar to huPEPT1 and wildtype mice. This study reports, for the first time, the development and characterization of mice humanized for PEPT1. This novel transgenic huPEPT1 mouse model should prove useful in examining the role, relevance, and regulation of PEPT1 in diet and disease, and in the drug discovery process.
Keywords: PEPT1, humanized mice, mRNA and protein expression, glycylsarcosine, in situ intestinal perfusions, in vivo oral pharmacokinetics
Introduction
Mammalian PEPT1 (SLC15A1), along with PEPT2 (SLC15A2), PHT1 (SLC15A4) and PHT2 (SLC15A3), belong to the solute carrier group of membrane transport proteins (i.e., SLC15) that mediate the cellular uptake of di- and tripeptides in addition to several peptidomimetic drugs. Following discovery of rabbit Pept1,1 the human and mouse orthologues were cloned (85% amino acid identity)2,3 in which they contained 708 and 709 amino acid residues, respectively. These Pept1 transporters have 12 transmembrane domains, C- and N-termini facing the cytoplasm, and Tyr12, His57, Tyr64, Trp294, Phe297, and Glu595 residues located within highly conserved transmembrane domains (H1, H2, H5, H7, and H10).4
In contrast to PEPT2, a high-affinity low-capacity transporter primarily responsible for the reabsorption of peptides/mimetics in kidney,5 PEPT1 is a low-affinity high-capacity transporter that is important in the absorption of digested peptides (mostly di- and tripeptides) from dietary protein in the small intestine. PEPT1 is also crucial for the intestinal uptake and absorption of therapeutic drugs such as the β-lactam antibiotic cefadroxil6 and the antiviral nucleoside prodrug valacyclovir.7 Previous studies using polymerase chain reaction (PCR) and immunoblot analyses have demonstrated that in rodents and humans PEPT1 is abundantly expressed in the apical membrane of enterocytes in duodenal, jejunal, and ileal regions.8−11 The expression of PEPT1 in colon is controversial and perhaps species dependent. Nevertheless, under normal conditions, PEPT1 is unlikely to have much impact on the absorption of peptides/mimetics from this region.
Species differences in PEPT1 expression and functional activity have been reported in mouse and human colonic tissue.11,12 Moreover, our laboratory demonstrated in vivo that both cefadroxil6 and valacyclovir7 exhibited dose-proportional absorption in wildtype and Pept1 knockout mice after oral dose escalation. The “apparent” dose linearity observed in these mouse studies is contrary to the nonlinear intestinal absorption kinetics reported in rats and humans for cefadroxil13,14 and in humans for valacyclovir.15 Interspecies differences in transporter-mediated activity are difficult to sort out given that studies are usually performed by different investigators and laboratories and especially under varying experimental conditions. For this reason, we demonstrated in a single system, yeast Pichia pastoris, a species difference in the affinity of glycylsarcosine (GlySar) for rat, mouse, and human PEPT1 transformants.16 These findings, and others, clearly illustrate that species differences may impact the intestinal absorption and pharmacokinetics of PEPT1 substrates, thereby, making it more difficult to predict systemic drug exposure. It is also clear that cell culture systems, naive or transfected with transporters of interest, as well as further in vitro or in situ methods, will not reflect what happens in humans under physiological conditions.
The past decade has shown a growing interest in the development of humanized mice to overcome species differences in drug metabolism, disposition, and regulation.17−21 Studies with humanized mouse models not only provide a mechanistic understanding of species differences but also improve our ability to optimize and predict the pharmacokinetic, therapeutic, and safety profiles of xenobiotics in humans. With this in mind, the primary aim of this study was to generate a humanized PEPT1 (huPEPT1) mouse model, which was nulled for the mouse Pept1 gene and expresses the human transporter in the tissues where Pept1 is normally expressed. The secondary aim was to characterize the huPEPT1 mice with respect to hPEPT1 expression and functional activity in the intestines, as examined by in situ permeability and in vivo oral absorption studies with the model dipeptide GlySar.
Experimental Section
Chemicals
[3H]-GlySar (98 mCi/mmol), [14C]-GlySar (113 mCi/mmol), and [14C]-inulin 5000 (1.1 mCi/g) were purchased from Moravek Biochemicals (Brea, CA). Unlabeled GlySar and inulin 5000 were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit antihuman PEPT1 antiserum was generously provided by Dr. Hannelore Daniel (Technische Universität München, Germany). Protease inhibitor cocktail was purchased from Roche (Seattle, WA) and Power SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA). All other chemicals were obtained from standard sources.
Animals
Gender- and weight-matched mice (8 to 10 weeks) were provided in-house for mPept1+/+ (wildtype), mPept1–/–/hPEPT1–/– (mPept1 knockout), and mPept1–/–/hPEPT1+/– (humanized or huPEPT1) genotypes. All animals were bred on a C57BL/6 background (>99% congenic) in which the Pept1 knockout and humanized mice were identified by genotyping and culled from the same litters. The mice were housed in a temperature-controlled environment with 12 h light and dark cycles and received a standard diet and water ad libitum (Unit for Laboratory Animal Medicine, University of Michigan, Ann Arbor, MI). All mouse studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Generation and Molecular Characterization of Humanized PEPT1 Mice
huPEPT1 mice were generated using an approach described previously.22 In brief, bacterial artificial chromosomes (BACs) containing the PEPT1 gene were obtained from a human BAC library (Empire Genomics, Buffalo, NY). A BAC clone [RP11-782G13; ∼179 kb; CHR13 (98,091,462–98,270,723)], containing the entire 5′-terminal regulatory elements, coding area, and 3′-terminal regulatory elements, was then microinjected into the male pronucleus of fertilized one-cell embryos obtained from Pept1–/– mice on a C57BL/6 background.23 The pronuclear stage embryos were then transferred into the uterus of pseudopregnant recipient animals. Founder mice were screened to identify an animal containing one copy of the human BAC after which these animals were bred (i.e., mPept1–/–/hPEPT1+/– × mPept1–/–/hPEPT1–/– mice) to maintain hemizygous transgenic lines.
Transgenic huPEPT1 alleles were detected in offspring by PCR using genomic DNA isolated from tail biopsies. The first set had a forward primer 5′-ATCTTCTTCATCGTGGTCAATG-3′ and a reverse primer 5′-CCCAGCTGATGAAATTTGTGAA-3′, with a product size of 200 bp. The second set had a forward primer 5′-CCAATCTGCTCACACAGGATAGAGAGGGCAGG-3′ and a reverse primer 5′-CCTTGAGGCTGTCCAAGTGATTCAGGCCATCG-3′, with a product size of 524 bp. The endogenous mPept1 gene was confirmed as nullified using a PCR approach described previously.23 The PCR conditions were 1 cycle at 94 °C for 2 min, 35 cycles at 94 °C for 30 s, 53 °C for 45 s, and 72 °C for 60 s, and then 72 °C for 10 min.
Initial Phenotypic Analysis
The huPEPT1 mice were evaluated for viability, fertility, serum clinical chemistry, and histology, as performed previously for wildtype and Pept1 knockout mice.23
Real-time PCR and Immunoblot Analyses
Quantitation of hPEPT1, mPept1, mPept2, mPht1, mPht2, and other relevant genes was performed in the small intestine, colon, and kidney of wildtype, mPept1 knockout, and humanized PEPT1 mice using a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA) as described before.24 In brief, 2.0 μg of total RNA, isolated using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA), was reversely transcribed into cDNA using the Omniscript RT Kit (Qiagen, Valencia, CA) with 16-mer random primers. The mouse Gapdh gene was used as an internal control of cDNA quality and quantity. The primers (Table 1) were designed using Primer 3.0 (Applied Biosystems, Foster City, CA) and synthesized by Integrated DNA Technologies (Coraville, IA). The real-time PCR thermal conditions were 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, and then 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The ΔCT method was used to calculate the relative levels of target gene transcripts in mice, where the ratio of target gene to mGapdh was equal to 2–ΔCT, ΔCT = CT(gene) – CT (mGapdh).
Table 1. Primers Used in Quantitative Real Time PCR.
| genea | forward primer (5′–3′) | reverse primer (5′–3′) |
|---|---|---|
| mGapdh (EC = 1.2.1.12) | GAGACAGCCGCATCTTCTTGT | CACACCGACCTTCACCATTTT |
| hPEPT1 (SLC15A1) | TGACCTCACAGACCACAACCA | GCCAGGCCGATCAAGGA |
| mPept1 (Slc15a1) | CCACGGCCATTTACCATACG | TGCGATCAGAGCTCCAAGAA |
| mPept2 (Slc15a2) | TGCAGAGGCACGGACTAGATAC | GGGTGTGATGAACGTAGAAATCAA |
| mPht1 (Slc15a4) | GCTGCCACCTGCATTACTACTTC | CGTACTTCACAGACACAATGAGGAA |
| mPht2 (Slc15a3) | GCTGAAGCTTGCGTTCCAA | AACAGGTGGGCACTTTCAGAGT |
| mBphl (EC = 3.1.-.-) | GCCAAGGTGGCTGTGAATG | GATCGCATGTTCCCCTTCTC |
| mAtb0,+ (Slc6a14) | TCAGGATTTGACTTGGCATTCA | CAAGGCCCAATGTTAAAAGCA |
| mOat1 (Slc22a6) | CCACCTGCTAATGCCAACCT | GATTCGGGTCGTCCTTGCT |
| mOat2 (Slc22a7) | TGTCGCAAAGACCCTCGTACT | ACATCATCATGCAGCACAGTGA |
| mOat3 (Slc22a8) | GCCCCAGCCTCACTGTCTATAT | ACATTCAAGATAATGGTGCTCAGAGA |
| mOct1 (Slc22a1) | TGGTGTTCAGGCTGATGGAA | GCCCAAAACCCCAAACAAA |
| mMate1 (Slc47a1) | TTCTGCTTGTGACACGCTCAT | AGTGTCCCCCTTTGCAGGAT |
| mMate2 (Slc47a2) | GACATCATTTCCCTTGTGAGTCAA | GCCCGCAAGTGCATCAA |
| mPat1 (Slc36a1) | TCTGCTGTGTCTACTTCGTGTTTCT | GGATCACGGTCACATTGTTGTT |
Shown as gene name (solute carrier group or enzyme commission number) for human (h) and mouse (m) transporters or enzymes.
Different segments of small intestine, colon, and kidney of wildtype, mPept1 knockout, and huPEPT1 mice were homogenized in 2.0 mL of Nonidet P40-lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P40, and proteinase inhibitor cocktail, pH 8.0), as described previously for immunoblot analyses.12 The homogenates were sonicated for 10 pulses in ice at half strength, and then centrifuged at 15,000g at 4 °C for 15 min. The final concentration of proteins was measured with a BCA Protein Assay Kit (Pierce, Rockford, IL). The proteins were denatured at 40 °C for 45 min, resolved using 10% SDS-PAGE, transferred to a PVDF membrane (Millipore, Billerica, MA), and then blotted with specific rabbit antihuman PEPT1 antiserum11 (1:3000 dilution) or specific rabbit antimouse PEPT1 antiserum12 (1:5000 dilution).
In Situ Single-Pass Intestinal Perfusion Studies
Wildtype, mPept1 knockout, and humanized PEPT1 mice were fasted overnight (∼12 h), but with free access to water, and anesthetized with sodium pentobarbital (40–60 mg/kg intraperitoneal). The permeability of GlySar in regional segments of the intestines (i.e., duodenum, jejunum, ileum, and colon) was then determined simultaneously, as described previously.12 In brief, the mouse was placed on top of a heating pad to maintain body temperature, the abdominal area sterilized with 70% ethanol, and the intestine exposed by making an incision along its midline. A 4 cm segment of duodenum, 8 cm segment of proximal jejunum (∼2 cm distal to the ligament of Treitz), 6 cm segment of ileum (∼1 cm proximal to the cecum), and 4 cm segment of colon (∼0.5 cm distal to the cecum) was isolated and followed by incisions at both the proximal and distal ends. After the segment was rinsed with 0.9% isotonic saline solution, a glass cannula (2.0 mm outer diameter) was inserted at each end of the intestinal segment and secured in place with silk sutures. The isolated intestinal segment was covered with saline-wetted gauze and parafilm to prevent dehydration. After cannulation, the animals were then transferred to a temperature-controlled chamber at 31 °C to maintain body temperature during the entire perfusion procedure. The cannulas were then connected to inlet tubing that was attached to a 30 mL syringe (BD, Franklin Lakes, NJ USA) on a perfusion pump (Model 22: Harvard Apparatus, South Natick, MA) and to outlet tubing that was placed in a collection vial.
The perfusate buffer contained 135 mM NaCl, 5 mM KCl, and 10 mM MES/Tris (pH 6.5), plus 10 μM [3H]-GlySar (0.5 μCi) and 0.01% (w/v) [14C]-inulin-5000 (0.25 μCi), which severed as a nonabsorbable marker to correct for water flux during the perfusions. Buffer was perfused through each intestinal segment at a flow rate of 0.1 mL/min, and the exiting perfusate was collected every 10 min over a 90 min period. A 100 μL aliquot from each collection was added to a vial containing 6.0 mL of scintillation cocktail (Ecolite, MP Biochemicals, Solon, OH), and the samples were measured for radioactivity by a dual-channel liquid scintillation counter (Beckman LS 6000 SC, Beckman Coulter Inc., Fullerton, CA). At the end of experimentation, the length of all four intestinal segments was measured.
Concentration-dependent studies were also performed in the jejunum of wildtype and huPEPT1 mice, by varying the perfusate concentrations of GlySar over a wide range (0.01–50 mM), to assess its saturable transport kinetics.
In Vivo Oral Pharmacokinetic Studies
Following an overnight fast (∼12 h), wildtype, mPept1 knockout, and huPEPT1 mice were anesthetized briefly with isoflurane and administered 5.0 nmol/g [14C]-GlySar (5.0 μCi/mouse in 0.2 mL normal saline) orally by gavage. After dosing, serial blood samples (15 μL) were collected at 5, 7.5, 15, 30, 45, 60, 90, 120, 180, 240, and 360 min via tail transections. The blood samples were placed in tubes containing 1.0 μL of EDTA-K3 and centrifuged at 3000g for 3 min to obtain plasma (∼5 μL). Animals were returned to their cages between blood sampling where they had free access to water and, 2 h after dosing, food. Radioactivity in the plasma samples was measured by a dual-channel liquid scintillation counter (Beckman LS 6000 SC, Beckman Coulter Inc., Fullerton, CA).
Data Analysis
The steady-state loss of drug from perfusate through the intestinal segments was achieved approximately 30 min after the start of perfusion. The effective permeability (Peff) of drug was calculated according to a complete radial mixing parallel tube model:25,26
where Q is the perfusion flow rate (0.1 mL/min), Cout is the outlet GlySar concentration after water flux correction, Cin is the inlet GlySar concentration, R is the internal radius (0.1 cm for small intestine and 0.2 cm for colon), and L is the length of intestinal segment.
The concentration-dependent flux (J) of GlySar in jejunum was best fit to a single Michaelis–Menten term in which12
where the parameters Vm′ and Km′ were referenced to the inlet concentrations (Cin) and the parameters Vm and Km were referenced to the intestinal wall concentrations (Cw) after correcting for the unstirred aqueous layer permeability.
Pharmacokinetic parameters, after oral dosing of GlySar, were determined using a noncompartmental approach (NCA, Phoenix WinNonlin v1.3, Certara, St. Louis, MO).
Data were reported as mean ± SE, unless otherwise noted. Statistical differences between two groups were determined by an unpaired t test. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test in which wildtype mice served as the control group (GraphPad Prism v5.0; GraphPad Software, Inc., La Jolla, CA). A value of p < 0.05 was considered significant.
Results
Identification of huPEPT1 Mice
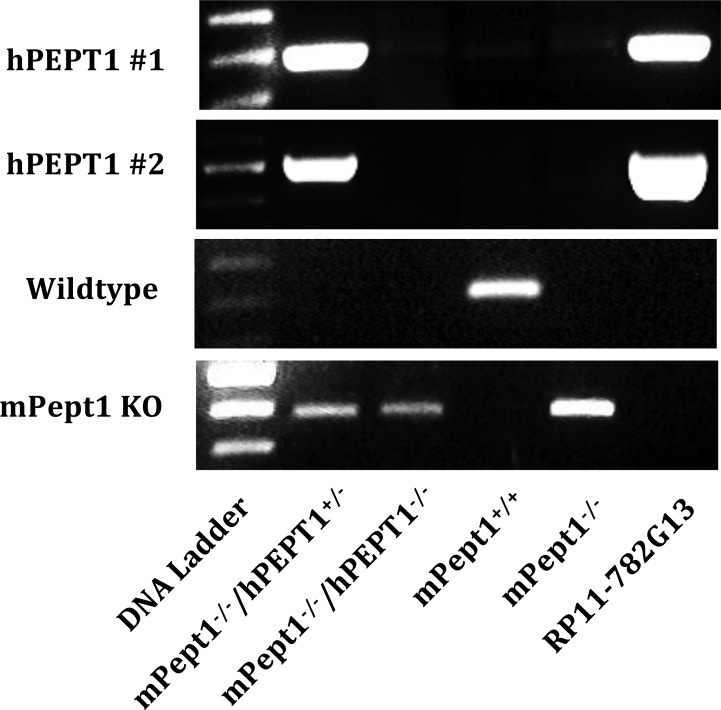
Mice humanized for PEPT1 were generated using a standard microinjection transgenic strategy with BAC DNA such that the entire hPEPT1 genome, comprising all regulatory and coding regions, was integrated into the mouse chromosome for inheritance. As shown in Figure 1, PCR analysis of genomic DNA extracted from tail biopsies demonstrated that hPEPT1 genomic DNA only appeared in huPEPT1 (mPept1–/–/hPEPT1+/–) mice and the BAC clone RP11-782G13, which served as a positive control. In contrast, hPEPT1 genomic DNA was not found in mouse genotypes mPept1+/+ (wildtype), mPept1–/–/hPEPT1–/– (mPept1 knockout mice bred to hemizygous huPEPT1 animals), and mPept1–/– (mPept1 knockout mice not bred to hemizygous huPEPT1 animals). Using wildtype primers, mouse Pept1 genomic DNA was neither detected in huPEPT1 nor Pept1 knockout mice (i.e., the mPept1–/–/huPEPT1–/– and mPept1–/– genotypes). Since mPept1 knockout primers were designed specifically to target the Neo gene, inserted during homologous recombination in the Pept1 knockout mouse model,23 a band was observed in mPept1–/–/hPEPT1+/–, mPept1–/–/huPEPT1–/–, and mPept1–/– mice, but not in mPept1+/+ animals.
Figure 1.
Genotyping results for the identification of humanized PEPT1 mice. Genomic DNA was extracted from mouse tail biopsies and genotyped by PCR using specific primers, as described previously.23 The DNA ladder, consisting of 100 bp repeats, was used to determine the size of PCR products. mPept1–/–/hPEPT1+/– represents the positive screen for humanized PEPT1 (huPEPT1) mice, mPept1–/–/hPEPT1–/– the negative screen for humanized PEPT1 mice, mPept1+/+ the wildtype mice, mPept1–/– the Pept1 knockout (KO) mice, and RP11–782G13 the purified BAC DNA (used to inject fertilized eggs in generating huPEPT1), which serves as a positive control.
During the process of generating huPEPT1 mice, six founder mice were identified as containing the RP11-782G13 BAC DNA for hPEPT1. However, only five of these mice succeeded in germline transmission of the transgenic gene, and only three mouse lines showed hPEPT1 transcripts (data not shown). The mouse line demonstrating the highest level of RNA was bred and maintained for subsequent studies. Using real-time PCR,27 the integration copy number of BAC DNA transferred from the RP11-782G13 clone was estimated as one in our humanized PEPT1 mouse genome.
Initial Phenotypic Analysis
Hemizygous huPEPT1 mice appeared normal with no obvious behavioral abnormality as compared to wildtype and mPept1 knockout (mPept1–/–) mice. These humanized mice had normal survival rates, fertility, litter size, gender distribution, and body weight. Moreover, as shown in Table 2, there were no significant differences in serum clinical chemistry between the wildtype, Pept1 knockout, and humanized PEPT1 mice. Histologic evaluation (i.e., hematoxylin and eosin staining) established normal morphology of the kidney, small intestine, and cecum/colon across the three genetic strains (data not shown).
Table 2. Serum Clinical Chemistry of Wildtype (WT), Pept1 Knockout (KO), and Humanized PEPT1 (huPEPT1) Micea.
| WT | Pept1 KO | huPEPT1 | |
|---|---|---|---|
| Body Weight | |||
| male, 7–8 weeks (g) | 21.5 ± 0.6 (12) | 21.3 ± 0.6 (12) | 22.1 ± 0.5 (12) |
| female, 7–8 weeks (g) | 17.9 ± 0.3 (12) | 17.7 ± 0.4 (12) | 18.0 ± 0.3 (12) |
| Serum | |||
| sodium (mmol/L) | 145 ± 1 (6) | 147 ± 1 (6) | 146 ± 1 (6) |
| potassium (mmol/L) | 7.3 ± 0.5 (6) | 7.4 ± 0.3 (6) | 7.7 ± 0.5 (6) |
| chloride (mmol/L) | 113 ± 1 (6) | 113 ± 1 (6) | 113 ± 1 (6) |
| calcium (mg/dL) | 9.7 ± 0.5 (6) | 9.5 ± 0.1 (6) | 9.8 ± 0.2 (6) |
| albumin (g/dL) | 3.4 ± 0.1 (6) | 3.5 ± 0.1 (6) | 3.6 ± 0.1 (6) |
| protein (g/dL) | 6.4 ± 0.1 (6) | 6.4 ± 0.1 (5) | 6.3 ± 0.1 (4) |
| creatinine (mg/dL) | 0.24 ± 0.03 (6) | 0.25 ± 0.01 (6) | 0.28 ± 0.05 (6) |
| bilirubin (mg/dL) | 0.16 ± 0.05 (5) | 0.08 ± 0.02 (5) | 0.14 ± 0.02 (5) |
| glucose (mg/dL) | 131 ± 22 (5) | 163 ± 18 (6) | 188 ± 6 (4) |
| BUN (mg/dL) | 29.5 ± 2.4 (6) | 27.5 ± 1.5 (6) | 33.3 ± 2.1 (6) |
| ALT (U/L) | 95.2 ± 16.0 (6) | 78.0 ± 4.8 (6) | 101 ± 6 (6) |
| ALP (U/L) | 186 ± 29 (6) | 94.2 ± 11.7 (6) | 149 ± 15 (6) |
| AST (U/L) | 124 ± 18 (6) | 124 ± 16 (6) | 104 ± 10 (6) |
Data are expressed as mean ± SE (n = number of mice). Pept1 KO (mPept1–/–) and huPEPT1 (mPept1–/–/hPEPT1+/–) mice were not significantly different than WT (mPept1+/+) mice, as evaluated by ANOVA/Dunnett’s analyses. BUN is urea nitrogen, ALT is alanine aminotransferase, ALP is alkaline phosphatase, and AST is aspartate aminotransferase.
Stable Expression of hPEPT1 in the Intestine of Humanized Mice
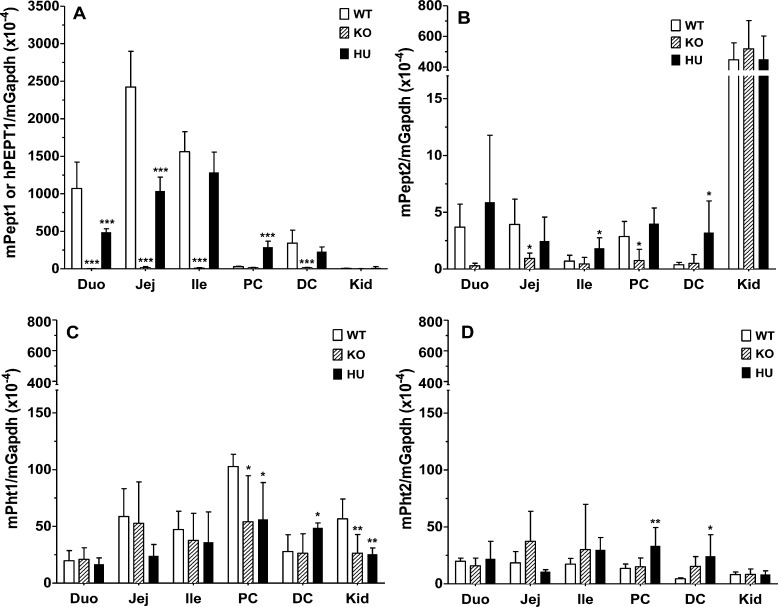
Quantitative real-time PCR (qPCR) demonstrated that human (and not mouse) PEPT1 transcripts were expressed in the small and large intestines of huPEPT1 mice (Figure 2A), but not in wildtype and Pept1 knockout animals (data not shown). Moreover, mouse Pept1 transcripts were expressed in wildtype animals but these same transcripts were not observed in Pept1 knockout mice (Figure 2A). A comparison of Pept1 expression in proximal colon further demonstrated that hPEPT1 mRNA was detectable in huPEPT1 mice, whereas mPept1 mRNA was not detectable in wildtype animals. Both hPEPT1 and mPept1 transcripts were observed in the distal colon, respectively, of huPEPT1 and wildtype mice.
Figure 2.
Real time-PCR analyses of mPept1 or hPEPT1 transcripts (A), mPept2 transcripts (B), mPht1 transcripts (C), and mPht2 transcripts (D) in the small intestine, colon, and kidney of wildtype (WT = mPept1+/+), Pept1 knockout (KO = mPept1–/–), and humanized PEPT1 (HU = mPept1–/–/hPEPT1+/–) mice. Gene expression was normalized by mGapdh. Duo represents the duodenum, Jej the jejunum, Ile the ileum, PC the proximal colon, DC the distal colon, and Kid the kidney. Data are expressed as mean ± SE (n = 4–6). *p < 0.05, **p < 0.01, and ***p < 0.001, as evaluated by ANOVA/Dunnett’s analyses in which WT was the control group. Note the discontinuous y-axis in panels B–D and the different scaling compared to panel A.
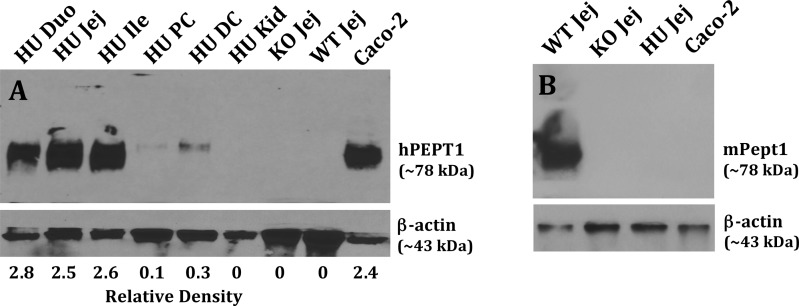
Immunoblot analyses of intestine and kidney were performed to assess whether the hPEPT1 transcripts would be translated into protein. As observed in Figure 3A, high expression levels of hPEPT1 protein were noted in the duodenum, jejunum, and ileum of humanized PEPT1 mice. In contrast, huPEPT1 animals had low expression of PEPT1 protein in the proximal and distal colon, and no expression in kidney. Specificity of the rabbit antihuman PEPT1 antibody was confirmed by the absence of signal in jejunal samples from wildtype and Pept1 knockout mice (Caco-2 cells served as a positive control). The presence of mPEPT1 protein was also tested in mice using a specific rabbit antimouse PEPT1 antibody, as shown in Figure 3B. In agreement with the qPCR results, mouse PEPT1 protein was expressed in the jejunum of wildtype mice, but not in the jejunum of huPEPT1 and mPept1 knockout animals (Caco-2 cells served as a negative control).
Figure 3.
Immunoblots of hPEPT1 protein in the small intestine, large intestine, and kidney of wildtype (WT = mPept1+/+), Pept1 knockout (KO = mPept1–/–), and humanized PEPT1 (HU = mPept1–/–/hPEPT1+/–) mice (A), and mPEPT1 protein in the jejunum of the same genotypes (B). Protein samples were separated by 10% SDS-PAGE, transferred onto PVDF membranes, and incubated for 1.5 h with rabbit antihuman hPEPT111 (1:3000) or antimouse mPEPT112 (1:5000) antiserum, and a mouse monoclonal antibody for β-actin (1:1000). The membranes were washed three times with TBST and then incubated for 1 h with an appropriate secondary antibody of IgG conjugated to horseradish peroxidase (1:3000). Caco-2 cells served as positive and negative controls, respectively, for hPEPT1 and mPEPT1. Duo represents the duodenum, Jej the jejunum, Ile the ileum, PC the proximal colon, DC the distal colon, and Kid the kidney.
Tissue Expression Profile of Select Transporters and Enzymes
It is crucial to know whether or not other proton-coupled oligopeptide transporters (POTs) will be dysregulated as a compensatory response to the mouse Pept1 gene being replaced by a human PEPT1 gene. As shown in Figure 2B–D, qPCR analyses indicated little to no expression of mouse Pept2, Pht1, and Pht2 transcripts in the small and large intestines. There were moderate levels of mPept2 mRNA in kidney; however, no change in expression was observed between the three genotypes (Figure 2B). In some cases, statistical differences were observed between the other three POT family members (Figures 2B–D). Nevertheless, given their extremely low expression levels, it is very unlikely that PEPT2 in the intestines and PHT1/2 in the intestines and kidney will have meaningful protein expression.
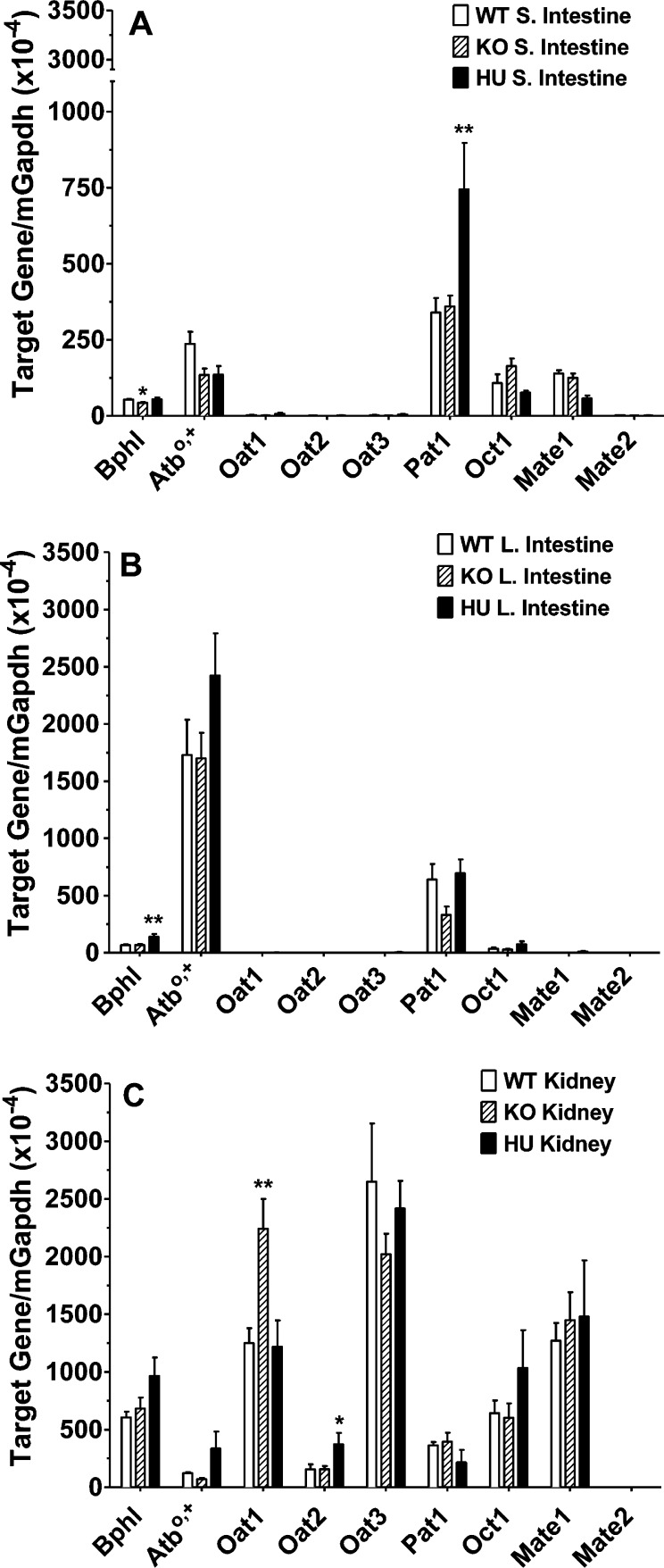
The tissue expression of several relevant peptide/mimetic transporters was also examined by qPCR. As shown in Figure 4, only minor changes were observed in mouse mRNA expression of transporters between the humanized PEPT1 and mPept1 knockout and wildtype animals. In this regard, mPat1 transcripts increased <2-fold in the small intestine of huPEPT1 (compared to wildtype or knockout mice; Figure 4A), mOat1 transcripts increased 2-fold in the kidney of Pept1 knockout mice (although no difference was observed between wildtype and huPEPT1 mice; Figure 4C), and mOat2 transcripts increased 2-fold in the kidney of huPEPT1 mice (compared to wildtype or knockout mice; Figure 4C). No differences were noted between the three genotypes in mouse mRNA expression of mAtb0,+, mOat3, mOct1, mMate1, and mMate2 in the small and large intestines and kidney.
Figure 4.
Real time-PCR analyses of select transporters and enzymes in the small intestine (A), large intestine (B), and kidney (C) of wildtype (WT = mPept1+/+), Pept1 knockout (KO = mPept1–/–), and humanized PEPT1 (HU = mPept1–/–/hPEPT1+/–) mice. Refer to Table 1 for gene identification. Data are expressed as mean ± SE (n = 4–6). *p < 0.05 and **p < 0.01, as evaluated by ANOVA/Dunnett’s analyses in which WT was the control group. Note the discontinuous y-axis in panel A.
The biphenyl hydrolase-like enzyme BPHL, important in activating the prodrug valacyclovir to acyclovir, was evaluated in the intestines and kidney by qPCR. A statistical decrease in mouse Bphl transcripts was observed in the small intestine of Pept1 knockout mice (although no difference was observed between wildtype and huPEPT1 mice; Figure 4A), and a statistical increase in mouse Bphl transcripts was observed in the large intestine of huPEPT1 mice (compared to wildtype or knockout mice; Figure 4B). It is very unlikely, however, that BPHL will have meaningful protein expression in the intestines given their extremely low expression levels. Finally, no difference in mouse Bphl transcripts was observed between genotypes in the kidney (Figure 4C).
Primers used in the qPCR analyses can be found in Table 1.
In Situ Single-Pass Intestinal Perfusion Studies
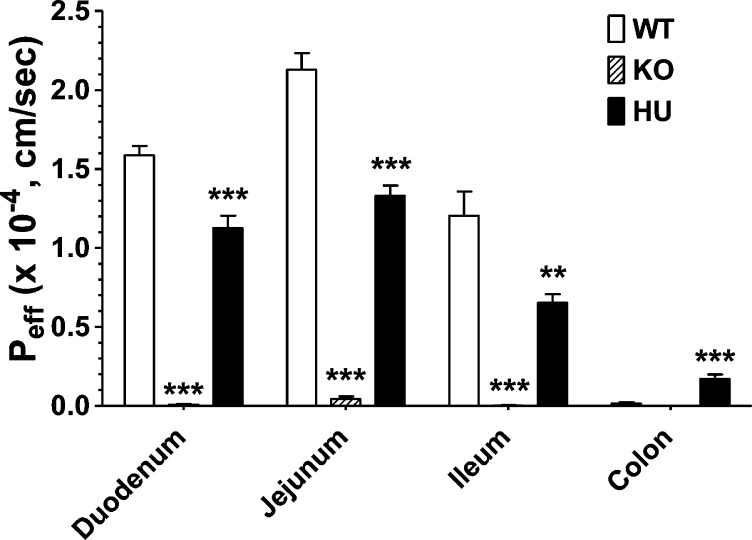
Intestinal perfusion studies were performed to evaluate the functional activity of PEPT1 in different regions of the small and large intestines, and to examine if differences exist between the three genotypes. As shown in Figure 5, there was substantial permeability of GlySar in the duodenum, jejunum, and ileum of huPEPT1 mice, although the permeability was lower than that observed in wildtype animals. As expected, GlySar permeability was minimal, at best, in Pept1 knockout mice in all intestinal regions. In agreement with the qPCR and immunoblot results, GlySar permeability was low, but measurable, in the colon of huPEPT2 mice and about 11-fold higher than the colonic permeability observed in wildtype animals.
Figure 5.
Effective permeability of 10 μM [3H]-GlySar in different intestinal regions of wildtype (WT = mPept1+/+), Pept1 knockout (KO = mPept1–/–), and humanized PEPT1 (HU = mPept1–/–/hPEPT1+/–) mice. All studies were performed in pH 6.5 buffer. Data are expressed as mean ± SE (n = 4–6). **p < 0.01 and ***p < 0.001, as evaluated by ANOVA/Dunnett’s analyses in which WT was the control group.
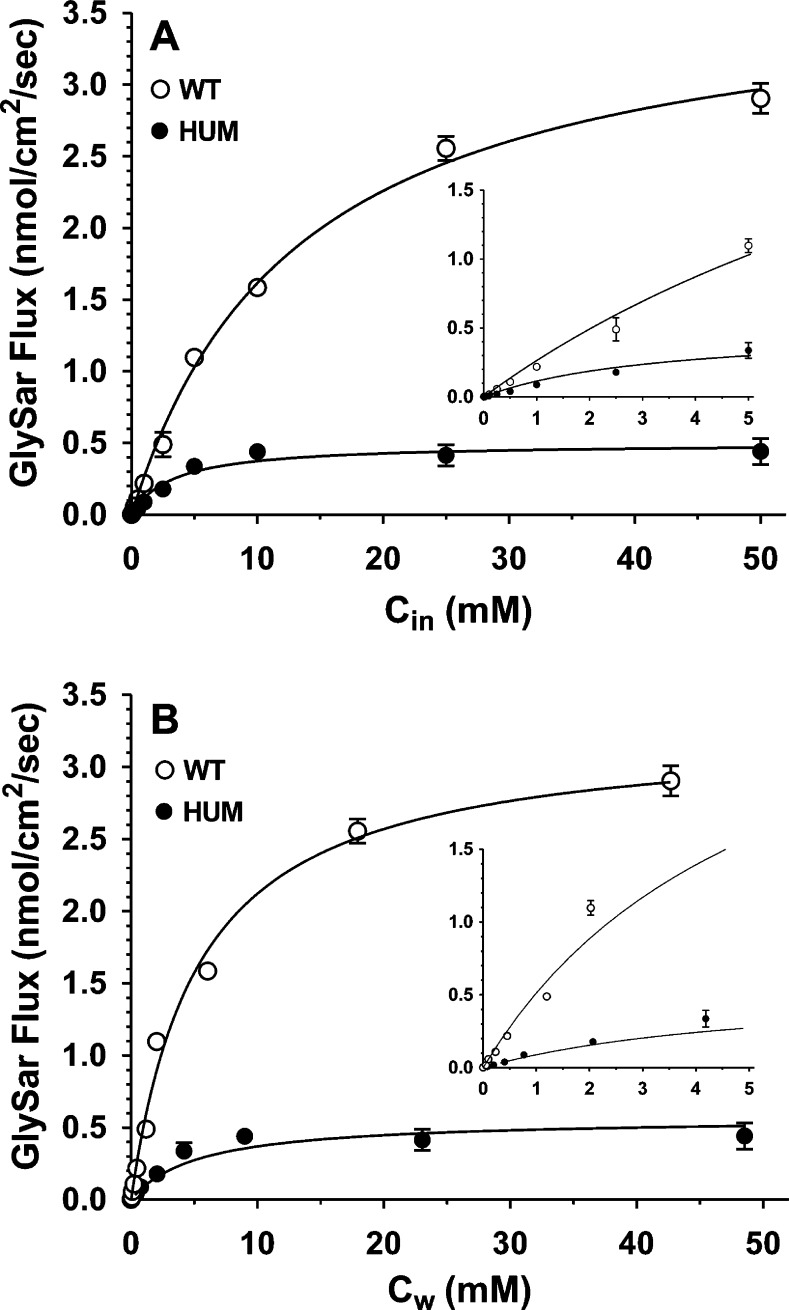
To assess if there were species-dependent differences in the transport kinetics of GlySar by PEPT1, concentration-dependent perfusion studies were performed in the jejunum of wildtype and huPEPT1 mice. As shown in Figure 6A, the maximal flux (Vm′ = 3.75 ± 0.11 nmol/cm2/sec for wildtype and 0.50 ± 0.04 nmol/cm2/sec for huPEPT1) and Michaelis constant (Km′ = 13.2 ± 1.0 mM for wildtype and 3.3 ± 0.9 mM for huPEPT1) of GlySar were substantially lower in mice humanized for the PEPT1 gene. When intestinal wall concentrations were used as the reference, after adjusting for the unstirred water layer, the maximal flux (Vm = 3.28 ± 0.13 nmol/cm2/sec for wildtype and 0.49 ± 0.03 nmol/cm2/sec for huPEPT1) and Michaelis constant (Km = 5.5 ± 0.7 mM for wildtype and 2.7 ± 0.6 mM for huPEPT1) were similarly lower in huPEPT1 mice as compared to wildtype animals (Figure 6B). These results demonstrate that a species difference exists in the transport kinetics of intestinal PEPT1.
Figure 6.
Concentration-dependent flux of [3H]-GlySar (0.01–50 mM total substrate) during jejunal perfusions of wildtype (WT = mPept1+/+) and huPEPT1 (HU = mPept1–/–/hPEPT1+/–) mice. Cin is the inlet concentration of GlySar in perfusate in which Vm′ = 3.75 ± 0.11 nmol/cm2/sec and Km′ = 13.2 ± 1.0 mM for WT mice, r2 = 0.988; Vm′ = 0.50 ± 0.04 nmol/cm2/sec and Km′ = 3.3 ± 0.9 mM for HU mice, r2 = 0.838 (A). Cw is the estimated concentration of GlySar at the membrane wall in which Vm = 3.24 ± 0.13 nmol/cm2/sec and Km = 5.5 ± 0.7 mM for WT mice, r2 = 0.993; Vm = 0.49 ± 0.03 nmol/cm2/sec and Km = 2.7 ± 0.6 mM for HU mice, r2 = 0.973 (B). All studies were performed in pH 6.5 buffer. Data are expressed as mean ± SE (n = 4–6).
In Vivo Oral Pharmacokinetic Studies
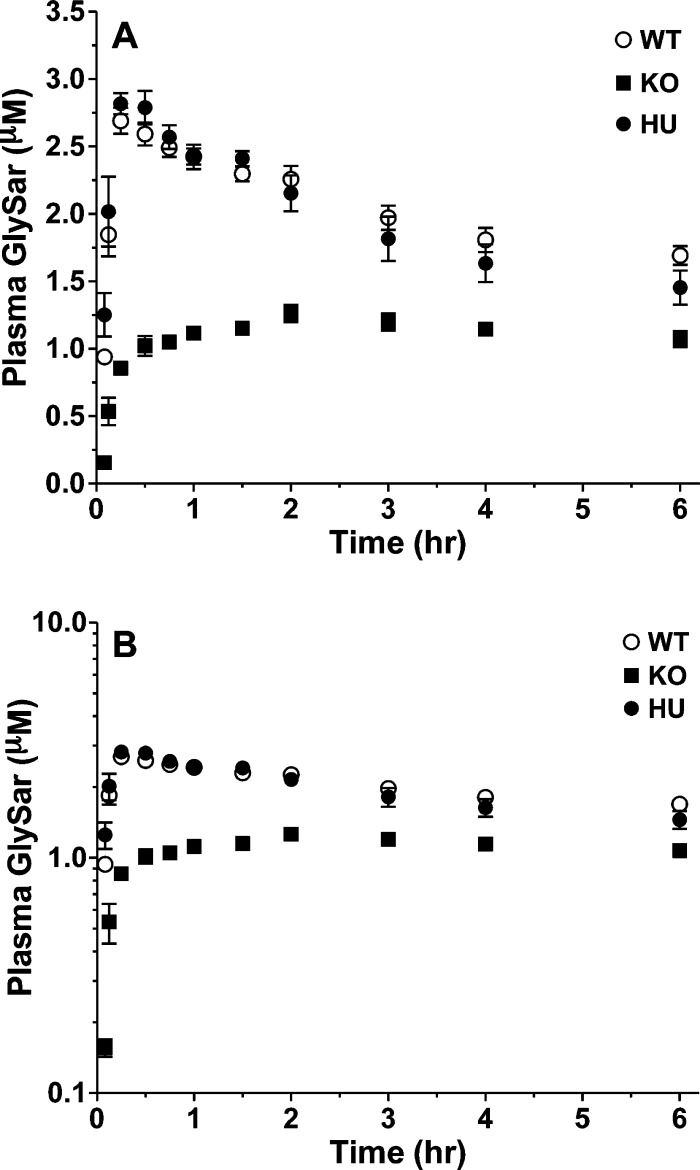
To assess the in vivo functional activity of hPEPT1 in humanized mice, the pharmacokinetics of the model dipeptide GlySar was evaluated after oral dosing. As shown in Figure 7, the plasma concentrations of GlySar were substantially reduced in Pept1 knockout mice, and according to Table 3, all pharmacokinetic parameters were significantly different than wildtype animals. The systemic exposure (AUC) of GlySar in Pept1 knockout mice was about 50% of that observed in wildtype animals. In contrast, the plasma concentration–time profiles of GlySar in huPEPT1 mice were virtually superimposable with that observed in wildtype animals. Humanized PEPT1 and wildtype mice had the same values for Cmax and Tmax, suggesting that the absorption rate was not different between the two genotypes. Moreover, the incremental AUC values (i.e., AUC0–t) in huPEPT1 mice were 93%, 97%, and 104% of that in wildtype mice at 0.5, 2, and 6 h, respectively, indicating that PEPT1 activity had been fully restored in these mice.
Figure 7.
Plasma concentration–time profiles of [14C]-GlySar in wildtype (WT = mPept1+/+), Pept1 knockout (KO = mPept1–/–), and humanized PEPT1 (HU = mPept1–/–/hPEPT1+/–) mice following a 5.0 nmol/g oral dose. Data are expressed as mean ± SE (n = 3) in which the y-axis is displayed on a linear scale (A) and on a logarithmic scale (B).
Table 3. Noncompartmental Pharmacokinetics of [14C]GlySar after a 5.0 nmol/g Oral Dose in Wildtype (WT), Pept1 Knockout (KO), and Humanized PEPT1 (huPEPT1) Micea.
| parameter (units) | WT | Pept1 KO | huPEPT1 |
|---|---|---|---|
| Cmax (μM) | 2.71 ± 0.09 | 1.27 ± 0.05b | 2.86 ± 0.09 |
| Tmax (h) | 0.33 ± 0.08 | 1.83 ± 0.17b | 0.33 ± 0.08 |
| AUC0–0.5 (μM·h) | 1.12 ± 0.02 | 0.34 ± 0.01b | 1.04 ± 0.04 |
| AUC0–2 (μM·h) | 4.76 ± 0.12 | 2.04 ± 0.04b | 4.61 ± 0.04 |
| AUC0–6 (μM·h) | 11.6 ± 0.6 | 6.66 ± 0.25b | 12.1 ± 0.3 |
Data are expressed as mean ± SE (n = 3).
***P < 0.001, as evaluated by ANOVA/Dunnett’s analyses of WT (mPept1+/+), Pept1 KO (mPept1–/–), and huPEPT1 (mPept1–/–/hPEPT1+/–) mice, where WT was the control group.
Discussion
Substantial progress has been made in generating and characterizing mice that contain human CYP450 and conjugation enzymes19,20 and nuclear receptors.20,21 However, with the exception of studies by Schinkel and co-workers28−30 in which several organic anion-transporting polypeptide (OATP) transporters were humanized in mice and Scheer et al.31 in which mice were humanized for multidrug resistance-associated protein 2 (MRP2), no other plasma membrane transporters have been humanized to date. Thus, the ability to generate a huPEPT1 mouse model containing the entire human genome offers an unparalleled opportunity to more reliably study in vivo systems in human PEPT1 absorption, transport, pharmacologic response, disease, and regulation.
In the present study, we developed and characterized a novel mouse line humanized for PEPT1. In doing so, we made the following observations: (1) huPEPT1 mice had no obvious behavioral or pathological phenotype; (2) mRNA and protein profiles indicated that huPEPT1 mice had substantial PEPT1 expression in all regions of the small intestine (i.e., duodenum, jejunum, and ileum) along with low but measurable expression in both proximal and distal segments of the colon; (3) the in situ permeability of GlySar in huPEPT1 mice was similar to but lower than wildtype animals in small intestine, and greater than wildtype mice in colon; (4) a species difference existed in the in situ transport kinetics of jejunal PEPT1, in which the maximal flux and Michaelis constant of GlySar were reduced in huPEPT1 compared to wildtype mice; and (5) the in vivo function of intestinal PEPT1 appeared fully restored (compared to Pept1 knockout mice) as indicated by the nearly identical pharmacokinetics and plasma concentration–time profiles of GlySar in huPEPT1 and wildtype mice following a single oral dose.
There is good agreement among species (e.g., rat, mouse, and human) regarding the abundant protein expression of PEPT1 in duodenal, jejunal, and ileal segments of small intestine, and its apical localization.8−12,32−35 However, the colonic expression of PEPT1 is controversial and may be the result of differences among species, antibody specificity, regional specificity, and the methods of preparation between different laboratories. Whereas some studies have reported the expression of PEPT1 protein in normal mouse, rat, and human colon,9,11,32 other studies have been unable to detect PEPT1 in normal colon.8,12,33−35 In particular, Wuensch et al.11 found a distinct spatial distribution of colonic PEPT1 in mice, rats, and humans in which immunostaining was not observed in proximal colon, but significant staining was observed in the distal colon. In our hands, we have consistently detected abundant expression of PEPT1 protein in all regions of mouse and rat small intestine, but not in the colon of rodents past 7 days of age.8,12 In addition, the functional activity of mouse PEPT1 was consistent with these expression levels, as determined by the permeability of GlySar,12 cefadroxil,36 and valacyclovir37 in wildtype compared to Pept1 knockout mice. In the present study, huPEPT1 mice had measurable expression of PEPT1 protein in the distal and proximal colon (distal > proximal) and 11-fold higher permeabilities of GlySar in colon as compared to wildtype mice. Still, the colonic permeability of GlySar was only about 25–30% of that in ileum. Therefore, it appears that huPEPT1 mice (under regulatory control of the human genome) could express a functional PEPT1 protein that transported GlySar across the colon, whereas wildtype mice (under regulatory control of the murine genome) did not have this capability.
A species-dependent difference was also reported in the affinity of PEPT1 for GlySar where the Km values varied over a 5.4-fold range in yeast Pichia pastoris expressing the human (0.86 mM), mouse (0.30 mM), and rat (0.16 mM) transformants.16 In the present study, this trend was reversed in which the Km of GlySar was 2- to 4-fold lower (i.e., greater affinity), and the Vm or Vm′ was 7-fold lower in huPEPT1 mice compared to wildtype animals during in situ jejunal perfusions of substrate. It is unclear, at present, why the Km values of GlySar “flip-flop” when studied in vitro in yeast expressing PEPT1 mouse and human homologues compared to in situ during intestinal perfusions in wildtype and huPEPT1 mice. Thus, it will be important to determine, in subsequent studies, if the in vivo intestinal absorption of GlySar (and other peptides/mimetics) is dose-dependent (nonlinear) following oral dose escalation. In doing so, the huPEPT1 mouse model might be useful in clarifying the discrepancy between the dose-proportional absorption of cefadroxil6 and valacyclovir7 in mice over an 8- to 10-fold oral dose range, respectively, and the nonlinear intestinal absorption observed for these compounds in humans.14,15 More important, perhaps, would be the ability of huPEPT1 mice to better predict the oral drug (and prodrug) performance of new chemical entities.
Previous studies have shown that, after oral dosing, the in vivo systemic exposure of GlySar in Pept1 knockout mice was only about 50% of that in wildtype mice even though in situ permeabilities in the proximal small intestine differed by >10-fold between genotypes.12,39 To explain this “apparent” discrepancy, the authors suggested that GlySar may be able to take advantage of the intestine’s residual length and long residence times so that passive absorption processes play a bigger role in the absence of PEPT1. Our current study corroborated these earlier findings where GlySar had a substantially reduced (>50-fold) in situ permeability in the small intestine of Pept1 knockout mice but an in vivo oral availability that differed by only 50%, compared to wildtype animals. Given the complexity of intestinal absorption (including membrane permeability, luminal drug concentration, and gastrointestinal residence time), it was not surprising that the pharmacokinetics and oral absorption profiles of GlySar were similar in wildtype and huPEPT1 mice (Figure 7 and Table 3), especially when in situ permeabilities in the small intestine of huPEPT1 mice were reduced by only 30–40% and higher in colon (Figure 5). However, since species differences were observed in the transport kinetics (i.e., Vm and Km) of GlySar, it will be interesting to see whether or not the pharmacokinetics are similar in wildtype and huPEPT1 mice when higher oral doses of substrate are administered and the chance of intestinal PEPT1 saturability increases.
It should be appreciated that transgenic mice were generated previously38 in which hPepT1 expression was regulated by the mouse β-actin or villin promoters as a model for studying the role of PEPT1 in inflammatory bowel disease. However, in these mice there was no deletion of endogenous mPEPT1. As a result, the concomitant protein expression of mouse and human PEPT1 in the intestines and other PEPT1-expressing tissues of the body make it impossible to separate the role of each species-specific transporter and, thereby, humanize the mouse. In our huPEPT1 mouse model, the purified BAC DNA, containing the entire hPEPT1 genome, was injected into eggs from Pept1 knockout mice. As a result, the huPEPT1 mice lacked endogenous mPEPT1 protein and, by maintaining the mice as hemizygotes, were able to avoid the potential interference of other endogenous genes. Another advantage of using genomic DNA was that transcripts of the huPEPT1 gene were regulated by their own regulatory elements and produced PEPT1 protein only if the gene translation mechanism was conserved among mammals.
In concluding, the present study reports, for the first time, the development and initial characterization of huPEPT1 mice. These mice are unique in that they contain a copy of the entire human genome in mice previously nulled for mPept1 and demonstrate the full restoration of PEPT1 function. There is excellent agreement between hPEPT1 expression in the intestines, the in situ intestinal permeability of GlySar, and the in vivo intestinal absorption of GlySar following a single oral dose. However, a clear species difference was observed in the maximal flux and affinity of GlySar during in situ jejunal perfusions of huPEPT1 mice as compared to wildtype animals. These humanized PEPT1 mice should prove a valuable model in future studies investigating the role, relevance, and regulation of PEPT1 in diet and disease and in the drug discovery process.
Acknowledgments
We greatly appreciate the expertise of the Transgenic Animal Model Core, University of Michigan, in helping us to generate the huPEPT1 mice. This work was supported by Public Health Service grant GM-035498 from the National Institute of General Medical Sciences (to D.E.S.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Fei Y. J.; Kanai Y.; Nussberger S.; Ganapathy V.; Leibach F. H.; Romero M. F.; Singh S. K.; Boron W. F.; Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 1994, 368, 563–566. [DOI] [PubMed] [Google Scholar]
- Liang R.; Fei Y. J.; Prasad P. D.; Ramamoorthy S.; Han H.; Yang-Feng T. L.; Hediger M. A.; Ganapathy V.; Leibach F. H. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 1995, 270, 6456–6463. [DOI] [PubMed] [Google Scholar]
- Fei Y. J.; Sugawara M.; Liu J. C.; Li H. W.; Ganapathy V.; Ganapathy M. E.; Leibach F. H. cDNA structure, genomic organization, and promoter analysis of the mouse intestinal peptide transporter PEPT1. Biochim. Biophys. Acta 2000, 1492, 145–154. [DOI] [PubMed] [Google Scholar]
- Smith D. E.; Clemencon B.; Hediger M. A. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol. Aspects Med. 2013, 34, 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocheltree S. M.; Shen H.; Hu Y.; Keep R. F.; Smith D. E. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J. Pharmacol. Exp. Ther. 2005, 315, 240–247. [DOI] [PubMed] [Google Scholar]
- Posada M. M.; Smith D. E. In vivo absorption and disposition of cefadroxil after escalating oral doses in wild-type and PepT1 knockout mice. Pharm. Res. 2013, 30, 2931–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Hu Y.; Smith D. E. Impact of peptide transporter 1 on the intestinal absorption and pharmacokinetics of valacyclovir after oral dose escalation in wild-type and PepT1 knockout mice. Drug Metab. Dispos. 2013, 41, 1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H.; Smith D. E.; Brosius F. C. 3rd. Developmental expression of PEPT1 and PEPT2 in rat small intestine, colon, and kidney. Pediatr. Res. 2001, 49, 789–795. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R.; Fernandez-Estivariz C.; Gu L. H.; Bazargan N.; Umeakunne K.; Wallace T. M.; Diaz E. E.; Rosado K. E.; Pascal R. R.; Galloway J. R.; Wilcox J. N.; Leader L. M. Distribution of the H+/peptide transporter PepT1 in human intestine: up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am. J. Clin. Nutr. 2002, 75, 922–930. [DOI] [PubMed] [Google Scholar]
- Walker D.; Thwaites D. T.; Simmons N. L.; Gilbert H. J.; Hirst B. H. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. 1998, 507Pt 3697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuensch T.; Schulz S.; Ullrich S.; Lill N.; Stelzl T.; Rubio-Aliaga I.; Loh G.; Chamaillard M.; Haller D.; Daniel H. The peptide transporter PEPT1 is expressed in distal colon in rodents and humans and contributes to water absorption. Am. J. Physiol.: Gastrointest. Liver Physiol. 2013, 305, G66–G73. [DOI] [PubMed] [Google Scholar]
- Jappar D.; Wu S. P.; Hu Y.; Smith D. E. Significance and regional dependency of peptide transporter (PEPT) 1 in the intestinal permeability of glycylsarcosine: in situ single-pass perfusion studies in wild-type and Pept1 knockout mice. Drug Metab. Dispos. 2010, 38, 1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pico A.; Peris-Ribera J. E.; Toledano C.; Torres-Molina F.; Casabo V. G.; Martin-Villodre A.; Pla-Delfina J. M. Non-linear intestinal absorption kinetics of cefadroxil in the rat. J. Pharm. Pharmacol. 1989, 41, 179–185. [DOI] [PubMed] [Google Scholar]
- Garrigues T. M.; Martin U.; Peris-Ribera J. E.; Prescott L. F. Dose-dependent absorption and elimination of cefadroxil in man. Eur. J. Clin. Pharmacol. 1991, 41, 179–183. [DOI] [PubMed] [Google Scholar]
- Weller S.; Blum M. R.; Doucette M.; Burnette T.; Cederberg D. M.; de Miranda P.; Smiley M. L. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 1993, 54, 595–605. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Chen X.; Smith D. E. Species-dependent uptake of glycylsarcosine but not oseltamivir in Pichia pastoris expressing the rat, mouse, and human intestinal peptide transporter PEPT1. Drug Metab. Dispos. 2012, 40, 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.; Gonzalez F. J. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J. Pharmacol. Exp. Ther. 2008, 327, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley L. A.; Horsburgh B. C.; Ross J.; Scheer N.; Wolf C. R. Drug transporters: gatekeepers controlling access of xenobiotics to the cellular interior. Drug Metab. Rev. 2009, 41, 27–65. [DOI] [PubMed] [Google Scholar]
- Jiang X. L.; Gonzalez F. J.; Yu A. M. Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metab. Rev. 2011, 43, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boverhof D. R.; Chamberlain M. P.; Elcombe C. R.; Gonzalez F. J.; Heflich R. H.; Hernández L. G.; Jacobs A. C.; Jacobson-Kram D.; Luijten M.; Maggi A.; Manjanatha M. G.; Benthem J.; Gollapudi B. B. Transgenic animal models in toxicology: historical perspectives and future outlook. Toxicol. Sci. 2011, 121, 207–233. [DOI] [PubMed] [Google Scholar]
- Scheer N.; Wolf C. R. Xenobiotic receptor humanized mice and their utility. Drug Metab. Rev. 2013, 45, 110–121. [DOI] [PubMed] [Google Scholar]
- Van Keuren M. L.; Gavrilina G. B.; Filipiak W. E.; Zeidler M. G.; Saunders T. L. Generating transgenic mice from bacterial artificial chromosomes: transgenesis efficiency, integration and expression outcomes. Transgenic Res. 2009, 18, 769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Smith D. E.; Ma K.; Jappar D.; Thomas W.; Hillgren K. M. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol. Pharmaceutics 2008, 5, 1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Xie Y.; Keep R. F.; Smith D. E. Divergent developmental expression and function of the proton-coupled oligopeptide transporters PepT2 and PhT1 in regional brain slices of mouse and rat. J. Neurochem. 2014, 129, 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou J. H.; Fleisher D.; Amidon G. L. Calculation of the aqueous diffusion layer resistance for absorption in a tube: application to intestinal membrane permeability determination. Pharm. Res. 1991, 8, 298–305. [DOI] [PubMed] [Google Scholar]
- Komiya I.; Park J. Y.; Kamani A.; Ho N. F. H.; Higuchi W. I. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int. J. Pharm. 1980, 4, 249–262. [Google Scholar]
- Chandler K. J.; Chandler R. L.; Broeckelmann E. M.; Hou Y.; Southard-Smith E. M.; Mortlock D. P. Relevance of BAC transgene copy number in mice: transgene copy number variation across multiple transgenic lines and correlations with transgene integrity and expression. Mamm. Genome 2007, 18, 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E.; van der Kruijssen C. M.; Wagenaar E.; Burggraaff J. E.; Mesman E.; Kenworthy K. E.; Schinkel A. H. Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1). Drug Metab. Dispos. 2009, 37, 277–281. [DOI] [PubMed] [Google Scholar]
- van de Steeg E.; Stránecký V.; Hartmannová H.; Nosková L.; Hřebíček M.; Wagenaar E.; van Esch A.; de Waart D. R.; Oude Elferink R. P.; Kenworthy K. E.; Sticová E.; al-Edreesi M.; Knisely A. S.; Kmoch S.; Jirsa M.; Schinkel A. H. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J. Clin. Invest. 2012, 122, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Steeg E.; van Esch A.; Wagenaar E.; Kenworthy K. E.; Schinkel A. H. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin. Cancer Res. 2013, 19, 821–832. [DOI] [PubMed] [Google Scholar]
- Scheer N.; Balimane P.; Hayward M. D.; Buechel S.; Kauselmann G.; Wolf C. R. Generation and characterization of a novel multidrug resistance protein 2 humanized mouse line. Drug Metab. Dispos. 2012, 40, 2212–2218. [DOI] [PubMed] [Google Scholar]
- Ford D.; Howard A.; Hirst B. H. Expression of the peptide transporter hPepT1 in human colon: a potential route for colonic protein nitrogen and drug absorption. Histochem. Cell Biol. 2003, 119, 37–43. [DOI] [PubMed] [Google Scholar]
- Ogihara H.; Saito H.; Shin B. C.; Terado T.; Takenoshita S.; Nagamachi Y.; Inui K.; Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem. Biophys. Res. Commun. 1996, 220, 848–852. [DOI] [PubMed] [Google Scholar]
- Groneberg D. A.; Doring F.; Eynott P. R.; Fischer A.; Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol.: Gastrointest. Liver Physiol. 2001, 281, G697–G704. [DOI] [PubMed] [Google Scholar]
- Merlin D.; Si-Tahar M.; Sitaraman S. V.; Eastburn K.; Williams I.; Liu X.; Hediger M. A.; Madara J. L. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 2001, 120, 1666–1679. [DOI] [PubMed] [Google Scholar]
- Posada M. M.; Smith D. E. Relevance of PepT1 in the intestinal permeability and oral absorption of cefadroxil. Pharm. Res. 2013, 30, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.; Smith D. E. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab. Dispos. 2013, 41, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G.; Nguyen H. T.; Ingersoll S. A.; Ayyadurai S.; Laroui H.; Charania M. A.; Yan Y.; Sitaraman S. V.; Merlin D. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology 2011, 141, 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jappar D.; Hu Y.; Smith D. E. Effect of dose escalation on the in vivo oral absorption and disposition of glycylsarcosine in wild-type and Pept1 knockout mice. Drug Metab. Dispos. 2011, 39, 2250–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]