Abstract
Objective
Our primary objective was to determine the sensitivity, specificity, and accuracy of fully quantitative stress perfusion CMR versus a reference standard of quantitative coronary angiography (QCA). We hypothesized that fully quantitative analysis of stress perfusion CMR would have high diagnostic accuracy for identifying significant coronary artery stenosis and exceed the accuracy of semi-quantitative measures of perfusion and qualitative interpretation.
Background
Relatively few studies apply fully quantitative CMR perfusion measures to patients with coronary disease and comparisons to semi-quantitative and qualitative methods are limited.
Methods
Dual bolus dipyridamole stress perfusion CMR exams were performed in 67 patients with clinical indications for assessment of myocardial ischemia. Stress perfusion images alone were analyzed with a fully quantitative method (QP) and 3 semi-quantitative methods including contrast enhancement ratio (CER), upslope index (SLP), and upslope integral (INT). Comprehensive exams (cine imaging, stress/rest perfusion, late gadolinium enhancement) were analyzed qualitatively with two methods including the Duke Algorithm and standard clinical interpretation. A 70% or greater stenosis by QCA was considered abnormal.
Results
The optimum diagnostic threshold for QP determined by receiver operating characteristic curve occurred when endocardial flow decreased to <50% of mean epicardial flow which yielded a sensitivity of 87% and specificity of 93%. The area under the curve (AUC) for QP was 0.92 which was superior to semi-quantitative methods: CER 0.78, SLP 0.82, and INT 0.75 (p=0.011, p=0.019, p=0.004 versus QP, respectively). AUC for QP was also superior to qualitative methods: Duke Algorithm 0.70 and clinical interpretation 0.78 (p<0.001 and p<0.001 versus QP, respectively).
Conclusions
Fully quantitative stress perfusion CMR has high diagnostic accuracy for detecting obstructive CAD. QP outperforms semi-quantitative measures of perfusion and qualitative methods that incorporate a combination of cine, perfusion, and late gadolinium enhancement imaging. These findings suggest a potential clinical role for quantitative stress perfusion CMR.
Keywords: Cardiac Magnetic Resonance, Myocardial Perfusion, Myocardial Ischemia, Quantitative Perfusion, Stress Testing
Introduction
CMR has potential advantages in the assessment of myocardial perfusion. Diagnostically, CMR appears superior to SPECT.(1–3) Comparisons to PET are favorable (4) and similar to PET, CMR can quantify perfusion in absolute terms.(5–7) However, CMR has finer resolution, wider availability, and can evaluate function, perfusion, and viability in the same exam without ionizing radiation.
Although semi-quantitative CMR perfusion has been evaluated in the setting of CAD, semi-quantitative techniques underestimate perfusion at high flow rates and have a potential diagnostic disadvantage compared with fully quantitative perfusion which increases linearly over a wide range of flow rates.(8) However, fully quantitative studies in humans with CAD have been limited and lack adequate comparisons to semi-quantitative and qualitative analyses.(9–15)
Quantifying myocardial perfusion utilizing a dual bolus first pass CMR method has been validated against microspheres in a canine model and all major findings have been confirmed in normal human volunteers.(8,16) To investigate the clinical utility of this technique, we applied the method to patients with known or suspected coronary disease.
Our primary objective was to determine the sensitivity, specificity, and accuracy of fully quantitative dual bolus stress perfusion CMR versus a reference standard of quantitative coronary angiography (QCA). We hypothesized that fully quantitative analysis of stress perfusion CMR would have high diagnostic accuracy for identifying significant coronary artery stenosis. Secondly, we hypothesized that the diagnostic accuracy of the fully quantitative method would exceed that of semi-quantitative and qualitative methods of interpretation. Finally, we sought to demonstrate that detailed stress perfusion analysis independently contains the diagnostic information necessary to detect the presence of significant coronary disease.
Methods
Patients
This study was approved by the institutional review boards of the National Institutes of Health and Suburban Hospital (Bethesda, Maryland). All patients were referred for stress myocardial perfusion imaging with clinical indications. Sixty-seven subjects with coronary angiography performed within 90 days of the CMR were included on a consecutive basis. Subjects with recent PCI, history of coronary bypass surgery, contraindication to dipyridamole, or contraindication to CMR were excluded.
Image Acquisition
Imaging was performed on a 1.5T Siemens (Erlangen, Germany) or General Electric (Waukesha, Wisconsin) scanner using a twelve-element or four-element phased array coil respectively. Gadolinium-DTPA (Magnevist, Bayer) was administered with a mechanical injector (MedRad, Indianola, Pennsylvania). The exam proceeded in the following sequence: stress perfusion, cine rest function, rest perfusion, and late gadolinium enhancement imaging (LGE).
The vasodilator stress protocol utilized dipyridamole 0.56 mg/kg infused intravenously over 4 minutes. Perfusion imaging commenced 4 minutes after the dipyridamole infusion. Dual bolus first pass perfusion was performed using gadolinium doses of 0.005 mmol/kg followed by 0.1 mmol/kg.(16) The 0.005 mmol/kg dose was diluted in saline to ensure equal volume and rates of injection for the two doses. Three short axis images were acquired using a saturation recovery, hybrid echo-planar perfusion sequence every heart beat for 50–60 cardiac cycles.(17) Typical imaging parameters were slice thickness 8 mm, FOV 360 × 270, TR 6.6–7.5 msec, TE 1.48–1.6 msec, echo train length 4, matrix 128 × 80–96, flip angle 20–25, and saturation recovery time 60–80 ms. Parallel imaging with rate 2 temporal sensitivity encoding was utilized in 69% of scans.(18) Aminophylline 100–150 mg was administered post-stress to mitigate residual vasodilator effects during rest imaging.
Subsequently, steady-state free precession cine images were acquired to assess left ventricular function. Thirty minutes after stress, rest perfusion imaging was performed repeating the dual bolus method as described. A final dose of 0.05 mmol/kg gadolinium was injected after the rest perfusion study in preparation for LGE imaging. Ten minutes after rest perfusion, LGE images were obtained with a phase sensitive inversion recovery fast gradient echo sequence.
Image Analysis
CMR exams were analyzed blinded to clinical history and cardiac catheterization results by a consensus of 2 readers. Qualitative interpretation of exams was performed by a standard clinical protocol using all imaging including cine, stress/rest perfusion, and LGE. Additionally, the published “Duke Algorithm” was applied in which LGE was used as an initial screen for obstructive CAD and stress/rest perfusion images were considered at secondary and tertiary levels of importance. (19)
For fully quantitative and semi-quantitiative analysis, three stress perfusion slices per patient were divided into 12 radial segments per slice and evaluated as endocardial, epicardial, and transmural regions. Endocardial and epicardial contours were drawn manually, automatically propagated, and manually corrected when necessary which required approximately 5–10 minutes per slice. Division into endocardial and epicardial regions was entirely computer derived. Absolute myocardial perfusion was quantified using Fermi function constrained deconvolution methods as described previously.(8,16) Semi-quantitative analysis of myocardial perfusion was performed as previously described.(4,8,20,21) The contrast enhancement ratio method (CER) involved the following calculation: CER = (SI peak − SI baseline)/SI baseline where SI peak is the mean peak signal intensity of the myocardial region and SI baseline is the mean baseline signal intensity of the myocardial region. The myocardial to left ventricular upslope ratio (SLP) was calculated by dividing the initial upslope of the myocardial time-intensity curve by the initial upslope of the left ventricular time-intensity curve. The upslope integral (INT) was calculated from the area under the curve from baseline to peak enhancement using baseline-adjusted myocardial time–intensity curves.
Endocardial flow was compared to normal flow within the slice as defined by median epicardial flow. In this manner, endocardial to epicardial ratios were generated for each segment. Similarly, semi-quantitative endocardial values were compared to median epicardial values within the slice. Studies were classified as abnormal when at least 2 segments had endocardial to epicardial ratios lower than the threshold in the distribution of the stenosed vessel. In addition to endocardial to epicardial ratio analysis, the diagnostic performance of absolute endocardial flow and absolute transmural flow was evaluated.
A cardiologist blinded to the CMR results performed quantitative coronary angiography (QCA) using Quantcor software by Siemens (Forchheim, Germany). All perfusion results were correlated to QCA on a per patient basis using a threshold of 70% stenosis.
Statistical Analysis
Categorical variables are expressed as numbers and percentages. Continuous variables are presented as mean +/− standard deviation (SD) unless otherwise specified. Receiver operating characteristic curves (ROC) for all methods were generated with MedCalc for Windows, version 12.2.1.0 (MedCalc Software, Mariakerke, Belgium). Diagnostic performance was ascertained from the area under the ROC curve (AUC). Comparison of ROC curves was performed by the method of Delong, et al. There was no correction for multiple comparisons of AUC curves. Optimal sensitivity and specificity were determined by the Youden index. Sensitivity and specificity between methods were compared with McNemar’s test. Normally distributed data was compared with the t-test. The Wilcoxon and Mann-Whitney tests were applied to non-normally distributed data.
Results
Patient Characteristics
Baseline characteristics summarized in Table 1 were reflective of patients routinely referred for stress imaging exams in clinical practice. The average age was 60 (range 38–85) and 33% were female. A history of myocardial infarction was present in 25%. Remote PCI had been performed in 25%. The prevalence of obstructive CAD by QCA (≥70% stenosis) was 34% (23/67) including 2 with three vessel disease and 5 with two vessel disease.
Table 1.
Patient Characteristics
| Characteristic | Number (%) |
|---|---|
| Age (years +/− SD) | 60 +/− 11 |
| Female | 22 (33) |
| Hypertension | 40 (60) |
| Hyperlipidemia | 50 (75) |
| Diabetes | 11 (16) |
| Smoking | 28 (42) |
| Family History | 31 (46) |
| Chest Pain | 48 (72) |
| Prior MI | 17 (25) |
| Prior PCI | 17 (25) |
| Any Stenosis ≥70% by QCA | 23 (34) |
| 3 Vessel Disease | 2 (3) |
| 2 Vessel Disease | 5 (7) |
| 1 Vessel Disease | 16 (24) |
Threshold for Abnormal Perfusion
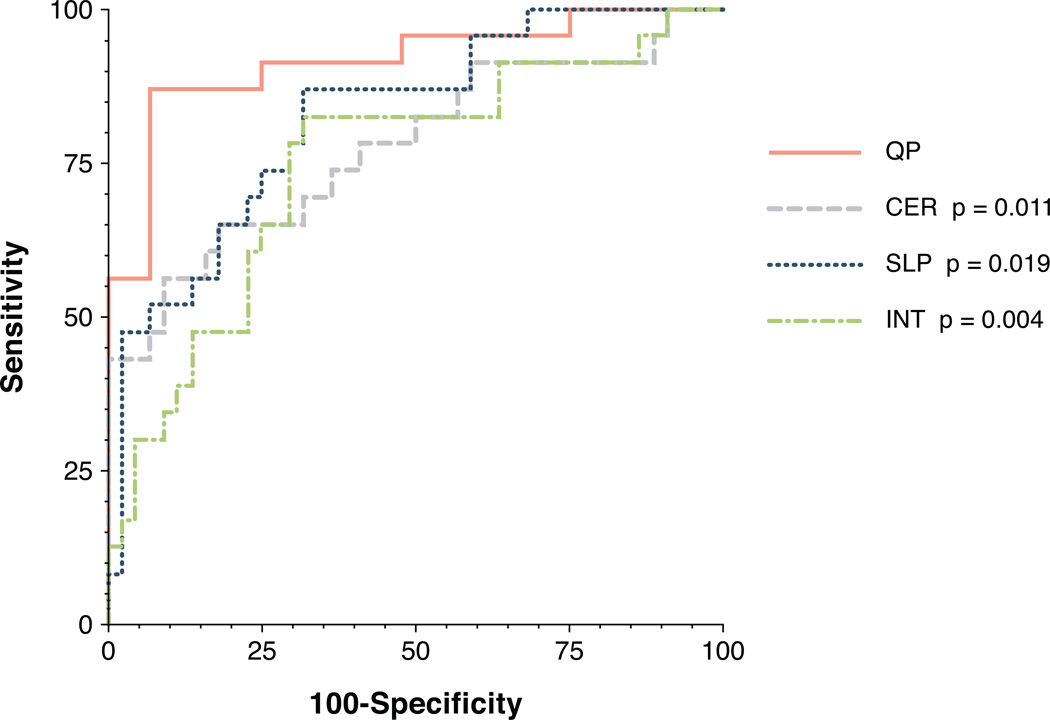
The threshold for abnormal perfusion was determined by ROC (Figure 1). The point of maximum sensitivity and specificity occurred when endocardial flow in two segments was less than 50% below normal as defined by median epicardial flow. Thus, an endocardial to epicardial ratio less than 0.50 identified segments with abnormal perfusion. Thresholds for semi-quantitative methods were determined in a similar manner from ROC analysis. The thresholds for semi-quantitative endocardial to median epicardial ratios were: CER 0.57, SLP 0.67, and INT 0.58.
Figure 1. ROC curves of QP and semi-quantitative methods.
The AUC for QP (0.92) was greater than the AUC for CER (0.78, p=0.011), SLP (0.82, p=0.019), and INT (0.75, p=0.004).
Diagnostic Performance
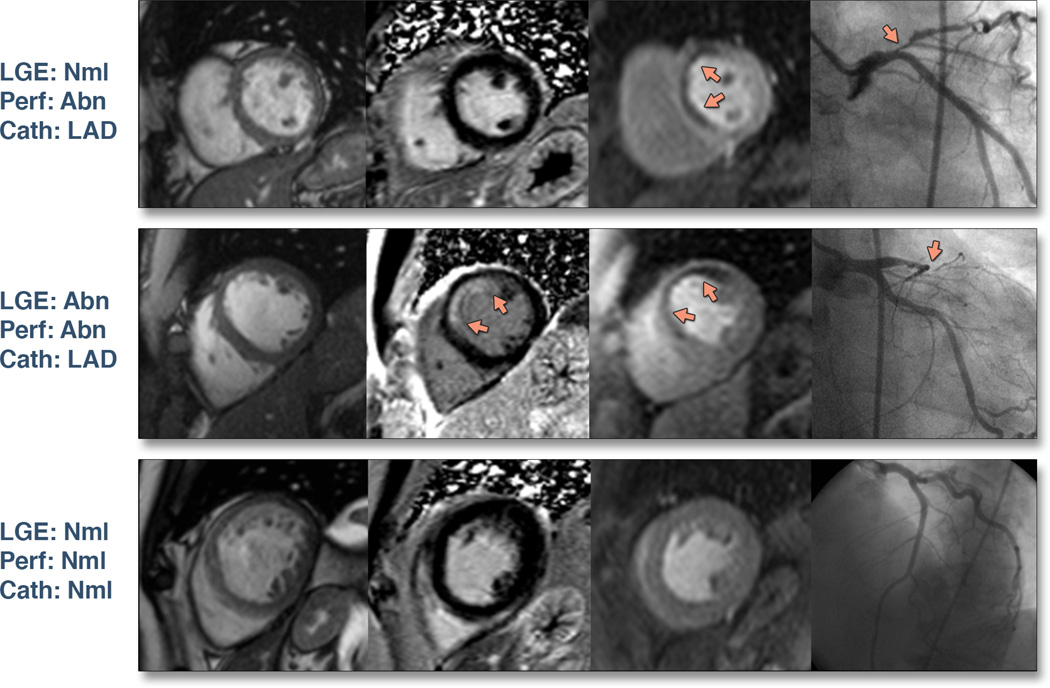
QP performed well against QCA with a sensitivity of 87% and specificity of 93%. There were 3 false negative patients by QP, all of whom had single vessel disease. All patients with multi-vessel disease (n=7) including one subject with left main disease were correctly identified as true positives by QP. There were 3 false positive subjects by QP who had stenoses of 67%, 65%, and two vessel disease with stenoses of 65% and 60%. No false positive patients had myocardial infarction. Invasive measures of fractional flow reserve were not available in any patients. Representative CMR images with angiographic correlation are shown in Figure 2.
Figure 2. Representative CMR images with angiographic correlation.
Columns from left to right display cine images, LGE images, stress perfusion images (Perf), and the invasive coronary angiogram (Cath). The first row demonstrates a subject with no myocardial infarction but a stress perfusion defect in the anterior and anteroseptal segments corresponding to a severe stenosis of the proximal left anterior descending coronary artery (LAD). The second row shows a subject with a subendocardial myocardial infarction and a stress perfusion defect in the anterior and anteroseptal segments which correlate to a sub-total occlusion of the LAD. The last row is an example of a normal CMR exam with normal coronary angiography.
The sensitivity of semi-quantitative methods was 57%, 87%, and 83% for CER, SLP, and INT, respectively. QP with a sensitivity of 87% was statistically higher than CER (p=0.016) but not SLP or INT. Compared to QP, qualitative methods had similar sensitivities of 87% and 83% for the Duke Algorithm and clinical interpretation, respectively.
The specificity of semi-quantitative methods was 91%, 68%, and 68% for CER, SLP, and INT, respectively. QP with a specificity of 91% was statistically higher than SLP and INT (p=0.001 and p=0.001, respectively), but not CER. Compared to QP, qualitative approaches had statistically lower specificities of 52% and 73% for the Duke Algorithm and clinical interpretation methods, respectively (p<0.001 and p=0.004, respectively)
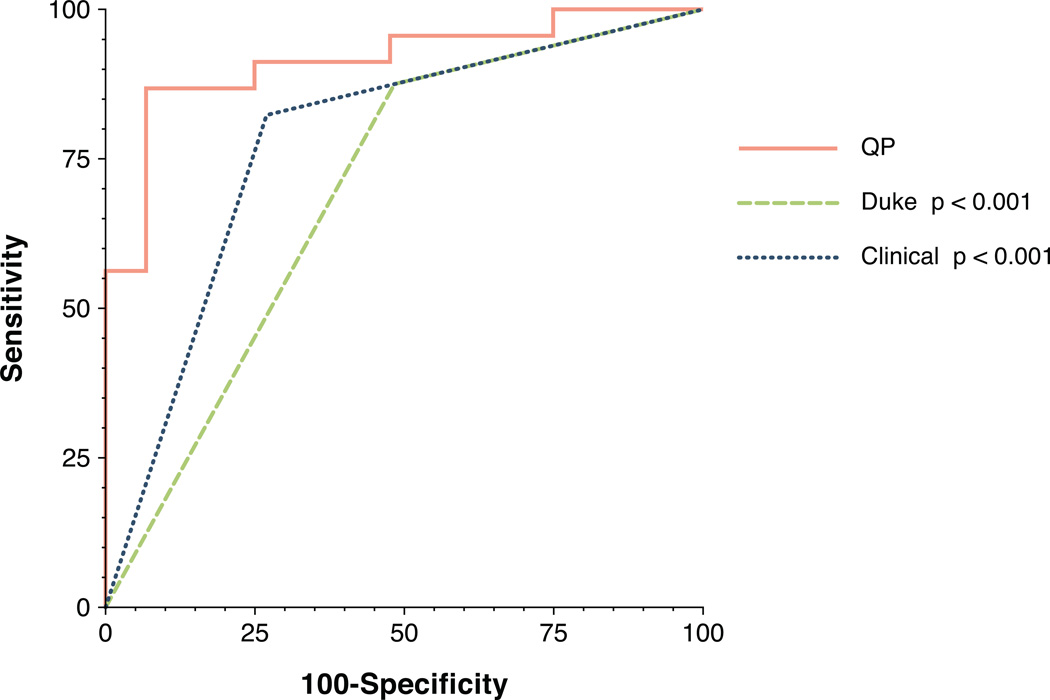
Diagnostic performance of QP as determined by AUC was 0.92 which was statistically superior to all semi-quantitative methods (Figure 1). AUC for CER, SLP, and INT was 0.78, 0.82, and 0.75, respectively (p=0.011, p=0.019, p=0.004 versus QP, respectively). AUC for QP was also statistically superior to qualitative methods (Figure 3). The AUC for the Duke Algorithm and for clinical interpretation were 0.70 and 0.78, respectively (p<0.001 and p<0.001 versus QP, respectively). Results are summarized in Table 2.
Figure 3. ROC curves of QP and qualitative methods.
The AUC for QP (0.92) was greater than the AUC for the Duke Algorithm (0.70, p<0.001) and for clinical interpretation (0.78, p < 0.001).
Table 2.
Diagnostic performance of fully quantitative, semi-quantitative, and qualitative methods to detect a 70% stenosis by QCA.
| Method | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| QP | 0.92 (82–97) | 87 (66–97) | 93 (81–99) | 87 (66–97) | 93 (81–99) |
| CER | 0.78* (66–87) | 57* (35–77) | 91 (78–98) | 76 (49–94) | 80 (66–90) |
| SLP | 0.82* (71–91) | 87 (66–97) | 68* (52–81) | 59 (41–75) | 91 (75–98) |
| INT | 0.75* (63–85) | 83 (61–95) | 68* (52–81) | 58 (39–75) | 88 (72–97) |
| Duke | 0.70* (57–80) | 87 (66–97) | 52* (37–68) | 49 (33–65) | 88 (70–98) |
| Clinical | 0.78* (66–87) | 83 (61–95) | 73* (57–85) | 61 (42–78) | 89 (74–97) |
p < 0.05 versus QP; statistical comparison of PPV and NPV was not performed. 95% confidence intervals are displayed in parenthesis.
Myocardial Perfusion: Absolute and Endocardial/Epicardial Ratio
Thus far, QP has represented the endocardial to median epicardial perfusion ratio. However, it is also important to understand the diagnostic performance of the raw endocardial and transmural perfusion values. The optimal absolute threshold for discriminating a 70% stenosis using stress endocardial perfusion was 1.98 ml/min/g which had an AUC of 0.82 (p=0.01 versus QP), sensitivity of 91%, specificity of 70%, PPV of 62%, and NPV of 94%. The optimal absolute threshold for stress transmural perfusion was 1.58 ml/min/g which had an AUC 0.77 (p=0.002 versus QP), sensitivity 70%, specificity 84%, PPV 70%, and NPV of 84%.
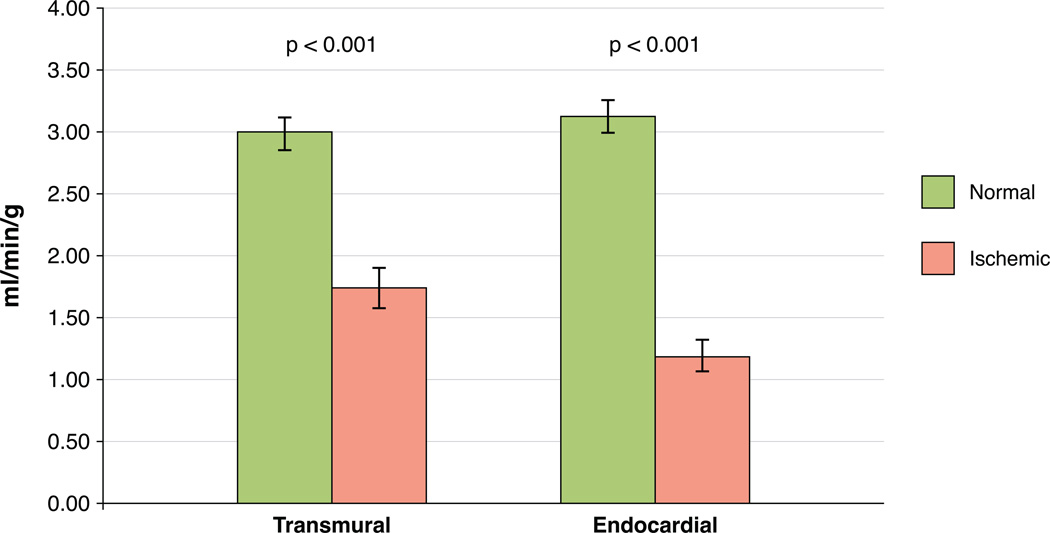
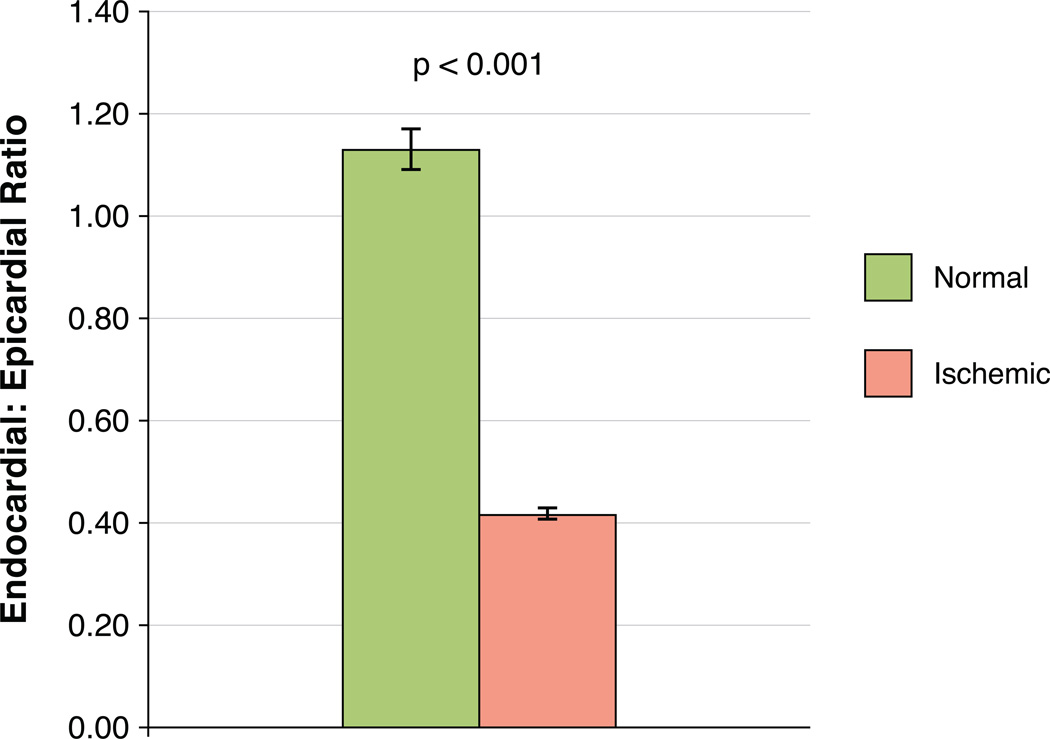
Absolute endocardial blood flow in patients with no coronary disease averaged 3.13 +/− 0.61 ml/min/g while endocardial blood flow in true positive perfusion defects averaged 1.20 +/− 0.53 ml/min/g (p<0.001). Absolute transmural blood flow in patients with no coronary disease averaged 2.99 +/−0.59 ml/min/g while transmural blood flow in true positive perfusion defects averaged 1.73 +/−0.71 ml/min/g (p<0.001). There were also significant differences between endocardial and transmural measurements among normal segments (p=0.015) and ischemic segments (p<0.001). The ratio of endocardial to median epicardial flow in patients with no coronary disease averaged 1.13 +/− 0.19 whereas flow ratios in true positive perfusion defects averaged 0.42 +/− 0.05 (p<0.001). Results are shown in Figures 4 and 5.
Figure 4. Absolute myocardial perfusion.
Absolute perfusion was significantly lower in patients with true positive ischemic segments relative to patients with no coronary disease (p<0.001 for both endocardial and transmural analysis, error bars represent standard deviation).
Figure 5. Endocardial/Epicardial ratio of absolute myocardial perfusion.
The endocardial to median epicardial perfusion ratio was significantly lower in patients with true positive ischemic segments compared to patients with no coronary disease (p<0.001, error bars represent standard deviation).
Discussion
The primary finding of this study is that a fully quantitative approach to stress perfusion CMR analysis has high diagnostic accuracy for detecting obstructive stenosis in patients with known or suspected coronary disease. Furthermore, QP outperforms semi-quantitative and qualitative interpretation methods used by experienced clinicians.
Quantitative CMR analysis could be applied in a manner similar to semi-quantitative SPECT software. Semi-quantitative SPECT analysis is equivalent or minimally better than expert visual interpretation and has become an important adjunctive tool in clinical practice by offering an objective approach to differentiate normal from abnormal.(22,23)
An objective approach to image analysis mitigates some of the intrinsic drawbacks of visual interpretation derived from artifact, subjective judgment, and bias. For example, discerning superimposed ischemia in the setting of myocardial infarction is a challenge in visual interpretation. However, QP performed well despite a population where a sizable portion had myocardial infarction.
QP independently has better diagnostic accuracy than qualitative methods that incorporate a combination of cine, perfusion, and LGE imaging. Simultaneous visual evaluation of stress and rest perfusion is used to identify perfusion artifacts and improve diagnostic accuracy.(19) Stress perfusion and LGE imaging are commonly compared to discriminate ischemia from infarct.(24) In contrast, QP utilizing stress perfusion alone performs well but without the other CMR methods. Thus, stress perfusion imaging may have all the necessary information to yield a highly accurate diagnosis of flow limiting stenosis.
With regards to other diagnostic parameters, although sensitivity was similar among methods with the exception of CER, QP specificity was significantly better than SLP, INT, and both visual methods. The improvement in specificity may help avoid unnecessary invasive testing and revascularization.
Absolute quantification of myocardial perfusion was comparable to previous data in patients with coronary disease. Transmural flow in ischemic segments averaged 1.73 ml/min/g which is similar to the value of 1.54 ml/min/g previously reported for CMR.(15) Endocardial flow in ischemic segments averaged 1.20 ml/min/g which is similar to the value of 1.0–1.2 ml/min/g reported by PET for regions supplied by a >70% stenosis.(25,26) No other CMR study has reported absolute endocardial flow in subjects with CAD. Our measurement of absolute endocardial flow is thus a unique aspect of this work.
In subjects without significant coronary disease, transmural myocardial blood flow averaged 2.99 ml/min/g which was somewhat lower than the 3.39 ml/min/g reported for normal volunteers.(16) However, our population likely had some degree of endothelial dysfunction caused by early atherosclerosis, diabetes, hypertension, and dyslipidemia or non-vascular factors including left ventricular hypertrophy.(27,28)
The threshold for abnormal perfusion was defined by an endocardial to mean epicardial ratio in this study and represented an approximate >50% reduction in flow. This threshold is consistent with previous studies.(21,29–32) The endocardial to epicardial ratio in patients without coronary disease averaged 1.13 which is similar to the previously reported value.(12)
The endocardial layer is known to be most susceptible to ischemia.(33) In fact, applying endocardial rather than transmural regions of interest demonstrated higher accuracy using SLP.(4) Prior studies utilizing INT have found relative sparing of epicardial layers even in severe stenosis.(21) Thus, epicardial regions are likely the best representation of preserved flow. Therefore our analysis focused on endocardial/epicardial flow ratios as the basis of diagnosis. The median epicardial value was used as the normal reference in order to minimize the contribution of segments where perfusion defects become transmural. This may be why our findings differ from previous data.(12) Furthermore, using an epicardial rather than remote endocardial reference may avoid problems with balanced ischemia.
Prior studies have concluded that relative perfusion measures may represent the physiological consequences of coronary stenosis better than absolute thresholds. Models have demonstrated absolute flow for a fixed stenosis can be variable due to multiple physiologic factors and that relative flow indices more accurately reflect stenosis severity.(34) Invasive fractional flow reserve relies on a relative ratio rather than an absolute value and identifies patients that benefit from revascularization.(35) An absolute cutoff for normal flow by PET is difficult to define given normal subject stress values that range from 1.86 +/− 0.27 to 5.05 +/− 0.90.(36) Despite this limitation, PET is still effective using relative scales of flow to assess functional significance of stenosis.(37,38)
This study differs from previous CMR studies in several ways. Much research has described semi-quantitative measures rather than a fully quantitative method.(20,21,39–42) Although studies using SLP have reported similar accuracy in humans, the threshold value for abnormal perfusion has been difficult to define.(40–42) Prior studies that utilized fully quantitative analysis in humans demonstrated moderate to high sensitivities of 78–93%, however specificities were low to modest at 50–75% at 70% stenosis.(9,12) Our improved performance could be due to multiple factors including the use of endocardial flow, more accurate calculation of the arterial input function, signal coil intensity correction, or validated custom software.(8,16) Unlike previous studies, this investigation has not excluded patients with known myocardial infarction or segments with LGE and thus is broadly applicable.(11,13–15) Furthermore, this is the largest study to date involving quantification (prior studies analyzed 20–49 subjects) and has over twice the population previously comparing fully quantitative, semi-quantitative, and qualitative methods of stress perfusion interpretation.(10) Finally, although previous data has suggested that quantification exceeds visual interpretation, this is the first study to demonstrate statistical superiority.
Overall, the qualitative results are in the expected ranges when comparing them to large studies such as CE-MARC (sensitivity/specificity = 86.5%/83.4%) and MR-IMPACT2 (sensitivity/specificity = 67%/61%).(3,43) The sensitivities from both visual methods are moderately high at 83%–87% similar to CE-MARC results. The specificity of clinical interpretation of 73% is somewhat lower than that reported in CE-MARC but higher than MR-IMPACT2. Of note, the high proportion of subjects with previous myocardial infarction and PCI likely contributes to the low specificity of the Duke algorithm which is validated in patients without known CAD. This is a recognized limitation of the Duke algorithm and a potential advantage of quantification as patients with CAD commonly undergo stress testing.
Limitations
An anatomic reference standard was used which may not reflect the flow limiting nature of coronary stenosis. Our study had a high proportion of single vessel disease which may have contributed to false negatives as is also true for nuclear imaging. Parallel imaging with rate 2 temporal sensitivity encoding became incorporated during the course of the study and while not uniformly employed, most perfusion exams (69%) utilized parallel imaging. Although QP could be applied in a similar manner as semi-quantitative SPECT, the use of manual contours makes this application less practical at this point in time although automated contour generation is currently under development. Automated curve analysis, although not used in this study, currently exists and would facilitate processing. Although a dual bolus approach was used for this study, a dual sequence approach would simplify acquisition in routine clinical practice. We did not address the prognostic value of quantitative CMR perfusion which has been reported for PET.(44)
Conclusions
Fully quantitative analysis of stress perfusion CMR has high diagnostic accuracy for detecting obstructive CAD. QP outperforms semi-quantitative measures of perfusion and qualitative methods that incorporate a combination of cine, perfusion, and LGE imaging. This objective, quantitative approach has a potential adjunctive role in clinical perfusion assessment.
Acknowledgments
Funding: Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health
Relationship with Industry: Andrew E. Arai receives research support from Siemens medical imaging (United States Government Cooperative Research and Development Award).
Abbreviations
- AUC
area under the curve
- CER
contrast enhancement ratio
- CMR
cardiac magnetic resonance
- INT
upslope integral
- LGE
late gadolinium enhancement
- PET
positron emission tomography
- QCA
quantitative coronary angiography
- QP
fully quantitative perfusion
- ROC
receiver operating characteristic
- SLP
myocardial to left ventricular upslope ratio
- SPECT
single photon emission computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishida N, Sakuma H, Motoyasu M, et al. Noninfarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229:209–216. doi: 10.1148/radiol.2291021118. [DOI] [PubMed] [Google Scholar]
- 2.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2011;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 5.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol. 1983;18:94–99. doi: 10.1097/00004424-198301000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Clough AV, al-Tinawi A, Linehan JH, Dawson CA. Regional transit time estimation from image residue curves. Ann Biomed Eng. 1994;22:128–143. doi: 10.1007/BF02390371. [DOI] [PubMed] [Google Scholar]
- 7.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 8.Christian TF, Rettmann DW, Aletras AH, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232:677–684. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 9.Costa MA, Shoemaker S, Futamatsu H, et al. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol. 2007;50:514–522. doi: 10.1016/j.jacc.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Futamatsu H, Wilke N, Klassen C, et al. Evaluation of cardiac magnetic resonance imaging parameters to detect anatomically and hemodynamically significant coronary artery disease. Am Heart J. 2007;154:298–305. doi: 10.1016/j.ahj.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Kurita T, Sakuma H, Onishi K, et al. Regional myocardial perfusion reserve determined using myocardial perfusion magnetic resonance imaging showed a direct correlation with coronary flow velocity reserve by Doppler flow wire. Eur Heart J. 2009;30:444–452. doi: 10.1093/eurheartj/ehn521. [DOI] [PubMed] [Google Scholar]
- 12.Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56:561–569. doi: 10.1016/j.jacc.2010.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockie T, Ishida M, Perera D, et al. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2011;57:70–75. doi: 10.1016/j.jacc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Groothuis JG, Kremers FP, Beek AM, et al. Comparison of dual to single contrast bolus magnetic resonance myocardial perfusion imaging for detection of significant coronary artery disease. J Magn Reson Imaging. 2010;32:88–93. doi: 10.1002/jmri.22231. [DOI] [PubMed] [Google Scholar]
- 15.Morton G, Chiribiri A, Ishida M, et al. Quantification of Absolute Myocardial Perfusion in Patients With Coronary Artery Disease: Comparison Between Cardiovascular Magnetic Resonance and Positron Emission Tomography. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu LY, Rhoads KL, Holly JE, Kellman P, Aletras AH, Arai AE. Quantitative myocardial perfusion analysis with a dual-bolus contrast-enhanced first-pass MRI technique in humans. J Magn Reson Imaging. 2006;23:315–322. doi: 10.1002/jmri.20502. [DOI] [PubMed] [Google Scholar]
- 17.Ding S, Wolff SD, Epstein FH. Improved coverage in dynamic contrast-enhanced cardiac MRI using interleaved gradient-echo EPI. Magn Reson Med. 1998;39:514–519. doi: 10.1002/mrm.1910390403. [DOI] [PubMed] [Google Scholar]
- 18.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45:846–852. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 19.Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 20.Kraitchman DL, Wilke N, Hexeberg E, et al. Myocardial perfusion and function in dogs with moderate coronary stenosis. Magn Reson Med. 1996;35:771–780. doi: 10.1002/mrm.1910350519. [DOI] [PubMed] [Google Scholar]
- 21.Klocke FJ, Simonetti OP, Judd RM, et al. Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation. 2001;104:2412–2416. doi: 10.1161/hc4501.099306. [DOI] [PubMed] [Google Scholar]
- 22.Slomka PJ, Nishina H, Berman DS, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77. doi: 10.1016/j.nuclcard.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: the Cedars-Sinai approach. J Nucl Cardiol. 2007;14:433–454. doi: 10.1016/j.nuclcard.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Gerber BL, Raman SV, Nayak K, et al. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson. 2008;10:18. doi: 10.1186/1532-429X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Carli M, Czernin J, Hoh CK, et al. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91:1944–1951. doi: 10.1161/01.cir.91.7.1944. [DOI] [PubMed] [Google Scholar]
- 26.Muzik O, Duvernoy C, Beanlands RS, et al. Assessment of diagnostic performance of quantitative flow measurements in normal subjects and patients with angiographically documented coronary artery disease by means of nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1998;31:534–540. doi: 10.1016/s0735-1097(97)00526-3. [DOI] [PubMed] [Google Scholar]
- 27.Schelbert HR, Wisenberg G, Phelps ME, et al. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilation. VI. Detection of coronary artery disease in human beings with intravenous N-13 ammonia and positron computed tomography. Am J Cardiol. 1982;49:1197–1207. doi: 10.1016/0002-9149(82)90045-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Jerosch-Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RF, Marcus ML, White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. 1987;75:723–732. doi: 10.1161/01.cir.75.4.723. [DOI] [PubMed] [Google Scholar]
- 30.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48–55. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 31.Gould KL. Pressure-flow characteristics of coronary stenoses in unsedated dogs at rest and during coronary vasodilation. Circ Res. 1978;43:242–253. doi: 10.1161/01.res.43.2.242. [DOI] [PubMed] [Google Scholar]
- 32.Klocke FJ. Measurements of coronary flow reserve: defining pathophysiology versus making decisions about patient care. Circulation. 1987;76:1183–1189. doi: 10.1161/01.cir.76.6.1183. [DOI] [PubMed] [Google Scholar]
- 33.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 34.Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459–474. doi: 10.1016/s0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 35.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 36.Sdringola S, Johnson NP, Kirkeeide RL, Cid E, Gould KL. Impact of unexpected factors on quantitative myocardial perfusion and coronary flow reserve in young, asymptomatic volunteers. JACC Cardiovasc Imaging. 2011;4:402–412. doi: 10.1016/j.jcmg.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein RA, Kirkeeide RL, Demer LL, et al. Relation between geometric dimensions of coronary artery stenoses and myocardial perfusion reserve in man. J Clin Invest. 1987;79:1473–1478. doi: 10.1172/JCI112976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Wilke N, Simm C, Zhang J, et al. Contrast-enhanced first pass myocardial perfusion imaging: correlation between myocardial blood flow in dogs at rest and during hyperemia. Magn Reson Med. 1993;29:485–497. doi: 10.1002/mrm.1910290410. [DOI] [PubMed] [Google Scholar]
- 40.Al-Saadi N, Nagel E, Gross M, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 41.Al-Saadi N, Nagel E, Gross M, et al. Improvement of myocardial perfusion reserve early after coronary intervention: assessment with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2000;36:1557–1564. doi: 10.1016/s0735-1097(00)00914-1. [DOI] [PubMed] [Google Scholar]
- 42.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 43.Schwitter J, Wacker CM, Wilke N, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2012;34:775–781. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 44.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]