Abstract
The renin-angiotensin-system (RAS) constitutes one of the most important hormonal systems in the physiological regulation of blood pressure through renal and non-renal mechanisms. Indeed, dysregulation of the RAS is considered a major factor in the development of cardiovascular pathologies including kidney injury and blockade of this system by the inhibition of angiotensin converting enzyme (ACE) or blockade of the angiotensin type 1 receptor (AT1R) by selective antagonists constitutes an effective therapeutic regimen. It is now apparent with the identification of multiple components of the RAS within the kidney and other tissues that the system is actually composed of different angiotensin peptides with diverse biological actions mediated by distinct receptor subtypes. The classic RAS can be defined as the ACE-Ang II AT1R axis that promotes vasoconstriction, water intake, sodium retention and other mechanisms to maintain blood pressure, as well as increase oxidative stress, fibrosis, cellular growth and inflammation in pathological conditions. In contrast, the non-classical RAS composed primarily of the AngII/Ang III–AT2R pathway and the ACE2-Ang-(1-7)-AT7R axis generally opposes the actions of a stimulated Ang II-AT1R axis through an increase in nitric oxide and prostaglandins and mediates vasodilation, natriuresis, diuresis, and a reduced oxidative stress. Moreover, increasing evidence suggests that these non-classical RAS components contribute to the therapeutic blockade of the classical system to reduce blood pressure and attenuate various indices of renal injury, as well as contribute to normal renal function.
Introduction
From the early pioneering studies of Tigerstedt and Bergman (144) that described “renin-like” activity in the canine kidney over 100 years ago to the identification of vasoactive peptide angiotensin II (Ang II) by Page, Braun-Menendez and their colleagues 50 years later (21; 22), the characterization of the renin-angiotensin system (RAS) within the kidney and other tissues continues unabated broadening our understanding of the functional aspects of this important hormonal system in blood pressure regulation and cardiovascular pathologies. Coupled with the discovery of angiotensin I converting enzyme (ACE) as the primary enzyme that forms Ang II in the circulation and tissue and the molecular identification of the Ang II type 1 receptor (AT1R) that confers the predominant actions of Ang II, the “classical” RAS was long-viewed as a sequential endocrine system to form the active peptide Ang II in the overall maintenance of blood pressure through various mechanisms including constriction of the renal vasculature and the reabsorption of sodium. Our current view now encompasses the RAS as a tissue system whose protein and peptide components are expressed in essentially every organ and whose actions are implicated in numerous physiological events that influence renal, neuronal, cardiac, pancreatic, vascular, adrenal, pituitary, cognitive, aging, inflammatory and reproductive functions (104). However, there is overwhelming evidence for a “non-classical” RAS that results in the formation of novel peptide products with functional properties distinct from that of the ACE-Ang II-AT1R pathway normally associated with maintenance of arterial pressure through renal hemodynamic and tubular mechanisms (20; 24; 27; 94; 125; 137). Indeed, our identification of Ang-(1-7) over 20 years ago as an endogenous and biologically active angiotensin peptide expressed in the circulation, kidney, brain, heart, and other tissues (29), as well as the subsequent discovery of angiotensin converting enzyme 2 (ACE2) (37; 41; 145) the Ang-(1-7)[AT7R] or Mas receptor (127) that are both evident within the kidney has revealed a far more complex system contributing to renal function than previously imagined (Figures 1 & 2). The current review considers the tenet that the components of the non-classical or alternative RAS influence renal function, as well as to attenuate the actions of an activated ACE-Ang II-AT1R axis within the kidney (Figure 2).
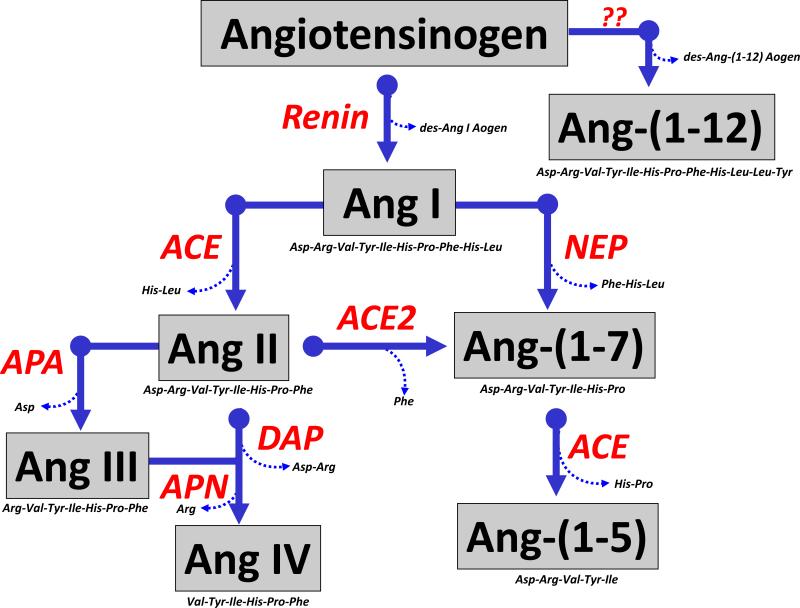
Figure 1. Enzymatic cascade of angiotensin peptide formation and metabolism.
Renin cleaves the precursor protein angiotensinogen (Aogen) at the Leu10-Leu11 bond to angiotensin-(1-10) (Ang I) which is further processed to the biologically active peptides Ang-(1-8) (Ang II) by angiotensin converting enzyme (ACE) and Ang-(1-7) by endopeptidases such as neprilysin (NEP). Ang II undergoes further processing at the carboxy terminus by the carboxypeptidase ACE2 to yield Ang-(1-7) and at the amino terminus by aminopeptidase A (APA) to form Ang-(2-8) or Ang III. Ang-(1-7) is metabolized by ACE to form Ang-(1-5) and Ang III is further hydrolyzed by aminopeptidase N (APN) to yield Ang-(3-8) or Ang IV. Ang II can be directly cleaved by dipeptidyl aminopeptidase IV (DAP) to Ang IV. The novel peptide Ang-(1-12) is derived from the hydrolysis of the Tyr12-Tyr12 bond of Aogen although the identity of the enzyme that forms the peptides is not known to date. Adapted from Chappell (27).
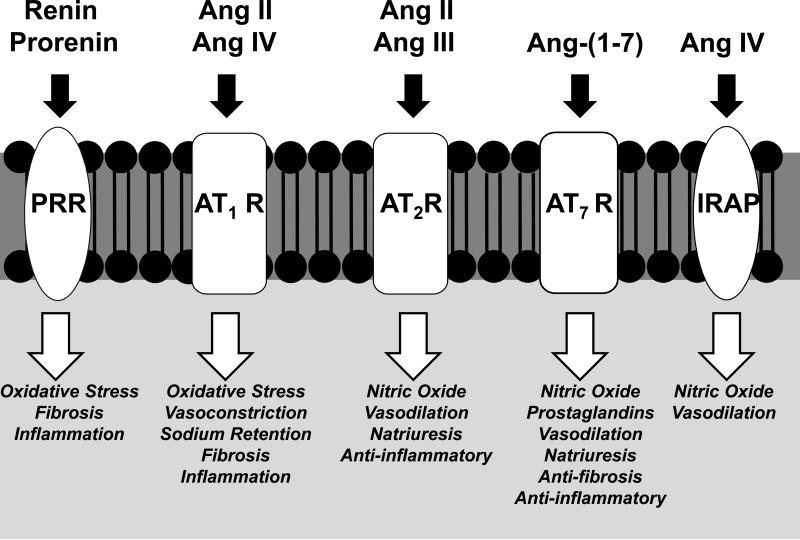
Figure 2. Renal actions of the classical and non-classical components of the reninangiotensin system.
Ang II interacts with the AT1 receptor (AT1R) to increase renal vasoconstriction, sodium reabsorption and promote inflammation and fibrosis. Ang IV may stimulate vasoconstriction through an interaction with the AT1R. Renin or prorenin binds to the prorenin receptor (PRR) to directly promote oxidative stress, fibrosis and inflammation. Ang II or Ang III stimulates vasodilation, reduced vascular resistance and natriuresis through activation of the AT2R and the generation of nitric oxide. Ang-(1-7) recognizes the AT7R to stimulate vasodilation, diuresis and natriuresis, but reduce inflammation and fibrosis through increased generation of nitric oxide and prostaglandins. Ang IV may interact with the insulin regulated aminopeptidase (IRAP) to reduce vascular resistance through an increase in nitric oxide.
RENAL SOURCE OF ANGIOTENSINS
Renin
The precursor for all angiotensin peptides is angiotensinogen, a 453 amino acid glycosylated protein that is primarily synthesized and secreted by the liver and other tissues to provide a significant circulating source of the protein. Within the kidney, angiotensinogen is synthesized and released into the tubular fluid by proximal tubule epithelial cells, although recent evidence importantly suggests the cellular uptake of the protein as well (108). The precursor protein is hydrolyzed between residues 10 and 11 (Leu10-Leu11 for rat, mouse, sheep, dog and Leu10-Ile11 for human) by the aspartyl protease renin to form the inactive peptide angiotensin-(1-10) (Ang I) and (des-Ang I)-angiotensinogen (Figure 1). The N-terminal region of angiotensinogen containing residues His6-Pro7-Phe8 is also highly conserved among species and is critical in the appropriate recognition by renin to form Ang I (92). Angiotensinogen is the only known substrate for renin; however, other proteases including tonin, kallikrein and cathepsin D are capable of metabolizing the precursor to Ang I or Ang II (104). The exact role these non-renin pathways contribute to an activated renal RAS is equivocal, although their participation may be important given the recent development of non-peptide inhibitors of renin such as aliskserin for the clinical treatment of hypertension (42; 54; 79). The catalytic function of renin involves the hydrolysis of angiotensinogen to Ang I has been well established; however, a receptor protein for prorenin was cloned by Nyguen and colleagues and found to be expressed in the kidney as well as other tissues (95). Receptor-bound prorenin exhibits increased proteolytic activity leading to Ang I formation (13), but prorenin may also induce distinct signaling pathways following binding and renin inhibitors such as aliskiren do not attenuate prorenin binding (121). Indeed, the prorenin-prorenin receptor pathway itself may be regarded as distinct component of the non-classical RAS within the kidney. Sakoda et al. (121) find that prorenin activated the MAP kinase pathway in human smooth muscle cells – actions similar to that for Ang II-AT1R activation (Figure 2). In isolated mesangial cells, exogenous renin increased TGF-β expression and the matrix proteins plasminogen activator inhibitor (PAI-1) and fibronectin that were not dependent on the formation of Ang II (68; 73). Increased expression of PAI-1 and other matrix proteins by prorenin may lead to progressive renal damage, which would not be inhibited by traditional agents that block the RAS such as ACE-inhibitors or AT1R antagonists. Moreover, prorenin is the major form of circulating and tissue forms of renin, and may further increase in pathological conditions. However, the actions of the prorenin receptor or following blockade of the protein with the “hinge peptide” were not confirmed and other issues regarding the localization and identify of the receptor remain (89; 113). Indeed, the cellular localization of the receptor is controversial particularly as several studies demonstrate the predominant intracellular distribution of the prorenin receptor in the perinuclear area (128). In this regard, the intracellular receptor may interact with internalized renin (106) or non-secreted forms of the enzyme (34). There is substantial evidence for an intracellular RAS, particularly within the kidney, and the predominant perinuclear distribution of the prorenin receptor may portend for intracellular actions of the protein (44; 55). Although the initial report on the prorenin receptor suggested the protein to be unique, the receptor is homologous with the protein ATP6AP2 that comprises the vacuolar +H-ATPase complex, a protein essential in the acidification of lysosomal vesicles and endosomes (11). ATP6AP2 is widely expressed within the kidney, particularly in the collecting duct where the +H-ATPase complex contributes to the acidification of urine (11). Thus, the nature of the interaction of prorenin or its blocking peptide with ATP6AP2 within the kidney is far from resolved at this time.
ACE
The inactive peptide Ang I is further processed by the metallopeptidase ACE to directly yield the active peptide Ang II. ACE is primarily characterized as a dipeptidyl carboxypeptidase that cleaves the Phe8-His9 bond of Ang I to form Ang II and the dipeptide His-Leu (Figure 1) (114; 148). Within the kidney, ACE is localized to various cell types including the tubular epithelial cells, vascular endothelial cells and glomerular mesangial cells. Although ACE is clearly critical to the generation of Ang II, the peptidase contributes to the metabolism of several vasoactive peptides within the kidney including bradykinin and Ang-(1-7) (27; 31). Shaltout et al. (129) demonstrate that ACE inhibition increases Ang-(1-7) and attenuates ACE dependent formation of the metabolite Ang-(1-5) in isolated proximal tubules from sheep (see Figures 1 & 3). This latter study emphasizes the alternative role of ACE to influence the metabolism of Ang-(1-7) in the kidney and circulation (27; 32). ACE likely represents the predominant Ang II-forming enzyme in the kidney under physiologic conditions; however, other peptidases including mast cell and smooth muscle chymases may contribute to tissue levels of Ang II in pathological states including Goldblatt hypertension (120). Harrison-Bernard and colleagues demonstrate that a chymase-like enzyme (chymostatin sensitive) resistant to ACE inhibitors accounted for the vascular conversion of Ang I to Ang II in the isolated afferent arterioles of the type II diabetic db/db mouse; however, ACE was the sole activity responsible for Ang II formation in the nondiabetic mice (103).
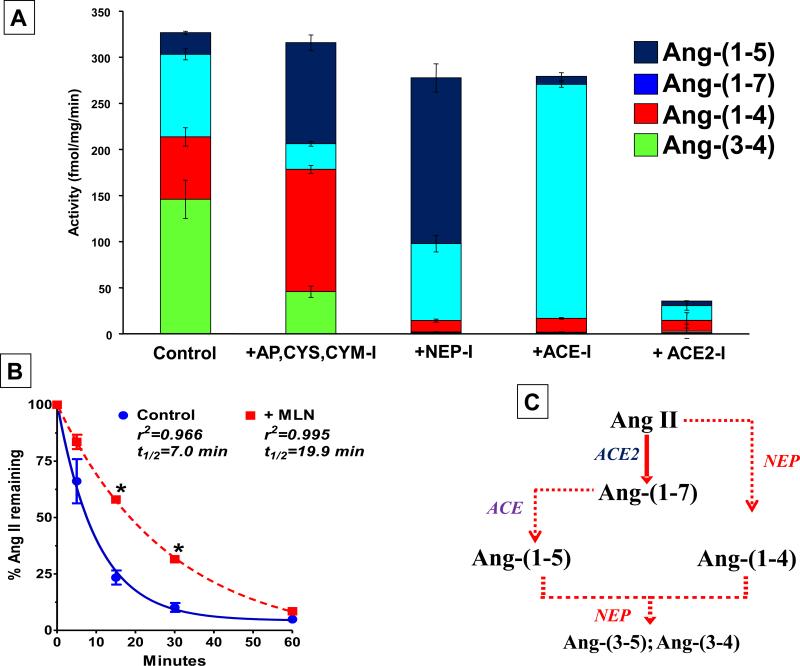
Figure 3. Enzymatic metabolism of 125I-Ang II in isolated sheep proximal tubules.
125I-Ang II (AII) was incubated with 50 Qg of proximal tubules membranes for 30 minutes at 37°C and the metabolites separated by HPLC. Panel A: Quantification of the peptidase activities for 125I-AII metabolism from the sheep proximal tubule membranes expressed as the rate of metabolism products formed (fmol/mg/min). Conditions: Control (no inhibitors); +AP,CYS,CHM-I (inhibitors for aminopeptidase, chymase, cysteine proteases); +NEP-I (addition of neprilysin inhibitor); +ACE-I (addition of ACE inhibitor); +ACE2-I (addition of ACE2 inhibitor). Data are means; n=4. Panel B: Influence of ACE2 inhibition on half-life (t1/2) of 125I-Ang II (AII) in proximal tubules. Conditions: Control (no inhibitors); +MLN (only the ACE2 inhibitor). Data are means; n=5; *P < 0.05 vs Control. Panel C: Metabolism pathway for Ang II and Ang-(1-7) in sheep proximal tubules. Adapted from Shaltout et al (129).
Aminopeptidases
Following the generation of Ang II, the peptide can undergo further metabolism at either the amino (N-terminus) or carboxyl (C-terminus) regions of the peptide (Figure 1). Aminopeptidase A (AP) activity is high in the kidney and converts Ang II directly to Ang-(2-8) or Ang III (3; 63; 78; 135; 158). AP is primarily localized to the glomerular (mesangial and endothelial cells) and brush border compartments of the kidney (77). In contrast, aminopeptidase N (AN) has preference for basic residues such as the N-terminal arginine and converts Ang III to Ang-(3-8) or Ang IV (78; 102). Although Ang III was shown to stimulate an AT1R-dependent increase of Ca++ in various tubular elements of the rat kidney (69), Carey and colleagues have presented additional findings that Ang III may be the endogenous ligand for the AT2 receptor within the rat kidney (99-101). Further N-terminal processing of Ang III by AN or direct conversion of Ang II by dipeptidyl aminopeptidase IV (DAP IV) yields another vasoactive peptide Ang-(3-8) (Ang IV) that convey biological actions not through a classical G-protein coupled receptor but by interacting with the insulin-regulated aminopeptidase (IRAP) to stimulate NO (6; 76). IRAP is membrane spanning protein of the M1 family of aminopeptidases (7). The enzyme is widely distributed in the kidney predominantly localized to the apical aspects of proximal tubules and thick ascending limbs in the cortex, as well as the principal cells of the medulla (8).
ACE2
Distinct from the amino terminal metabolism of Ang II by aminopeptidases, the octapeptide is also processed on the C-terminus by a homolog of ACE termed ACE2 within the kidney (Figures 1 & 3). ACE2 is a metallopeptidase similar to ACE, but exhibits carboxypeptidase activity cleaving the Pro7-Phe8 bond of Ang II to generate Ang-(1-7). ACE2 also hydrolyzes the His9-Leu10 bond of Ang I to form Ang-(1-9); however, the ratio of the catalytic to the Michelis-Menten constant (kcat/Km, efficiency constant) is approximately 500 fold greater for the conversion of Ang II to Ang-(1-7) (152); ACE2 exhibits the highest kcat/Km among a number of enzymes that convert Ang I, Ang II or Ang-(1-9) directly to Ang-(1-7). Furthermore, the abundance of these peptidases within the kidney in addition to their catalytic properties and cellular access to peptide substrates will determine the extent that they may participate in the formation of Ang-(1-7). As observed for ACE, the homologue ACE2 is found in both soluble and membrane-associated forms within a number of tissues including the kidney, heart, brain, lung and testes (27). In the kidney, ACE2 is primarily localized to the apical side of the proximal tubules with expression also evident in the podocytes of the glomerulus (111; 150; 153; 163). ACE2 is the primary enzyme responsible for the conversion of Ang II to Ang-(1-7) in the sheep proximal tubules (Figure 3) and isolated podocytes from rat (129; 150). It has become increasingly apparent that ACE2 influences the expression of Ang II and Ang-(1-7) within the kidney as well as other tissues ultimately impacting the functional output of the RAS (96; 159; 163). Indeed, the augmentation of ACE2 by administration of the active peptidase may constitute a novel and effective therapy to lower blood pressure and reduce renal injury by enhancing the Ang-(1-7) to Ang II peptide ratio (97; 165; 166).
Endopeptidases
Endopeptidases such as neprilysin, prolyl oligopeptidase and thimet oligopeptidase utilize Ang I as substrate to generate Ang-(1-7) that is independent of Ang II formation within the kidney (9; 28; 30; 151; 161) (Figure 1). Neprilysin is a metallopeptidase that is richly expressed on the brush border of the proximal tubules within the kidney and exhibits high catalytic activity for the conversion of Ang I to Ang-(1-7), a well as other vasoactive peptides including kinins, endothelins, ANP and BNP (114). Although the kidney expresses multiple peptidases that potentially form Ang-(1-7), the issue of whether Ang-(1-7) is derived directly from Ang I or Ang II remains equivocal. In ACE2 knockout mice, renal content of Ang-(1-7) is reduced but clearly not abolished (96). Moreover, our findings in the tissue ACE knockout (tisACE−/−) mice, originally developed by Bernstein and colleagues that deleted the membrane spanning region of the enzyme, revealed an 80% reduction in renal Ang II; Ang-(1-7) tissue content and urinary levels were surprisingly unchanged (88) (Figure 4). These studies suggest that the renal formation of Ang-(1-7) is not entirely dependent on ACE2 or other carboxypeptidase-like enzymes and alternative pathways for Ang-(1-7) generation are functionally present in the kidney. Interestingly, the tisACE−/− mice cannot adequately concentrate urine and do not respond to subsequent Ang II treatment to conserve water or sodium which may reflect the increased ratio of Ang-(1-7) to Ang II in this model (45; 74).
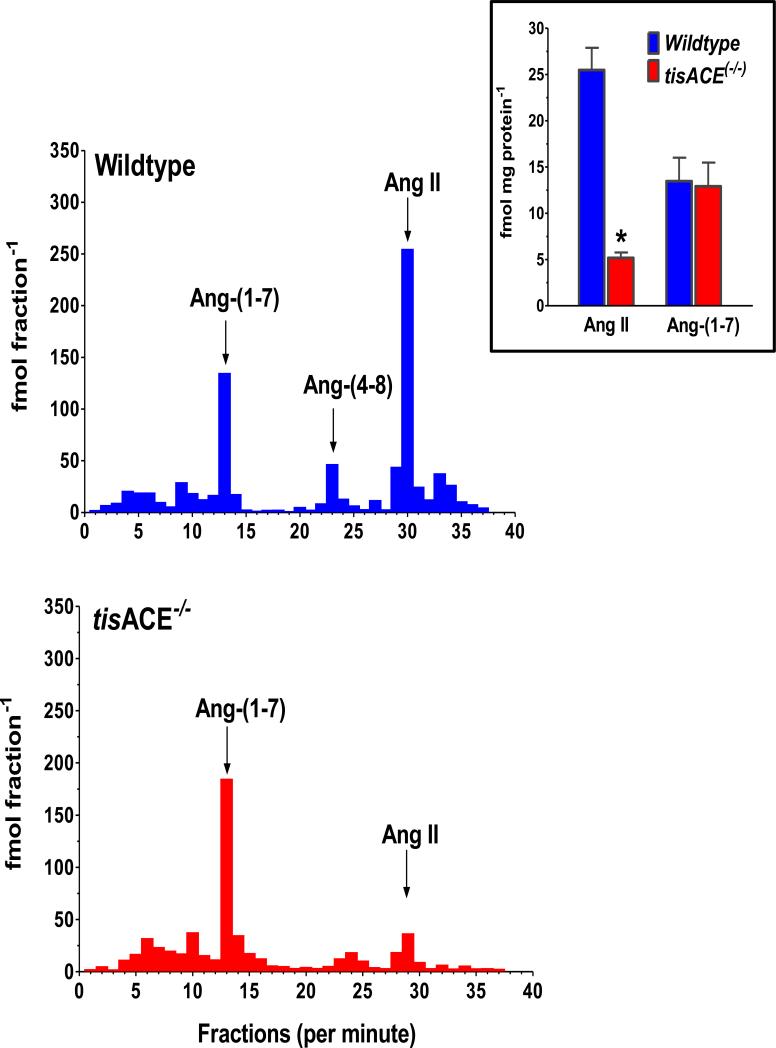
Figure 4. Deletion of tissue ACE significantly reduces levels of Ang II but not Ang-(1-7) in mouse kidney.
HPLC/RIA analysis of pooled mouse kidney samples from Wildtype (upper panel) and tissue ACE knockout (tisACE−/−) mice (lower panel). The HPLC fractions were measured with Ang-(1-7) (fractions 1-20) and Ang II (fractions 21-40) RIAs, respectively. The arrows indicate the elution peak times for Ang-(1-7), Ang-(4-8) and Ang II. Inset: intrarenal concentration of Ang II and Ang-(1-7) expressed as fmol/mg protein in Wildtype and tisACE−/− mice, n = 8 per group; *P < 0.001 vs Wildtype. Adapted from Modrall et al. (88).
In lieu of the data supporting Ang II-independent formation of Ang-(1-7), it should be noted that Nagata and colleagues have isolated and identified a novel endogenous angiotensin peptide termed Ang-(1-12) that contains the first 12 amino acids of the N-terminal sequence of the secreted form of angiotensinogen (90). Utilizing antibodies to the N and C-terminal sequences of Ang-(1-12), these investigators found that Ang-(1-12) was expressed in essentially all tissues that contain Ang II with the highest peptide concentrations in the intestine, heart, plasma and kidney (90). Differential expression of Ang-(1-12) was recently demonstrated in the heart and kidney of the spontaneously hypertensive rat (SHR) and the normotensive control Wistar Kyoto strain (WKY) (72). The site of hydrolysis rat for formation of Ang-(1-12) from angiotensinogen occurs at residues Tyr12-Tyr13 which is distinct from the Leu10-Leu11 sequence cleaved by renin to form Ang I. Thus, the generation of Ang-(1-12) is likely through a non-renin dependent pathway and may be particularly apparent in conditions of low or suppressed renin activity, and particularly with the clinical use of selective renin inhibitors (49; 53). Studies to date suggest that Ang-(1-12) is processed to Ang II by ACE or chymase; however, our preliminary data find that neprilysin converts Ang-(1-12) directly to Ang-(1-7) in kidney tissues (4; 26; 90; 146). Moreover, circulating or renal renin does not appear to participate in the metabolism of Ang-(1-12) suggesting that the peptide is not a substrate for renin (26). The conversion of Ang-(1-12) to Ang II by ACE in the circulation is consistent with the acute increase in blood pressure following an infusion of Ang-(1-12) in normotensive rats, as well as the blockade of the pressor response by either an ACE inhibitor or AT1R antagonist (90). Therefore, Ang-(1-12) is likely not biologically active, but may serve as an intermediate precursor to Ang II or Ang-(1-7) via a renin-independent mechanism. Clearly, additional studies are required to evaluate the role of this novel peptide in the processing of Ang II or Ang-(−7), identify the enzyme(s) that catalyze the formation of Ang-(1-12) and importantly, determine whether Ang-(1-12) is a constituent of the human RAS within the kidney and circulation.
RENAL ACTIONS
The Ang II/Ang III – AT2R axis
Signaling pathways
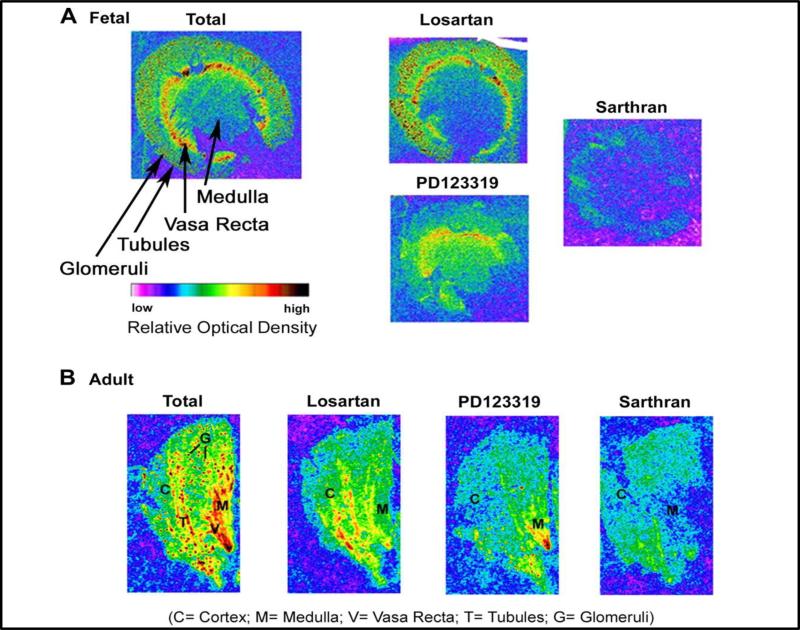
Ang II primarily interacts with two G-protein coupled receptors identified as the AT1 and AT2 subtypes. Intense research has focused on the signaling mechanisms of Ang II-AT1R receptor axis with more recent attention to the generation of reactive oxygen species (ROS) that underlies the vasoconstrictor and sodium retaining actions of this pathway. In contrast to the AT1R, Ang II dependent activation of the AT2R subtype is closely linked to the stimulation of NO and the activation of cellular phosphatases (50; 86; 130; 133; 140). Within the adult kidney, the AT2R is expressed primarily on the interlobular arterioles and other vascular elements, as well as in the tubular portions of the nephron but glomerular expression was neglible (57; 87; 98). As shown in Figure 5, we demonstrate AT2 sites primarily in the tubulointerstial areas of both the fetal and adult sheep kidney by autoradiography binding of the non-selective antagonist 125I-[Sar1,Thre8]-Ang II binding that is sensitive to the AT2R antagonist PD123319. In the adult renal cortex, the glomerular binding sites were primarily blocked by the AT1R antagonist losartan (Figure 5).
Figure 5. Autoradiography of Ang II binding sites in the fetal and adult sheep kidney.
Frozen-thawed kidney sections were incubated with receptor antagonists to the AT2 receptor (PD123319) or the AT1 receptor (losartan) in the presence of the non-selective antagonist 125I Sarthran (0.2 nM). Nonspecific labeling was obtained by pre-incubation with the unlabeled Sarthran antagonist (5 VM). Adapted from Gwathmey et al (57).
Hemodynamic actions
The majority of studies suggest that the AT2R exhibits vasorelaxant actions within the kidney. Genetic studies of global AT2R knockout mice find a mild increase in blood pressure, but an exaggerated pressure response to Ang II administration suggesting a modulatory influence on AT1R-dependent vasoconstriction. Indeed, there is good evidence that the AT2R may contribute to the blood pressure and vasorelaxant actions of AT1R receptor blockade, particularly in hypertensive models (12; 18; 83). The vasorelaxant actions of the AT2R appear primarily dependent on the generation of NO, although dilation of pre-constricted afferent arterioles may reflect an NO-independent mechanism (75). Additional studies support a role for bradykinin activation and/or release in the functional actions of the AT2R, although AT2-dependent effects remain in the B2R knockout mouse suggesting that at least a subset of actions are not kinin dependent and directly increase cGMP and cGMP kinase (1; 133; 147).
Tubular actions
In addition to alterations in blood pressure, the pressure-natriuresis relationship is shifted rightward in the AT2R null mice. Activation of the AT2R primarily invokes a natriuretic response that likely involves the generation of NO and cGMP (100; 131). A potential target for the tubular actions of AT2R is the direct inhibition of the Na+,K+,ATPase activity on the proximal tubules (60). Moreover, concomitant blockade of oxidative stress potentiates the natriuretic response to the AT2R agonist CGP42112A (118). Pathological conditions such as hypertension or obesity are associated with upregulation of the AT2R within the kidney and potentially suggest a greater role of the receptor to offset the actions of an activated Ang II- AT1R axis within the RAS that include an increase in oxidative stress (117). In contrast to the AT1R, intracellular pools of the AT2R are evident within the tubules that await stimulation and insertion into the plasma membrane to promote activation of the AT2R pathway (101). These AT2R pools may function in the conjunction with the renal dopaminergic system to promote tubular natriuresis and diuresis (122). Similar to the other components of the RAS that may compromise an intracellular system within the kidney, there is evidence for AT2R on cortical nuclei and mitochondria within the kidney (2; 57). We find that nuclear AT2R are coupled to the generation of NO and co-localize with nuclear endothelial NOS (eNOS) and soluble guanylate cyclase that may generate intracellular or nuclear cGMP (57).
Renoprotective actions
Accumulating evidence suggests a renoprotective role of the AT2R that is associated with reduced inflammation and cytokine production (14; 91; 115; 132; 155). Benndorf and colleagues show that AT2R deletion in the FVB/N mouse strain (that do not exhibit developmental abnormalities) markedly exacerbates renal injury in the 5/6 nephrectomy model of progressive injury (14). Moreover, deletion of the AT2R in this model significantly increases the mortality rate; however, the renal phenotype was not associated with altered AT1R or eNOS expression (14). More recent studies have taken advantage of a new non-peptide agonist for the AT2R to establish the protective actions of the receptor without relying on Ang II as the ligand in conjunction with AT1R blockade or following treatment with the AT2R antagonist PD12319, which may exhibit other properties (136; 155). Indeed, the AT2R agonist compound 21 (C21) reduces a number of indices for renal damage in the stroke-prone spontaneously hypertensive rat (SP-SHR) independent of changes in blood pressure. Interestingly, the AT2R agonist significantly reduced plasma renin activity supporting earlier data that the AT2R activation may reduce renin release and/or synthesis (70; 133). In early Goldblatt hypertension, Siragy and colleagues also find that the AT2R agonist C21 is renoprotective. The C21 agonist increased NO and cGMP content in the non-clipped kidney, as well as reduced expression of key inflammatory and fibrotic cytokines TNFα, TGF-β and IL6 (85).
The Ang-(1-7) – AT7R axis
Peptide expression
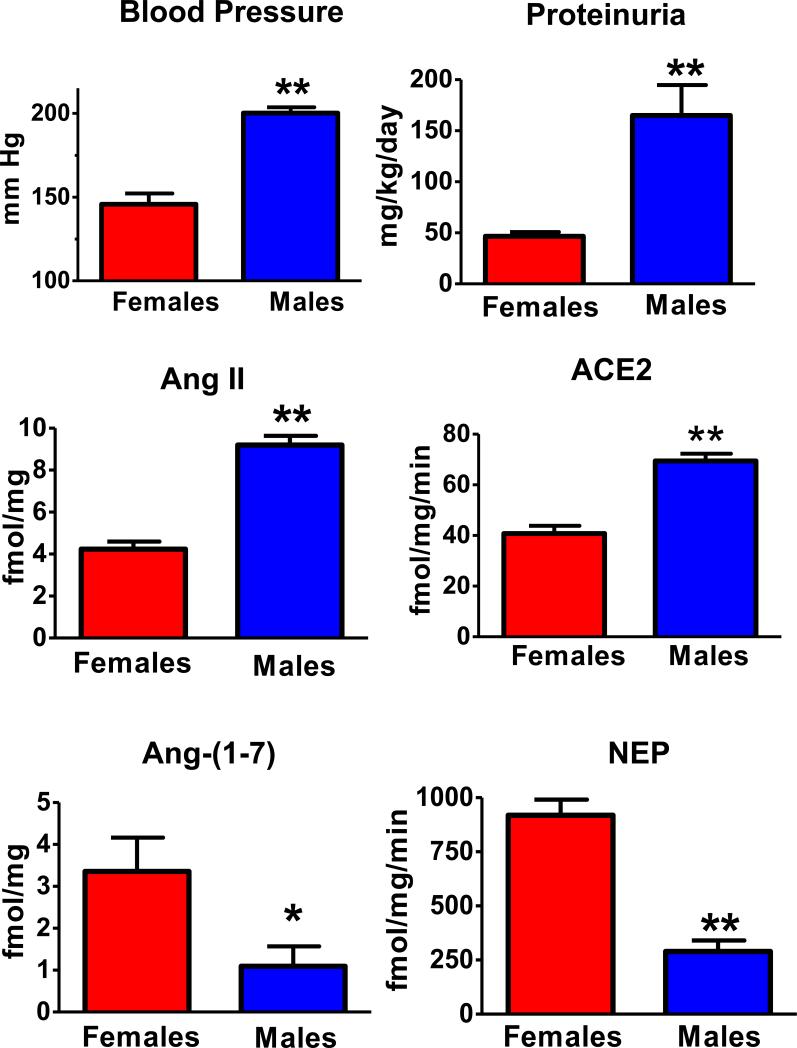
Ang-(1-7) is endogenously expressed within the kidney of various species at tissue levels that are comparable to that of Ang II (105; 109; 143). In the hypertensive mRen2.Lewis congenics that exhibit significant sex differences in blood pressure and renal injury, cortical levels of Ang-(1-7) are significantly lower in the male congenics than their female littermates while cortical content of Ang II is higher in males (Figure 6). However, cortical ACE2 activity is higher in males that may reflect a compensatory response to the higher pressure and extent of injury in the male kidney (105). In contrast, the female mRen2 congenic exhibit significantly higher neprilysin content and activity than the males that may contribute to both greater Ang-(1-7) formation and Ang II metabolism (Figure 6). Sullivan and colleagues also find higher renal Ang-(1-7) content in female SHRs and that treatment with the Ang-(1-7) antagonist abolishes differences in the blood pressure response to Ang II (139). Furthermore, the development of Goldblatt hypertension in male rats is associated with a lower ratio of Ang-(1-7) to Ang II as well as ACE2/ACE activities in the kidney (109). Similar to Ang II, significant levels of Ang-(1-7) are found in the urine and are found to increase with either ACE inhibition or AT1R blockade (35; 47; 160). The source of urinary Ang-(1-7) and Ang II may reflect either release from renal epithelial cells such as the proximal tubule or the processing of tubular fluid angiotensinogen that may arise from tubular secretion or glomerular filtration, the latter mechanism may be particularly important in pathologies associated with glomerular injury (35; 93). Clinical studies demonstrate lower excretion of Ang-(1-7) in hypertensive patients as compared to the normotensive cohort (48). Moreover, diabetes-induced renal injury is associated with a marked reduction in the renal content of Ang-(1-7), and chronic inhibition of ACE2 exacerbates indices of damage injury, as well as attenuates the protective effects of ACE inhibitor treatment (134; 143; 159).
Figure 6. Sex differences in systolic blood pressure, proteinuria and components of the renin-angiotensin system in the renal cortex of mRen2.Lewis congenic rats.
Systolic blood pressure is expressed in mm Hg and proteinuria as mg per kilogram body weight per day (mg/kg/day). Intrarenal concentrations of Ang II and Ang-(1-7) are expressed as fmol peptide per mg protein (fmol/mg) and enzyme activities as fmol product per mg protein per min (fmol/mg/min) in 15 week old hemizygous mRen2.Lewis, n = 5-8 per group; **P < 0.01 or *P < 0.01. Adapted from Pendergrass et al. (105).
Signaling pathways
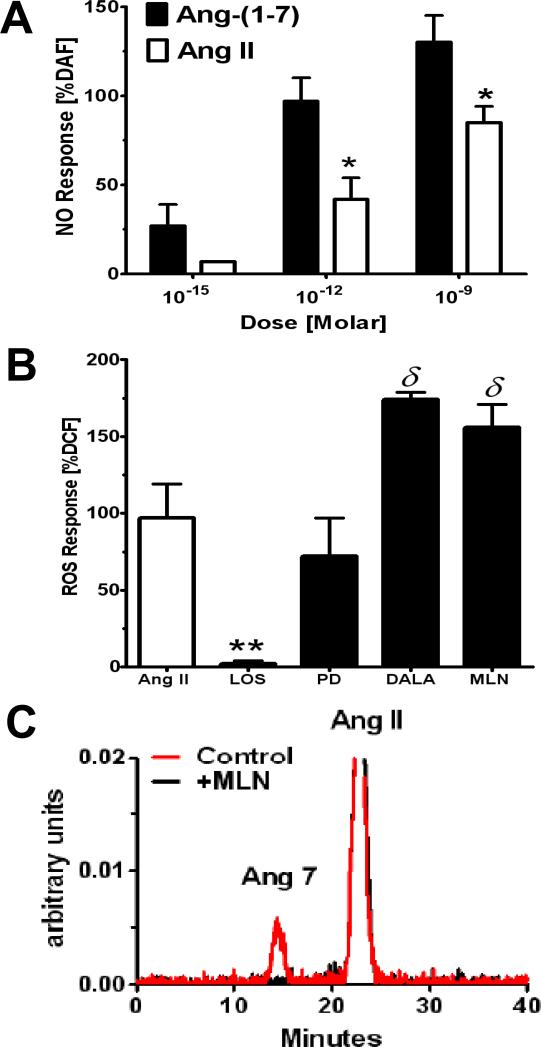
The evidence to-date suggests that the Ang-(1-7)–AT7R axis and its associated signaling pathways may antagonize the actions of Ang II-AT1R pathway (27; 46). Similar to the AT2R, Ang-(1-7) stimulates NO production via enhanced phosphorylation of Akt and increased levels of cGMP that may or may not depend on the release of bradykinin (123; 154). Ang-(1-7) also activates cellular phosphatases including the dual specificity phosphatase MKP-1 in various cells that attenuates MAP kinase activity (124; 138). In addition, Ang-(1-7) directly increases the production of prostaglandin species that likely contribute to the vasodilatory and natriuretic actions of the peptide (71). Recent studies by Benter and colleagues reveal that Ang-(1-7) attenuates the transactivation of the EGF receptor (EGFR) following exposure to Ang II or high glucose; however, the intracellular mechanism for attenuated EGFR phosphorylation is not known (5). In contrast to either AT1R or AT2R subtypes, which bind Ang II with high affinity, Ang-(1-7) activates at least one other unique receptor protein (126). Originally cloned as an orphan receptor, the G-protein coupled Mas protein exhibits little affinity for Ang II, but mediates a number of actions associated with Ang-(1-7) (27). In addition, a nonpeptide agonist (AVE9971) and two peptide antagonists [D-Ala7-Ang-(1-7) or A779; D-Pro7-Ang-(1-7)] selective for the AT7R are available that generally do not antagonize the actions of Ang II at either AT1 or AT2 receptor sites. Several selective antibodies that recognize the Mas receptor in various species have facilitated studies on the expression and distribution of the protein within the kidney of the rat and sheep (38; 59). As shown in Figure 7, Mas protein staining was evident in the proximal tubules and cortical collecting ducts of the cortex, the thick ascending limb of Henle and the vasa recta of the medullary area of adult male sheep (59). The selective pattern of the Mas receptor within kidney is consistent with the hemodynamic and tubular actions of the Ang-(1-7) AT7R axis. A similar pattern for Mas expression is also evident in the rat kidney, although following acute renal ischemia/reperfusion, the glomerular expression of the receptor was markedly more robust suggesting the dynamic regulation of the Mas receptor following renal injury and the potential amelioratory effects of Ang-(1-7) in pathological conditions (38). As evident for both AT1 and AT2 receptors, the intracellular or nuclear localization AT7R was also reported in the kidney (59). Similar to the AT2R, the nuclear AT7/Mas receptor is coupled to the formation of NO; however, we find that far lower concentrations of Ang-(1-7) are required to stimulate NO than that for Ang II acting at the AT2R (56; 58; 59) (Figure 8A). Furthermore, significant intracellular or nuclear activities for the processing enzymes ACE, ACE2 and neprilysin as well as immunoreactive angiotensinogen and renin are evident within proximal tubules and mesangial cells suggesting the potential for intracellular generation of Ang-(1-7), as well as for Ang II (23; 56; 58; 59). Indeed, we show that blockade of either ACE2 with the ACE2 inhibitor MLN or the AT7R significantly augments the Ang II-dependent formation of ROS within renal cortical nuclei, as well as abolishes the conversion of Ang II to Ang-(1-7) (Figure 8B-8C).
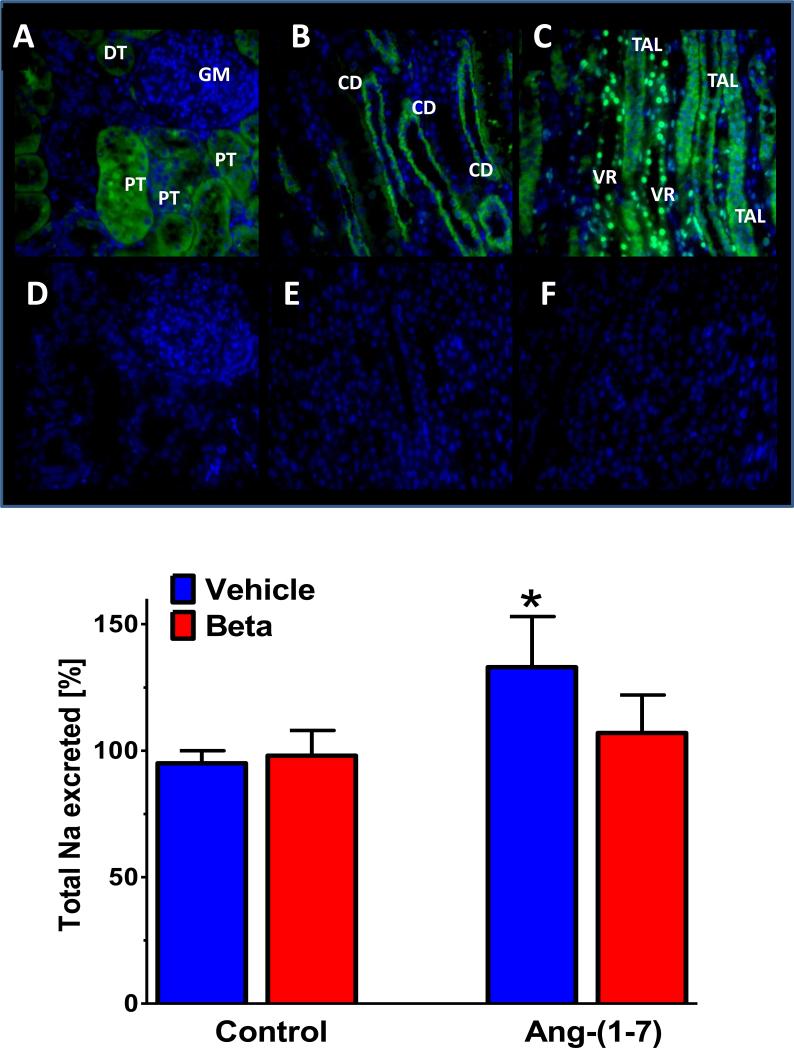
Figure 7. Immunocytochemical distribution of the Mas receptor in the adult sheep kidney and natriuretic influence of Ang-(1-7).
Upper panel: Signal for Mas receptor in proximal tubules (PT) and distal tubules but not glomerulus in renal cortex (A); positive staining of collecting ducts in cortex (B); Mas staining of thick ascending limb of Henle (TAL) and vasa recta (VR) in renal medulla; antigenic peptide for primary antibody abolishes Mas staining in adjacent tissue sections (D-F). Binding of the primary antibody against the Mas receptor protein was followed by the secondary antibody conjugated to Alexa Fluor 488 (green fluorescence) and the nuclear marker stain DAPI (blue). Adapted from Gwathmey et al. (59).
Lower panel: Ang-(1-7) infusion increases sodium excretion (% of an acute sodium load) in control sheep as compared to saline infusion (Vehicle). The natriuretic response to Ang-(1-7) was absent in sheep prenatally exposed to the glucocorticoid betamethasone (Beta). Data are means, n=11-12 sheep. Adapted from Tang et al (142).
Figure 8. Ang-(1-7) increases nitric oxide and attenuates Ang II-dependent increase in reactive oxygen species in isolated nuclei from renal cortex.
Panel A: Ang-(1-7) exhibits greater potency than Ang II at the AT2R to stimulate nitric oxide (NO) as detected by diaminofluorescein [DAF; *P<0.05 vs. Ang-(1-7)]. Panel B: The AT1R antagonist losartan (LOS) blocks the Ang II stimulation of ROS; the AT7R antagonist D-Ala7-Ang-(1-7) (DALA) and ACE2 inhibitor MLN4760 (MLN) exacerbate the Ang II response (δ P<0.05 vs. Ang II); the AT2R antagonist PD had no effect. Panel C: HPLC chromatograph of conversion of Ang II to Ang-(1-7) [Ang7] in isolated nuclei from sheep proximal tubules and inhibition by the ACE2 inhibitor MLN. Adapted from Gwathmey et al (56).
Hemodynamic actions
For Ang-(1-7), the majority of the renal actions of the peptide are in opposition to those of Ang II. Acute infusion of Ang-(1-7) generally increases GFR and increases renal blood flow. In contrast to Ang II, the increase in GFR by Ang-(1-7) likely reflects the vasorelaxant properties of the peptide. In pre-constricted afferent arterioles of the rabbit kidney, Ang-(1-7) induces vasodilation that is dependent on the release of NO (112). In higher species including dog and sheep, administration of Ang-(1-7) increases renal blood flow (65; 141). The vascular response was absent in adult sheep exposed to glucocorticoids during fetal development and the lack of a renal Ang-(1-7) response may contribute to the sustained elevation in blood pressure in the ovine model of fetal programming (141; 141). The vasorelaxant effects of Ang-(1-7) within the kidney are consistent with the actions of the peptide on other vascular beds and the ability of the peptide to acutely stimulate the formation of NO (64; 156). In endothelial cells, Ang-(1-7) stimulates the phosphorylation of eNOS and the associated kinase Akt (123). Inhibition of phosphoinositol 3 (PI3) kinase attenuates the Ang-(1-7)/NO response suggesting that Ang-(1-7) shares a similar pathway with other vasodilatory peptides such as bradykinin that release NO. As to the chronic actions of Ang-(1-7), long-term administration of the peptide is associated with the enhanced expression of eNOS in pre glomerular afferent arterioles of the ApoE knockout mice but not in the wildtype littermates (40). Ang-(1-7) treatment also reduced components of the NAD(P)H oxidase complex (p47phox, NOX2) in the afferent vessels that was associated with lower cortical ROS (H2O2) and 8-isoprostance excretion (40). Importantly, the long-term effects of Ang-(1-7) were abolished by the AT7R antagonist D-Ala7-Ang-(1-7) (40). These studies support the initial work by Benter and colleagues in the diabetic SHR kidney where chronic Ang-(1-7) treatment attenuated NAD(P)H oxidase activity and NOX4 expression, the most abundant NAD(P)H isoform in the kidney (16). Thus, the vascular actions of may reflect the stimulation of NO and the attenuation of oxidative stress that ultimately increases the bioavailability of NO within the renal vasculature. In addition to the influence on NO, the vasorelaxant actions of Ang-(1-7) may also reflect the ability of the peptide to release vasoactive prostaglandins including prostacyclin and PGE2 (31). In the perfused rat kidney, the Ang-(1-7)-dependent increase in prostacyclin was attenuated by the cyclooxygenase inhibitor indomethacin (67). Finally, genetic studies in the Mas knockout model support the contribution of Ang-(1-7) to the renal vasculature as these mice exhibit increased vascular resistance and reduced renal blood flow (107). In turn, genetic overexpression of circulating Ang-(1-7) in the rat results in a sustained increase in renal blood flow and a reduction in renal vascular resistance (19).
Tubular Actions
Infusion of Ang-(1-7) into the renal artery leads to diuresis and natriuresis accompanied by a modest increase in glomerular filtration rate (GFR) (39; 62; 65-67; 149). The natriuretic actions of Ang-(1-7) likely involve the influence of the peptide on the activity of various sodium channels. Indeed, immunocytochemical studies of the AT7R/Mas localize the protein primarily to tubular elements of the kidney including the proximal tubule and thick ascending limb of Henle (38; 59). Ang-(1-7) inhibits Na+,K+-ATPase activity in isolated convoluted proximal tubules and homogenates of the renal cortex (61; 62; 84). Ang-(1-7) also inhibits the transcellular flux of sodium in renal tubular epithelial cells which is associated with activation of phospholipase A2 and the release of arachidonic acid (10). Low picomolar doses of Ang-(1-7) stimulate phosphatidylcholine incorporation in the renal cortex, providing a potential mechanism for the arachidonic acid substrate in the kidney (25; 52; 64). Furthermore, the inhibition of sodium transport by Ang I is markedly potentiated by ACE inhibitor captopril suggesting either a shift to Ang-(1-7) formation or reduced metabolism of the peptide in the proximal tubules of the kidney (10). Actions at the Na+/H+ exchanger may also occur (10). The potent vasopeptidase inhibitor omapatrilat produces a chronic and pronounced diuresis associated with large increases in urinary excretion of Ang-(1-7) and enhanced immunocytochemical staining of the peptide as well as ACE2 in the kidney of the SHR (28; 47). The omapatrilat-induced diuresis consists of dilute urine and is consistent with the localization of the Ang-(1-7) in all segments of the renal tubules (47). In sheep, low systemic doses of Ang-(1-7) induce rapid natriuresis without overall changes in systemic blood pressure and the response is blocked by the AT7 antagonist D-Ala7-Ang-(1-7) (142). However, the acute natriuretic response to Ang-(1-7) is absent in the female offspring of maternal sheep exposed to antenatal glucocorticoids, a model of fetal programming (Figure 7). Moreover, the NO response to Ang-(1-7) is reduced and the Ang II-dependent stimulation of oxidative stress is enhanced in renal cortical nuclei of the glucocorticoid exposed sheep (58). These studies again emphasize the divergent actions of these two pathways within the RAS and that an altered Ang II to Ang-(1-7) response may contribute to fetal programmed events within the kidney. The genetic studies in Mas knockout mouse also support the overall natriuretic and diuretic properties of the Ang-(1-7). Deletion of the Mas protein results in the reduction of the fractional excretion of sodium and lower urine volume, as well reduced renal blood flow and increased renal vascular resistance; however, there was no overall change in mean arterial blood pressure in this model (107).
Renoprotective Actions
The sustained activation of the ACE-Ang II-AT1R axis within the kidney leads to chronic renal inflammation and fibrosis that is likely mediated by increases in oxidative stress (116; 119; 157). In contrast, Ang-(1-7) opposes this pathway and generally exhibits anti-inflammatory and anti-fibrotic effects to attenuate tissue injury within the kidney that are in certain instances independent of an overall change in blood pressure (15; 17). In young Mas knockout mice (9-10 weeks of age), the extent of urinary albumin excretion was significantly higher in comparison to the male wildtype littermates (107). Consistent with the anti-fibrotic actions of Ang-(1-7), the Mas null mice exhibit an increase in collagen and fibronectin deposition in both cortical and medullary areas of the kidney, as well as enhanced gene expression of the cytokine TGF-β and the AT1R that may promote the progression of fibrosis (107). Inducing renal hypertension in the Mas knockout mice exacerbates the increase in blood pressure but reduces excretion of urinary nitrate/nitrite ratio which was reversed by antioxidant treatment with the NAD(P)H inhibitor Apocynin or the SOD mimetic tempol (110). These data again emphasize the functional role of the Ang-(1-7)-AT7R axis to influence NO tone within the kidney. Chronic administration of Ang-(1-7) in the stroke-prone SHR corrected a number of renal indices of renal damage including fibrosis and proteinuria that was associated with the normalization of nephrin expression, a key component in the integrity of the glomerular barrier (51). Ang-(1-7) treatment also reduced glomerular expression for the pro-inflammatory cytokines IL-6 and TNF-α, as well as normalized the early transcriptional response protein NFkB that is commonly associated with inflammatory events (51). In these studies, Ang-(1-7) significantly reduced blood pressure in the SP-SHR; however, it is not clear to what extent the improvement in renal injury contributes to the sustained reduction in pressure in this model. In diabetic SHR, Benter and colleagues demonstrated that administration of Ang-(1-7) improved proteinuria and increased the H2O2 scavenging enzyme catalase, but reduced NAD(P)H oxidase activity (16; 40). In this case, the renoprotective effects of Ang-(1-7) were associated with the increased expression of the PPAR-γ, the therapeutic target for the class of thiazolidenone activators that improve cardiovascular and renovascular pathologies (40). Additional studies in the anti-Thy-1 model of glomerulonephritis reveal that chronic Ang-(1-7) treatment significantly attenuated the extent of proteinuria although the peptide did not completely normalize protein excretion to the control group (164). Associated with the reduction in proteinuria, Ang-(1-7) reduced the glomerular expression of the cytokines plasminogen activating inhibitor-1 (PAI-1) and TGF-β1, as well as the extracellular matrix protein collagen and fibronectin (164). Ang-(1-7) also exhibited anti-inflammatory actions in this model by reducing the presence of ED-1 positive cells within the glomerulus. Interestingly, Ang-(1-7) treatment in the anti-Thy-1 model significantly augmented plasma renin activity as well as glomerular renin gene expression, but tended to further lower ACE2 gene levels (164). The mechanism for the renin response is not known; however, the increase may reflect downregulation of the AT1R as reported by Clark and colleagues in renal cortical slices perfused with Ang-(1-7) and the subsequent disinhibiton of the AT1R negative feedback loop (33). These studies potentially reveal an important consequence of the Ang-(1-7)-AT7R axis regarding the long-term regulation of the AT1R within the kidney. Indeed, the activation of circulating and glomerular renin by Ang-(1-7) may limit the renoprotective actions of the peptide such that additional therapies to block the ACE-Ang II-AT1R axis are required. The ex vivo studies in cultured mesangial cells revealed similar effects of Ang-(1-7) that were attenuated by the AT7R antagonist D-Ala7-Ang-(1-7) suggesting direct actions of the peptide to reduce glomerular inflammation, fibrosis and cytokine production (164). The intracellular pathways involved in the Ang-(1-7)-dependent protective actions within mesangial cells remains to be established.
The Ang IV – AT4R/IRAP axis
The role of Ang-(3-8) or Ang IV within the kidney is quite controversial at this time. An initial report demonstrated that the peptide increased renal blood flow by an increase in NO within the cortex that was independent of either AT1 or AT2 receptors (36). Subsequent binding studies suggested that Ang IV does not interact with a conventional receptor, but the insulin-regulated aminopeptidase or IRAP (80). However, more recent reports find that Ang IV increases renal vascular resistance and the vasoconstriction is abolished by an AT1R antagonist (43; 81; 162). Receptor binding studies by Zhuo and colleagues also demonstrate that Ang IV effectively competes for 125I-[Sar1,Ile8]-Ang II binding at AT1R sites within the kidney (167; 168). Moreover, Ang IV stimulates similar signaling pathways common to the Ang II-AT1R pathway including an increase in intracellular Ca++ and MAP kinase activation in various renal cells types (168). Indeed, Ang IV stimulation of the MAP kinase ERK1/2 in mesangial cells was abolished by an AT1R antagonist (81; 82; 168). At this point, the contribution of the Ang IV IRAP pathway to renal function or under pathological conditions is far from resolved.
Concluding Remarks
The vast literature of the RAS clearly supports a dominant role of the ACE-Ang II- AT1R axis within the kidney to maintain blood pressure through hemodynamic and tubular processes. So too, does activation of this axis participate in pathological conditions including hypertension, diabetes and salt sensitivity that contribute to the progression of renal injury and blockade of ACE or the AT1R are effective therapies to attenuate renal damage, as well as manage blood pressure. However, these therapeutic approaches result in the activation of other components of the non-classical RAS that may oppose those pathways stimulated by the Ang II- AT1R axis. The extent that the alternative pathways within the RAS contribute to normal renal function is best supported by genetic studies on the AT7R and AT2R studies that reveal increases in blood pressure and altered renal parameters. Cleary, the significant expression of an Ang-(1-7) receptor distinct from the AT1 or AT2 subtypes and arguably a specific Ang-(1-7) converting enzyme such as ACE2 of multiple species strengthens a physiological role for the ACE2-Ang-(1-7)-AT7R axis within the kidney and in other tissues, as well as the potential for novel therapeutic approaches that target this pathway in conjunction with blockade of the ACE-Ang II- AT1R arm of the RAS. Indeed, the novel experimental approach for overexpression of ACE2 or administration of the active form of the enzyme has now established the cardiovascular benefit of increasing the hormonal ratio of Ang-(1-7) to Ang II.
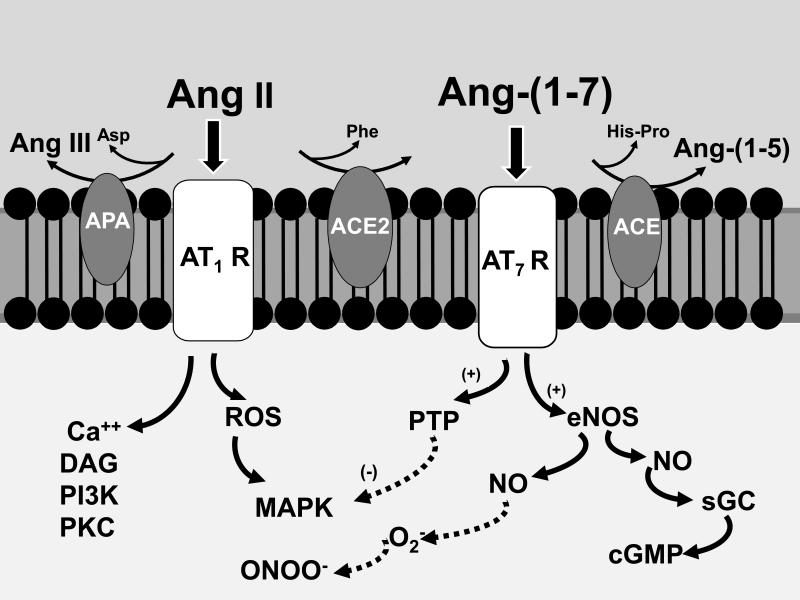
Figure 9. Scheme for the attenuation of the Ang II-AT1 receptor signaling by Ang-(1-7).
Ang II stimulates various signaling pathways including reactive oxygen species (ROS) that culminates in the activation of intracellular kinases (MAPK). Attenuation of Ang II signaling within the kidney occurs through amino and carboxy terminal metabolism to Ang III and Ang-(1-7) by aminopeptidase A (APN) and ACE2, respectively. Formation of Ang-(1-7) will stimulate the generation of nitric oxide (NO) and cGMP that may antagonize the actions of Ang II, as well as complex superoxide (O2−) to form peroxynitrite (ONOO−). In addition, Ang-(1-7) may activate intracellular phosphatases (PTP) to attenuate the Ang II-induced phosphorylation of kinases. ACE may abrogate Ang-(1-7) signaling by enzymatic conversion to Ang-(1-5) which likely does not interact with the AT7/Mas receptor. Although not depicted, generation of Ang III from Ang II may contribute to increased formation of NO by stimulation of the AT2 receptor pathway. Additional abbreviations: calcium (Ca++); diacylglycerol (DAG); phosphoinositol 3 kinase (PI3 kinase); protein kinase C (PKC); endothelial NO synthase (eNOS); soluble guanylate cyclase (sGC). Adapted from Chappell (27).
Acknowledgements
These studies were supported in part by grants from the National Institute of Health grants (HL-56973, HL-51952, HL112237, HD047584 and HD017644) and the American Heart Association (AHA-151521 and AHA-355741). An unrestricted grant from the Unifi Corporation (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC) is also acknowledged.
Bibliography
- 1.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 2.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad A, Ward PE. Role of aminopeptidase activity in the regulation of the pressor activity of circulating angiotensins. J Pharmacol Exp Ther. 1990;252:643–650. [PubMed] [Google Scholar]
- 4.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhtar S, Yousif MH, Dhaunsi GS, Chandrasekhar B, Al-Farsi O, Benter IF. Angiotensin-(1-7) inhibits epidermal growth factor receptor transactivation via a Mas receptor-dependent pathway. Br J Pharmacol 2011. doi: 10.1111/j.1476-5381.2011.01613.x. Epub Aug. 11 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FAO, Simpson RJ, Connolly LM, Chai SY. Evidence that the angiotensin IV(AT4) receptor is the enzyme insulin regulated aminopeptidase. The Journal of Biological Chemistry. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 7.Albiston AL, Ye S, Chai SY. Membrane bound members of the M1 family: more than aminopeptidases. Protein Pept Lett. 2004;11:491–500. doi: 10.2174/0929866043406643. [DOI] [PubMed] [Google Scholar]
- 8.Albiston AL, Yeatman HR, Pham V, Fuller SJ, Diwakarla S, Fernando RN, Chai SY. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul Pept. 2011;166:83–89. doi: 10.1016/j.regpep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1-7) metabolism in pulmonary and renal tissues. Am J Physiol. 2000;279:F841–F850. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 10.Andreatta-Van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7). Kidney Int. 1993;44:932–936. doi: 10.1038/ki.1993.334. [DOI] [PubMed] [Google Scholar]
- 11.Bader M. The second life of the (pro)renin receptor. J Renin Angiotensin Aldosterone Syst. 2007;8:205–208. doi: 10.3317/jraas.2007.031. [DOI] [PubMed] [Google Scholar]
- 12.Barber MN, Sampey DB, Widdop RE. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- 13.Batenburg WW, Krop M, Garrelds IM, de VR, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 14.Benndorf RA, Krebs C, Hirsch-Hoffmann B, Schwedhelm E, Cieslar G, Schmidt Haupt R, Steinmetz OM, Meyer-Schwesinger C, Thaiss F, Haddad M, Fehr S, Heilmann A, Helmchen U, Hein L, Ehmke H, Stahl RA, Boger RH, Wenzel UO. Angiotensin II type 2 receptor deficiency aggravates renal injury and reduces survival in chronic kidney disease in mice. Kidney Int. 2009;75:1039–1049. doi: 10.1038/ki.2009.2. [DOI] [PubMed] [Google Scholar]
- 15.Benter IF, Yousif MH, Al-Saleh FM, Raghupathy R, Chappell MC, Diz DI. Angiotensin-(1-7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J Cardiovasc Pharmacol. 2011;57:559–567. doi: 10.1097/FJC.0b013e31821324b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 17.Benter IF, Yousif MHM, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684–H691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botelho-Santos GA, Sampaio WO, Reudelhuber TL, Bader M, Campagnole-Santos MJ, Souza dos Santos RA. Expression of an angiotensin-(1-7)-producing fusion protein in rats induced marked changes in regional vascular resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2485–H2490. doi: 10.1152/ajpheart.01245.2006. [DOI] [PubMed] [Google Scholar]
- 20.Bradford CN, Ely DR, Raizada MK. Targeting the vasoprotective axis of the renin-angiotensin system: a novel strategic approach to pulmonary hypertensive therapy. Curr Hypertens Rep. 2010;12:212–219. doi: 10.1007/s11906-010-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun-Menendez E, Fasciolo JC, Leloir LF, Munoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283–298. doi: 10.1113/jphysiol.1940.sp003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bumpus FM, Green AA, Page IH. Purification of angiotonin. J Biol Chem. 1954;210:287–294. [PubMed] [Google Scholar]
- 23.Camargo de Andrade MC, Di Marco GS, de Paulo CT,V, Mortara RA, Sabatini RA, Pesquero JB, Boim MA, Carmona AK, Schor N, Casarini DE. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F364–F375. doi: 10.1152/ajprenal.00110.2005. [DOI] [PubMed] [Google Scholar]
- 24.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor. The AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 25.Chansel D, Vandermeersch S, Oko A, Curat C, Ardaillou R. Effects of angiotensin IV and angiotensin-(1-7) on basal and angiotensin II-stimulated cytosolic Ca2+ in mesangial cells. Eur J Pharmacol. 2001;414:165–175. doi: 10.1016/s0014-2999(01)00791-9. [DOI] [PubMed] [Google Scholar]
- 26.Chappell MC, Westwood BM, Pendergrass KD, Jessup J, Ferrario CM. Distinct processing pathways for the novel peptide angiotensin-(1-12) in the serum and kidney of the hypertensive mRen2.Lewis rat. Hypertension. 2007;50:E139, P228. [Abstract] [Google Scholar]
- 27.Chappell MC. Emerging evidence for a functional angiotesin-converting enzyme 2-angiotensin-(1-7) mas receptor axis; more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 28.Chappell MC, Allred AJ, Ferrario CM. Pathways of angiotensin-(1-7) metabolism in the kidney. Nephrol Dial Transplant. 2001;16:22–26. doi: 10.1093/ndt/16.suppl_1.22. [DOI] [PubMed] [Google Scholar]
- 29.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain: evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 30.Chappell MC, Gomez MN, Pirro NT, Ferrario CM. Release of angiotensin-(1-7) from the rat hindlimb: influence of angiotensin-converting enzyme inhibition. Hypertension. 2000;35:348–352. doi: 10.1161/01.hyp.35.1.348. [DOI] [PubMed] [Google Scholar]
- 31.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1-7), ACE2 and blood pressure regulation. In: Suzuki H, Saruta T, editors. Kidney and Blood Pressure Regulation. Basel; Karger: 2004. [DOI] [PubMed] [Google Scholar]
- 32.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 33.Clark MA, Tommasi EN, Bosch SM, Tallant EA, Diz DI. Angiotensin-(1-7) reduces renal aniotensin II receptors through a cyclooxygenase dependent pathway. J Cardiovasc Pharmacol. 2003;41:276–283. doi: 10.1097/00005344-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Clausmeyer S, Sturzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res. 1999;84:337–344. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2).Lewis strain. Am J Physiol Renal Physiol. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman JKM, Krebs LT, Hamilton TA, Ong B, Lawrence KA, Sardinia MF, Harding JW, Wright JW. Autoradiographic identification of kidney angiotensin IV-binding sites and angiotensin IV induced renal cortical blood flow changes in rats. Peptides. 1998;19:269–277. doi: 10.1016/s0196-9781(97)00291-x. [DOI] [PubMed] [Google Scholar]
- 37.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santo AJ, da Costa J, Zhang L, Pei Y, Scholey J, Bray MR, Ferrario CM, Backx PH, Manoukian AS, Chappell MC, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 38.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA, Simoes e Silva AC, Ribeiro Vieira MA. ACE2-angiotensin-(1-7) Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 2010;119:385–394. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 39.DelliPizzi A, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin (1-7). Br J Pharmacol. 1994;111:1–3. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol. 2010;638:108–114. doi: 10.1016/j.ejphar.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robinson K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 42.Drummond W, Munger MA, Rafique EM, Maboudian M, Khan M, Keefe DL. Antihypertensive efficacy of the oral direct Renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. J Clin Hypertens (Greenwich ) 2007;9:742–750. doi: 10.1111/j.1524-6175.2007.06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont AG, Yang R, Smolders I, Vanderheyden P. IRAP and AT(1) receptor mediated effects of angiotensin IV. J Intern Med. 2009;265:401–403. doi: 10.1111/j.1365-2796.2008.02027.x. [DOI] [PubMed] [Google Scholar]
- 44.Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a funcitonal intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00487.2011. Epub Dec 4 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esther CR, Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 46.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 47.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. Vasopeptidase inhibition and Ang-(1-7) in the spontaneously hypertensive rat. Kid Int. 2002;62:1349–1357. doi: 10.1111/j.1523-1755.2002.kid559.x. [DOI] [PubMed] [Google Scholar]
- 48.Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov S, Pinillas C, Luque M. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- 49.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gendron L, Cote F, Payet MD, Gallo-Payet N. Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108-15 cells. Neuroendocrinology. 2002;75:70–81. doi: 10.1159/000048222. [DOI] [PubMed] [Google Scholar]
- 51.Giani JF, Munoz MC, Pons RA, Cao G, Toblli JE, Turyn D, Dominici FP. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F272–F282. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 52.Gironacci MM, Fernandez Tome MC, Speziale E, Sterin-Speziale N, Pena C. Enhancement of phosphatidylcholine biosynthesis by angiotensin-(1-7) in the rat renal cortex. Biochem Pharmacol. 2002;63:507–514. doi: 10.1016/s0006-2952(01)00920-0. [DOI] [PubMed] [Google Scholar]
- 53.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gradman AH, Pinto R, Kad R. Current concepts: renin inhibition in the treatment of hypertension. Curr Opin Pharmacol. 2008;8:120–126. doi: 10.1016/j.coph.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Invited Review: Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00525.2011. Epub Dec 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–626. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–F990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+ATPase activity via a NO/cGMP-dependent pathway. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 61.Handa RK. Angiotensin-(1-7) can interact with the rat proximal tubule AT(4) receptor system. Am J Physiol. 1999;277:F75–F83. doi: 10.1152/ajprenal.1999.277.1.F75. [DOI] [PubMed] [Google Scholar]
- 62.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7) in vivo and in vitro studies. Am J Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- 63.Healy DP, Song L. Kidney aminopeptidase A and hypertension, part I: spontaneously hypertensive rats. Hypertension. 1999;33:740–745. doi: 10.1161/01.hyp.33.2.740. [DOI] [PubMed] [Google Scholar]
- 64.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7) stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 65.Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1-7) in the dog. Kidney Blood Press Res. 2000;23:89–94. doi: 10.1159/000025959. [DOI] [PubMed] [Google Scholar]
- 66.Heyne N, Beer W, Muhlbauer B, Osswald H. Renal response to angiotensin (1-7) in anesthetized rats. Kidney Int. 1995;47:975–976. [Google Scholar]
- 67.Hilchey SD, Bell-Quilley CP. Association between the natriuretic action of angiotensin-(1-7) and selective stimulation of renal prostaglandin I2 release. Hypertension. 1995;25:1238–1244. doi: 10.1161/01.hyp.25.6.1238. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-B1 matrix proteins through receptor mediated, angiotensin II-independent mechanisms. Kid Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 69.Hus-Citharel A, Gasc JM, Zini S, Marchetti J, Roques B, Corvol P, Llorens-Cortes C. Aminopeptidase A activity and angiotensin III effects on [Ca2+]i along the rat nephron. Kidney Int. 1999;56:850–859. doi: 10.1046/j.1523-1755.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 70.Ichihara A, Hayashi M, Hirota N, Okada H, Koura Y, Tada Y, Kaneshiro Y, Tsuganezawa H, Saruta T. Angiotensin II type 2 receptor inhibits prorenin processing in juxtaglomerular cells. Hypertens Res. 2003;26:915–921. doi: 10.1291/hypres.26.915. [DOI] [PubMed] [Google Scholar]
- 71.Iyer SN, Yamada K, Diz DI, Ferrario CM, Chappell MC. Evidence that prostaglandins mediate the antihypertensive actions of angiotensin (1-7) during chronic blockade of the renin angiotensin system. J Cardiovasc Pharmacol. 2000;36:109–117. doi: 10.1097/00005344-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the Novel Angiotensin Peptide, Angiotensin-12 [Ang-(1-12)], in Heart and Kidney of Hypertensive and Normotensive Rats. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 74.Klein JD, Le Quach D, Cole JM, Disher K, Mongiu AK, Wang X, Bernstein KE, Sands JM. Impaired urine concentration and absence of tissue ACE: involvement of medullary transport proteins. Am J Physiol Renal Physiol. 2002;283:F517–F524. doi: 10.1152/ajprenal.00326.2001. [DOI] [PubMed] [Google Scholar]
- 75.Kohagura K, Arima S, Endo Y, Chiba Y, Ito O, Abe M, Omata K, Ito S. Involvement of cytochrome P450 metabolites in the vascular action of angiotensin II on the afferent arterioles. Hypertens Res. 2001;24:551–557. doi: 10.1291/hypres.24.551. [DOI] [PubMed] [Google Scholar]
- 76.Kramar EA, Krishnan R, Harding JW, Wright JW. Role of nitric oxide in angiotensin IV-induced increases in cerebral blood flow. Regul Pept. 1998;74:185–192. doi: 10.1016/s0167-0115(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 77.Kugler P. Aminopeptidase A is angiotensinase A-I. Quantitative histochemical studies in the kidney glomerulus. Histochemistry. 1982;74:229–245. doi: 10.1007/BF00495833. [DOI] [PubMed] [Google Scholar]
- 78.Kugler P. Aminopeptidase A is angiotensinase A. II. Biochemical studies on aminopeptidase A and M in rat kidney homogenate. Histochemistry. 1982;74:247–261. doi: 10.1007/BF00495834. [DOI] [PubMed] [Google Scholar]
- 79.Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL. Aliskiren, a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertension. Hypertens Res. 2006;29:997–1005. doi: 10.1291/hypres.29.997. [DOI] [PubMed] [Google Scholar]
- 80.Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP). J Neurochem. 2003;86:344–350. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- 81.Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am J Physiol Renal Physiol. 2006;290:F1024–F1033. doi: 10.1152/ajprenal.00221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol. 2011;300:F1076–F1088. doi: 10.1152/ajprenal.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li XC, Widdop RE. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142:821–830. doi: 10.1038/sj.bjp.0705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez O, Gironacci M, Rodriguez d, Pena C. Effect of angiotensin-(1-7) on ATPase activities in several tissues. Regulatory Peptides. 1998;77:135–139. doi: 10.1016/s0167-0115(98)00113-x. [DOI] [PubMed] [Google Scholar]
- 85.Matavelli LC, Huang J, Siragy HM. Angiotensin AT receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsubara H, Shibasaki Y, Okigaki M, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T. Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-Jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem Biophys Res Commun. 2001;282:1085–1091. doi: 10.1006/bbrc.2001.4695. [DOI] [PubMed] [Google Scholar]
- 87.Matsubara H, Sugaya T, Murasawa S, Nozawa Y, Mori Y, Masaki H, Maruyama K, Tsutumi Y, Shibasaki Y, Moriguchi Y, Tanaka Y, Iwasaka T, Inada M. Tissue-specific expression of human angiotensin II AT1 and AT2 receptors and cellular localization of subtype mRNAs in adult human renal cortex using in situ hybridization. Nephron. 1998;80:25–34. doi: 10.1159/000045121. [DOI] [PubMed] [Google Scholar]
- 88.Modrall JG, Sadjadi J, Brosnihan KB, Gallagher PE, Ya C-H, Kramer GL, Bernstein KE, Chappell MC. Depletion of tissue ace differentially influences the intrarenal and urinary expression of angiotensins. Hypertension. 2003;43:4849–4853. doi: 10.1161/01.HYP.0000121462.27393.f6. [DOI] [PubMed] [Google Scholar]
- 89.Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51:676–681. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- 90.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Comm. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 91.Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han JY, Kon V, Fogo AB. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298:F683–F691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakagawa T, Akaki J, Satou R, Takaya M, Iwata H, Katsurada A, Nishiuchi K, Ohmura Y, Suzuki F, Nakamura Y. The His-Pro-Phe motif of angiotensinogen is a crucial determinant of the substrate specificity of renin. Biol Chem. 2007;388:237–246. doi: 10.1515/BC.2007.026. [DOI] [PubMed] [Google Scholar]
- 93.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen G. Renin/prorenin receptors. Kidney Int. 2006;69:1503–1506. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Patho. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ozono R, Wang Z Q, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subytpe 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 99.Padia SH, Howell NL, Kemp BA, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition restores defective angiontesin II type 2-mediated natriuresis in spontaneously hypertensive rats. Hypertension. 2010;55:474–480. doi: 10.1161/HYPERTENSIONAHA.109.144956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 101.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53:338–343. doi: 10.1161/HYPERTENSIONAHA.108.124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palmieri FE, Bausback HH, Ward PE. Metabolism of vasoactive peptides by vascular endothelium and smooth muscle aminopeptidase M. Biochem Pharmacol. 1989;38:173–180. doi: 10.1016/0006-2952(89)90165-2. [DOI] [PubMed] [Google Scholar]
- 103.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 105.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2).Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res. 2002;90:1135–1141. doi: 10.1161/01.res.0000019242.51541.99. [DOI] [PubMed] [Google Scholar]
- 107.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 108.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prieto MC, Gonzalez-Villalobos RA, Botros FT, Martin VL, Pagan J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1-7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F749–F755. doi: 10.1152/ajprenal.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rakusan D, Burgelova M, Vaneckova I, Vanourkova Z, Huskova Z, Skaroupkova P, Mrazova I, Opocensky M, Kramer HJ, Netuka I, Maly J, Alenina N, Bader M, Santos RA, Cervenka L. Knockout of angiotensin 1-7 receptor mas worsens the course of two-kidney, one-clip goldblatt hypertension: roles of nitric oxide deficiency and enhanced vascular responsiveness to angiotensin II. Kidney Blood Press Res. 2010;33:476–488. doi: 10.1159/000320689. [DOI] [PubMed] [Google Scholar]
- 111.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 112.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 113.Reudelhuber TL. Prorenin, Renin, and their receptor: moving targets. Hypertension. 2010;55:1071–1074. doi: 10.1161/HYPERTENSIONAHA.108.120279. [DOI] [PubMed] [Google Scholar]
- 114.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rompe F, Unger T, Steckelings UM. The angiotensin AT2 receptor in inflammation. Drug News Perspect. 2010;23:104–111. doi: 10.1358/dnp.2010.23.2.1475901. [DOI] [PubMed] [Google Scholar]
- 116.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 117.Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol. 2011;300:F700–F706. doi: 10.1152/ajprenal.00616.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabuhi R, Asghar M, Hussain T. Inhibition of NAD(P)H oxidase potentiates AT2 receptor agonist-induced natriuresis in Sprague-Dawley rats. Am J Physiol Renal Physiol. 2010;299:F815–F820. doi: 10.1152/ajprenal.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 120.Sadjadi J, Kramer GL, Yu C, Welborn MB, Chappell MC, Modrall JG. Angiotensin converting eyzyme-independent angiotensin II production by chymase is up regulated in the ischemic kidney in renovascular hypertension. J Surg Res. 2005;127:65–69. doi: 10.1016/j.jss.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 121.Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 2007;30:1139–1146. doi: 10.1291/hypres.30.1139. [DOI] [PubMed] [Google Scholar]
- 122.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 123.Sampaio WO, dos Santos RA, Faria-Silva R, de Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 124.Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 125.Santos RA, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 126.Santos RAS, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Bul I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss H-P, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santos RAS, Simoes-e-Silva A, Maric C, Silva DMR, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SVB, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss H-P, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G-protein coupled receptor Mas. PNAS. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 129.Shaltout HA, Westwood B, Averill DB, Ferrario CM, Figueroa J, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: Evidence for ACE2-dependent processing of Angiotensin II. Am J Physiol Renal Physiol. 2006;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 130.Shibasaki Y, Matsubara H, Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T. Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension. 2001;38:367–372. doi: 10.1161/01.hyp.38.3.367. [DOI] [PubMed] [Google Scholar]
- 131.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monopphosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 133.Siragy HM, Inagami T, Carey RM. NO and cGMP mediate angiotensin AT2 receptor-induced renal renin inhibition in young rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1461–R1467. doi: 10.1152/ajpregu.00014.2007. [DOI] [PubMed] [Google Scholar]
- 134.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin induced diabetic mice. Kid Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 135.Song L, Healy DP. Kidney aminopeptidase A and hypertension, part II: effects of angiotensin II. Hypertension. 1999;33:746–752. doi: 10.1161/01.hyp.33.2.746. [DOI] [PubMed] [Google Scholar]
- 136.Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, Namsolleck P, Dahlof B, Unger T. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol. 2011;11:187–192. doi: 10.1016/j.coph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 137.Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol. 2009;23:693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 138.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibitis angiotensin II stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 139.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin-(1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension. 2010;56:658–666. doi: 10.1161/HYPERTENSIONAHA.110.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takahasi K, Bardhan S, Kambayashi Y, Shirai H, Inagami T. Protein tyrosine phosphatase inhibition by angiotensin II in rat pheochromocytoma cells through type 2 receptor, AT2. Biochem Biophys Res Commun. 1994;198:60–66. doi: 10.1006/bbrc.1994.1009. [DOI] [PubMed] [Google Scholar]
- 141.Tang L, Bi J, Valego NK, Carey LC, Figueroa JP, Chappell MC, Rose JC. Prenatal betamethasone exposure alters renal function in immature sheep: Sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol. 2009;296:R309–R317. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tikellis C, Bialkowski K, Pete J, Sheehy K, Su Q, Johnston C, Cooper M, Thomas M. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 144.Tikkanen I, Tikkanen T, Cao Z, Allen TJ, Davis BJ, Lassila M, Casley D, Johnston CI, Burrell LM, Cooper ME. Combined inhibition of neutral endopeptidase with angiotensin converting enzyme or endothelin converting enzyme in experimental diabetes. J Hypertens. 2002;20:707–714. doi: 10.1097/00004872-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 145.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidse. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 146.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an Alternate Substrate for Angiotensin Peptide Production in the Heart. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyama A, Takahashi H, Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. TIPS. 2002;23:177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 149.Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-D-ALA]-Angiotensin 1-7 blocks renal actions of angiotensin 1-7 in the anesthetized rat. J Cardiovasc Pharmacol. 1998;32:164–167. doi: 10.1097/00005344-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 150.Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol. 2007;293:F398–F407. doi: 10.1152/ajprenal.00050.2007. [DOI] [PubMed] [Google Scholar]
- 151.Velez JC, Ryan KJ, Harbeson CE, Bland AM, Budisavljevic MN, Arthur JM, Fitzgibbon WR, Raymond JR, Janech MG. Angiotensin I is largely converted to angiotensin (1-7) and angiotensin (2-10) by isolated rat glomeruli. Hypertension. 2009;53:790–797. doi: 10.1161/HYPERTENSIONAHA.109.128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 153.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280:39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 154.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in ApoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 155.Wenzel UO, Krebs C, Benndorf R. The angiotensin II type 2 receptor in renal disease. J Renin Angiotensin Aldosterone Syst. 2010;11:37–41. doi: 10.1177/1470320309347787. [DOI] [PubMed] [Google Scholar]
- 156.Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40:847–852. doi: 10.1161/01.hyp.0000037979.53963.8f. [DOI] [PubMed] [Google Scholar]
- 157.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 158.Wolf G, Assmann KJ, Stahl RA. Overexpression of aminopeptidase A abolishes the growth promoting effects of angiotensin II in cultured mouse mesangial cells. Kidney Int. 1997;52:1250–1260. doi: 10.1038/ki.1997.450. [DOI] [PubMed] [Google Scholar]
- 159.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamada K, Iyer SN, Chappell MC, Brosnihan KB, Fukuhara M, Ferrario CM. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept. 1999;80:57–66. doi: 10.1016/s0167-0115(99)00005-1. [DOI] [PubMed] [Google Scholar]
- 161.Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- 162.Yang R, Smolders I, De BD, Fouyn R, Halberg M, Demaegdt H, Vanderheyden P, Dupont AG. Brain and peripheral angiotensin II type 1 receptors mediate renal vasoconstrictor and blood pressure responses to angiotensin IV in the rat. J Hypertens. 2008;26:998–1007. doi: 10.1097/HJH.0b013e3282f5ed58. [DOI] [PubMed] [Google Scholar]
- 163.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotension-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminemia in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 164.Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1-7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F579–F588. doi: 10.1152/ajprenal.00548.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–28. doi: 10.1161/CIRCULATIONAHA.110.955369. 18. [DOI] [PubMed] [Google Scholar]
- 166.Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 167.Zhuo J, Moeller I, Jenkins T, Chai SY, Allen AM, Ohishi M, Mendelsohn FA. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1998;16:2027–2037. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 168.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32:1551–1565. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]