Summary
An abnormal circulating ganglioside was found in patients with neuroblastoma. This ganglioside appeared as a single band by resorcinol-HCl staining of thin-layer chromatograms of purified total gangliosides isolated from as little as 1 ml of patient plasma. It is a major ganglioside of neuroblastoma tumour tissue and was present (250–1500 pmol lipid-bound sialic acid/ml) in the plasma of five patients with widespread neuroblastoma. In contrast, the ganglioside was not detected (<50 pmol/ml) in plasma samples of six patients in complete remission, nor in plasma samples of seventeen healthy children and adults. Measurement of this circulating tumour-associated ganglioside should be clinically useful in neuroblastoma, offering a new approach to the detection of tumour and the evaluation of therapy.
INTRODUCTION
Gangliosides, sialic-acid-containing glycosphingolipids, occur mainly in the cell surface membrane. The concentrations of gangliosides are highest in brain tissue, but they are present in all tissues, including tumour tissue. These membrane-bound glycolipids are released or shed in vitro by many cells, especially proliferating cells such as tumour cells.1,2 It has been suggested that high concentrations of gangliosides, present in the circulation of tumour-bearing hosts,3–7 may be tumour-related.4 To date, however, there has been no report of direct visualisation and identification of a ganglioside in the plasma of patients with cancer that has not also been detected in normal plasma. We report here the direct visualisation of a specific tumour-associated ganglioside in the plasma of patients with neuroblastoma.
PATIENTS AND METHODS
Human Plasma and Tissue Samples
This study was approved by the UCLA human subject protection committee. Samples were obtained after written consent had been given by the donor or parents. Heparinised blood samples (2–15 ml) were obtained by venepuncture from children (aged 2–6 years) with histology-confirmed neuroblastoma, and from healthy normal subjects (aged 2–51 years). Plasma was separated by centrifugation. Tumour tissue was obtained at the time of surgical diagnosis. Samples were stored at −70°C and processed within 72 h.
Isolation and Purification of Gangliosides
Total lipid extracts of tumour tissue (0.5–1.0 g homogenised in distilled water) and plasma (1–8 ml) were prepared by extraction in 10–20 volumes of chloroform/methanol (1/1).8 The extract was clarified by centrifugation and dried under a stream of nitrogen. For purification of the gangliosides9 the dried total lipid extract was partitioned in di-isopropylether, 1-butanol, 50 mmol/1 aqueous sodium chloride (in the ratio 6/4/5 by volume: 3 vol/ml plasma, 30 vol/g tissue). This partitioning separates most other lipids from gangliosides, which are recovered in the lower aqueous phase. The aqueous phase was repartitioned with fresh organic phase, lyophiiised, and dissolved in distilled water, and the gangliosides were separated from low-molecular-weight contaminants by `Sephadex G-50' gel filtration.10 Gangliosides were recovered in the void volume, which was lyophiiised. The gangliosides were dissolved in chloroform/methanol (1/1). Trace residual protein was removed by centrifugation. The ganglioside-containing clear supernatant was stored at −20°C under nitrogen.
Thin-layer Chromatography
Thin-layer chromatograms of gangliosides were prepared on 10× 10 or 10× 20 cm precoated silica gel 60 high-performance plates (E. Merck, Darmstadt, West Germany) preactivated by heating to 90°C for 45 min. The gangliosides isolated from 1 ml plasma were spotted per lane. The plates were developed in chloroform, methanol, and 0.2% aqueous calcium chloride (in the ratio 60/40/9 by volume). Gangliosides were visualised as purple bands with resorcinol-HCl reagent8 and were quantified by scanning densitometry. Single ganglioside bands of 50 pmol lipid-bound sialic acid can be detected by this method.
Preparative thin-layer chromatography was carried out with the same developing solvent system. Individual bands were visualised in iodine vapour and scraped from the plate. The gangliosides were recovered by repeated sonication of the gel in chloroform, methanol, water (50/50/15). The solution was clarified by centrifugation then dried. Gangliosides were redissolved in chloroform/methanol (1/1) and stored at −20°C under nitrogen.
Enzymic Degradation of Gangliosides
Purified gangliosides (10–40 nmol lipid-bound sialic acid) were dissolved in 100 μl 0.1 mol/1 sodium acetate buffer, pH 5.5, containing 0.1% calcium chloride and 20 mU neuraminidase (purified, type V, Sigma, St Louis, Missouri).11 The reaction mixture was incubated at 37°C for 18 h. The reaction products were purified by sephadex G-50 gel filtration to remove free sialic acid and salt, with distilled water as the eluting solvent. The ganglioside-containing fraction was lyophiiised, redissolved in chloroform/methanol, and analysed by thin-layer chromatography.
RESULTS
In fig 1, the total gangliosides isolated and purified from plasma and tumour-tissue samples of a patient with metastatic neuroblastoma are compared with those isolated from normal human plasma and brain tissue. Differences in the intensity of several bands can be seen in the plasma ganglioside patterns, but the most striking difference was the presence in the patient's plasma (lane C) of a ganglioside that was not detected in the control plasma sample (lane D). This ganglioside had the same mobility on thin-layer chromatography as that of the most prominent ganglioside of the tumour-tissue sample (lane B). The ganglioside was also readily apparent in two other biopsy specimens available for study (not shown). The mobility of this tumour-associated ganglioside is identical to that of the disialoganglioside GD2 (II3α(NeuAc)2-GgOse3Cer; gangliosides are identified by the nomenclature of Svennerholm12) a minor ganglioside of brain13 and the very faint band just below GDla ganglioside in lane A of fig 1.
Fig 1. Total gangliosides purified from a neuroblastoma biopsy sample (lane B) and neuroblastoma patient's plasma (lane C) compared with normal human plasma gangliosides (lane D) and human brain gangliosides (lane A).
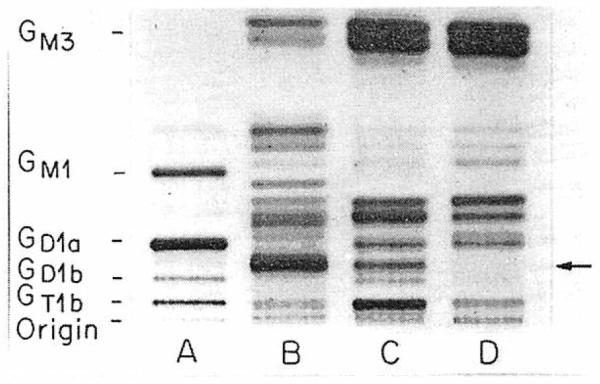
All bands above origin arc resorcinol positive. Migration of standard brain gangliosides is shown on left; arrow indicates neuroblastoma-associated ganglioside detected in patient's but not normal plasma.
To test further the possibility that by the method we used, human neuroblastoma tumour gangliosides could be detected in the plasma of patients with advanced disease, gangliosides were purified from the plasma of four other patients with widespread neuroblastoma (fig 2). Each patient's plasma ganglioside pattern clearly showed a band of the same mobility as that of the major tumour ganglioside (lane B). Scanning densitometry showed lanes 1–5 to contain 900, 250, 450, 1000, and 400 pmol lipid-bound sialic acid of the neuroblastoma-associated ganglioside. The ganglioside was not detected in the two normal plasma samples shown (lanes A and C).
Fig 2. Total gangliosides purified from plasma of five patients with widespread neuroblastoma (lanes 1–5) compared with plasma gangliosides of a healthy adult (lane A) and child (lane C) and neuroblastoma tissue gangliosides (lane B).
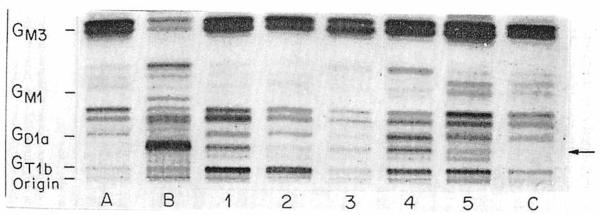
Lane B and lane 1 show gangliosides of same samples as lanes B and C in fig 1. Arrow as fig 1.
To investigate their carbohydrate structure, the major tumour ganglioside and the abnormal circulating ganglioside were purified by preparative thin-layer chromatography and subjected to enzymic degradation with neuraminidase in the absence of detergent (fig 3). Several observations can be made about the neuraminidase treatment products of the purified neuroblastoma tissue (lane A) and abnormal circulating (lane B) gangliosides. Each of these gangliosides gave a single resorcinol-positive product when treated with neuraminidase. These products had identical thin-layer-chromatographic mobility (lanes AN and BN), which is further evidence of identity between the tumour ganglioside and the abnormal circulating ganglioside. Furthermore, the mobility of the neuraminidase treatment product was identical to that of ganglioside GM2 isolated from Tay-Sachs disease brain tissue. Since under the conditions we used neuraminidase cleaves one of the two sialic acids from the disialoganglioside GD2 to yield the monosialoganglioside GM2,11 these results suggest that the carbohydrate structure of the neuroblastoma-associated ganglioside is the same as that of GD2.
Fig 3. Structural studies of neuroblastoma-associated gangliosidc.
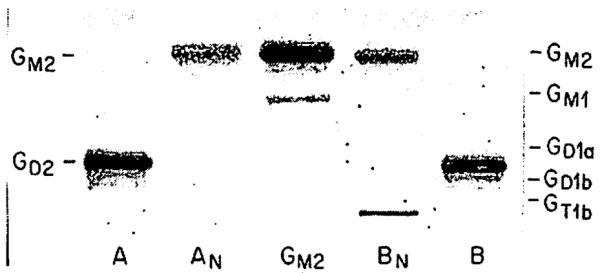
Reaction products (lanes AN and BN) of neuroblastoma tissue ganglioside (lane A) and of plasma ganglioside (lane B) had mobility identical with that of standard GM2 ganglioside, isolated from Tay-Sachs disease brain tissue (centre lane). Migration of standard brain gangliosides indicated on right.
Total gangliosides were purified from the plasma of seventeen healthy subjects, aged 2–51 years (eight male, nine female). The neuroblastoma-associated ganglioside was not detected in any of these samples, indicating that it is not a significant component ganglioside of normal plasma. To provide a specific and more sensitive test for the presence of this ganglioside in normal plasma, an experiment using the approach shown in fig 3 was carried out. The region in which standard GD2 and the neuroblastoma-associated ganglioside migrate was recovered by preparative thin-layer chromatography of the total gangliosides from 15 ml of normal plasma. The major, resorcinol-positive reaction products resulting from neuraminidase treatment of these normal plasma gangliosides migrated similarly to GM1. A faint band with similar migration to GM2 was visible, corresponding to 75 pmol lipid-bound sialic acid of the monosialoganglipside GM2, the product of the neuraminidase treatment of 150 pmol lipid-bound sialic acid of the disialoganglioside GD2. Thus, GD2 is present in normal plasma only in trace amounts (10 pmol lipid-bound sialic acid/ml), in contrast to ≥250 pmol in the patient plasma samples (fig 2).
The abnormal circulating ganglioside was not detected in the plasma of any of six neuroblastoma patients who were in complete clinical remission. One clinically symptomless patient, who unexpectedly had neuroblastoma cells infiltrating the bone marrow, did have the abnormal circulating ganglioside in his plasma (100 pmol lipid-bound sialic acid/ml). These results suggest that detection of the ganglioside reflects the presence of tumour in patients with neuroblastoma. The study of plasma of one patient undergoing treatment for metastatic neuroblastoma further supports this conclusion. Plasma was obtained from this patient immediately before and 2 months after a course of therapy which substantially reduced the tumour burden. The thin-layer chromatogram of the gangliosides purified from these samples was analysed by scanning densitometry to determine the quantity of the abnormal ganglioside present. The concentration of the ganglioside fell greatly, from 1500 to 250 pmol lipid-bound sialic acid/ml plasma. Thus, quantification of the abnormal circulating ganglioside in patients diagnosed as having neuroblastoma may be useful in assessing their response to therapy.
DISCUSSION
Changes in circulating gangliosides associated with the presence of malignant tumour in vivo have been the subject of much interest, stemming partly from the possibility that such changes specifically reflect the presence of tumour. As such, abnormalities in circulating gangliosides could be useful as markers of malignant disease. An association between abnormalities in circulating gangliosides and the presence of tumour is suggested by the knowledge that membrane-bound gangliosides are, among other molecules on the cell surface, released by proliferating cells.1,2 Findings of high concentrations of gangliosides in the circulation of tumour-bearing hosts,3–7 which fall towards normal on removal of tumour,4,5 support this association, as does the indirect evidence that binding between a monoclonal antibody and a colon-carcinoma-associated ganglioside is inhibited by the serum of patients with this tumour.14
To date, however, direct thin-layer-chromatographic visualisation of gangliosides isolated and purified from the plasma of patients with cancer has not shown the presence of any ganglioside which was not also detected though perhaps in lower concentrations in the plasma of healthy normal donors. For example, levels of GM3 and GD3, prominent constituent gangliosides of normal human plasma, were slightly raised in the plasma of patients with melanoma,5 as were those of GM3 in plasma of patients with cerebral astrocytoma.6 In contrast, our study shows that a particular ganglioside, a major ganglioside of neuroblastoma tumour tissue not detectable in normal human plasma samples by standard thin-layer-chromatographic methods, is present in high concentrations in the plasma of patients with this malignant disorder. These findings support the hypothesis that changes in circulating gangliosides in patients with cancer are related to the tumour gangliosides themselves.
The thin-layer-chromatographic migration characteristics and the neuraminidase susceptibility of this neuroblastoma-associated ganglioside suggest that its carbohydrate structure is that of the disialoganglioside GD2. This is a major ganglioside of neuroblastoma tissue,15,16 comprising up to 28% of the total gangliosides of this tumour,16 and it is found in other tumours of neuroectodermal origin.17–20 Tumour-derived gangliosides whose carbohydrate structures are the same as those of gangliosides of normal tissues such as brain may nevertheless show minor differences in the ceramide (lipid) portion of the molecule.21 Therefore, the complete molecular characterisation of the neuroblastoma-associated ganglioside described here will be of interest.
Our findings of only trace concentrations of GD2 in normal plasma accord with those of others who have not detected GD2 in normal human plasma, either by standard thin-layer-chromatographic methods22 or in a detailed study of the composition and structure of human plasma gangliosides.23 Lack of detectable quantities (ie, <50 pmol lipid-bound-sialic acid/ml) of the tumour-associated ganglioside in plasma samples of patients in complete remission and the high levels (≥250 pmol lipid-bound sialic acid/ml) of this ganglioside in plasma samples of patients with widespread neuroblastoma suggest that the abnormal circulating ganglioside is a tumour marker in patients with neuroblastoma.
The ideal tumour marker is a molecule of tumour-cell origin, released by the tumour cell in quantities easily detectable in the circulation of the host and proportional to the tumour burden, and not present in substantial concentrations in the circulation of non-tumour-bearing hosts. Carcinoembryonic antigen, human chorionic pnadotropin, and alpha-fetoprotein are examples of such molecules, which have already been shown to be valuable in the diagnosis and sequential monitoring of patients with certain malignant disorders. All the above criteria for a tumour marker are met by the circulating tumour-associated ganglioside described here. It is necessary to confirm that the detection and quantification of the abnormal circulating ganglioside are clinically useful in managing patients with neuroblastoma.
Acknowledgments
We thank Eileen Schwartz for assistance with thin-layer chromatography, Dr Michael Kaback for the sample of Tay-Sachs disease brain tissue, Dr R. Seeger, Dr S. Feig, Dr C. Lenarsky, and Dr R. Shearer for cooperation in obtaining patient samples, and Donna Lopez for preparation of the typescript. This work was supported by grants from the National Cancer Institute (CA27701), the American Cancer Society (PDT 270), and the Cancer Research Coordinating Committee, University of California. S. L. is recipient of research career development award CA00821 from the National Cancer Institute and a scholar of the Leukemia Society of America. Z.L.W. received a Silbert International Scholarship and support from the Concern Foundation, and is on sabbatical leave from Guangzhou Medical College, Canton, People's Republic of China.
REFERENCES
- 1.Black PH. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- 2.Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- 3.Skipski VP, Katapodis N, Prendergast JS, Stock CC. Gangliosides in blood serum of normal rats and Morris hepatoma 5123tc-bearing rats. Biochem Biophys Res Commun. 1975;67:1122–27. doi: 10.1016/0006-291x(75)90790-1. [DOI] [PubMed] [Google Scholar]
- 4.Kloppel TM, Keenan TW, Freeman MJ, Morre DJ. Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc Natl Acad Sci USA. 1977;74:3011–13. doi: 10.1073/pnas.74.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portoukalian J, Zwingelstein G, Abdul-Malak N, Dore JF. Alteration of gangliosides in plasma and red cells of humans bearing melanoma tumours. Biochem Biophys Res Commun. 1978;85:916–20. doi: 10.1016/0006-291x(78)90630-7. [DOI] [PubMed] [Google Scholar]
- 6.Lo HS, Hogan EL, Koontz MS, Traylor TD. Serum gangliosides in cerebral astrocytoma. Ann Neurol. 1980;8:534–38. doi: 10.1002/ana.410080511. [DOI] [PubMed] [Google Scholar]
- 7.Lenglc EE. Increased levels of lipid-bound sialic acid in thymic lymphocytes and plasma of leukemic AKR/J mice. J Natl Cancer Inst. 1979;82:1565–67. [PubMed] [Google Scholar]
- 8.Ledeen RW, Yu RK. Gangliosides: structure, isolation and analysis. Method Enzymol. 1982;83:139–91. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- 9.Ladisch S, Gillard B. Anal Biochem. A solvent partition method for microscale ganglioside purification. in press. [DOI] [PubMed] [Google Scholar]
- 10.Ueno K, Ando S, Yu RK. Gangliosides of human, cat and rabbit spinal cords and cord myclin. J Lipid Res. 1978;19:863–71. [PubMed] [Google Scholar]
- 11.Wenger DA, Wardell S. Action of neuraminidase (EC 3.2.1.18) from Clossridium perfringens on brain gangliosides in the presence of bile salts. J Neurochem. 1973;20:607–12. doi: 10.1111/j.1471-4159.1973.tb12159.x. [DOI] [PubMed] [Google Scholar]
- 12.Svenncrholm L. Chromatographic separation of human brain gangliosides. J Neurochem. 1963;10:613–23. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 13.Klenk E, Hof L, Georgias L. Zur kenntnis der gehirnganglioside. Hoppe Seyler's Z Physiol Chem. 1967;348:149–66. [PubMed] [Google Scholar]
- 14.Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 15.Schochat SJ, Abt AB, Schengrund CL. VCN-releasable sialic acid and gangliosides in human neuroblastomas. J Pediatr Surg. 1977;12:413–18. doi: 10.1016/0022-3468(77)90019-7. [DOI] [PubMed] [Google Scholar]
- 16.Yates AJ, Thompson DK, Boesel CP, Albrightson C, Hart RW. Lipid composition of human neural tumours. J Lipid Res. 1979;20:428–36. [PubMed] [Google Scholar]
- 17.Traylor TD, Hogan EL. Gangliosides of human cerebral astrocytomas. J Neurochem. 1980;34:126–31. doi: 10.1111/j.1471-4159.1980.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Pukel CS, Takeyama H, et al. Human melanoma antigen AH is an autoantigenic ganglioside related to GD2. J Exp Med. 1982;156:1884–89. doi: 10.1084/jem.156.6.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portoukalian J, Zwingelstein G, Dore JF. Lipid composition of human malignant melanoma tumours at various levels of malignant growth. Eur J Biochem. 1979;14:19–23. doi: 10.1111/j.1432-1033.1979.tb12866.x. [DOI] [PubMed] [Google Scholar]
- 20.Cahan LD, trie RF, Singh R, Cassidenti A, Paulson JC. Identification of human neuroectodermal tumour antigen (OFA-I-2) as ganglioside GD) Proc Natl Acad Sci USA. 1982;79:7629–33. doi: 10.1073/pnas.79.24.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakomori S, Kannagi R. Glycosphingolipids as tumour-associated and differentiation markers. J Natl Cancer Inst. 1983;71:231–51. [PubMed] [Google Scholar]
- 22.Sela BA, Konat G, Offner H. Elevated ganglioside concentration in serum and peripheral blood lymphocytes from multiple sclerosis patients in remission. J Neurol Sci. 1982;54:143–48. doi: 10.1016/0022-510x(82)90226-x. [DOI] [PubMed] [Google Scholar]
- 23.Kundu SK, Diego I, Marcus DN. Glycosphingolipids of human plasma. Proceedings Seventh International Symposium on Glycoconjugates. 1983:256–57. abstr. [Google Scholar]


