Abstract
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. It often precedes the development of food allergy and asthma. Recent insights into AD reveal abnormalities in terminal differentiation of the epidermal epithelium leading to a defective stratum corneum, which allows enhanced allergen penetration and systemic IgE sensitization. Atopic skin is also predisposed to colonization or infection by pathogenic microbes, most notably Staphylococcus aureus and herpes simplex virus (HSV). Causes of this abnormal skin barrier are complex and driven by a combination of genetic, environmental and immunologic factors. These factors likely account for the heterogeneity of AD onset, severity and natural history of this skin disease. Recent studies suggest prevention of AD can be achieved by early interventions protecting the skin barrier. Onset of lesional AD requires effective control of local and systemic immune activation for optimal management. Early intervention may improve long term outcomes for AD and reduce the systemic allergen sensitization leading to associated allergic diseases in the gastrointestinal and respiratory tract.
Keywords: atopic dermatitis, eczema, skin epithelium, immune, infection, filaggrin
Introduction
AD is the most common chronic inflammatory skin disease (1,2). Recent studies reveal strong associations between mental health disorders and AD, suggesting the need to effectively manage this disease for patient's general well being and their family's quality of life (3). AD is often associated with food allergy and asthma (4,5). The abnormal skin barrier in AD may allow epicutaneous absorption of environmental allergens through the skin and promote systemic allergen sensitization, which predisposes to the development of food allergy and asthma. In this month's journal, researchers report that AD increases the impact of environmental peanut exposure in AD children (6-8). Because there are currently no cures for food allergy and asthma, the development of effective treatments for AD may be an important strategy for prevention of the Atopic March. Elucidation of the underlying mechanisms of AD therefore provides a critical opportunity for early intervention.
AD is a complex disease with a genetic predisposition that is strongly influenced by innate and adaptive immune responses as well as environmental factors including allergen exposure, irritants, microbes, diet, stress, and air quality (9-13). Although it is commonly referred to as a single disease (14), recent studies suggest that the time has come to distinguish various AD phenotypes and endotypes (15,16) in much the same manner in which attempts have been made to categorize asthma and rhinosinusitis into different subtypes based on a constellation of the onset, biomarkers, immune polarization, gene variants and natural history of the disease (17-19). Identification of immune pathway polarity will be of particular importance as biologics become more readily available to target specific immune pathways such as Th2 and Th22 pathways as well as various inflammatory cytokines and mediators (20, 21).
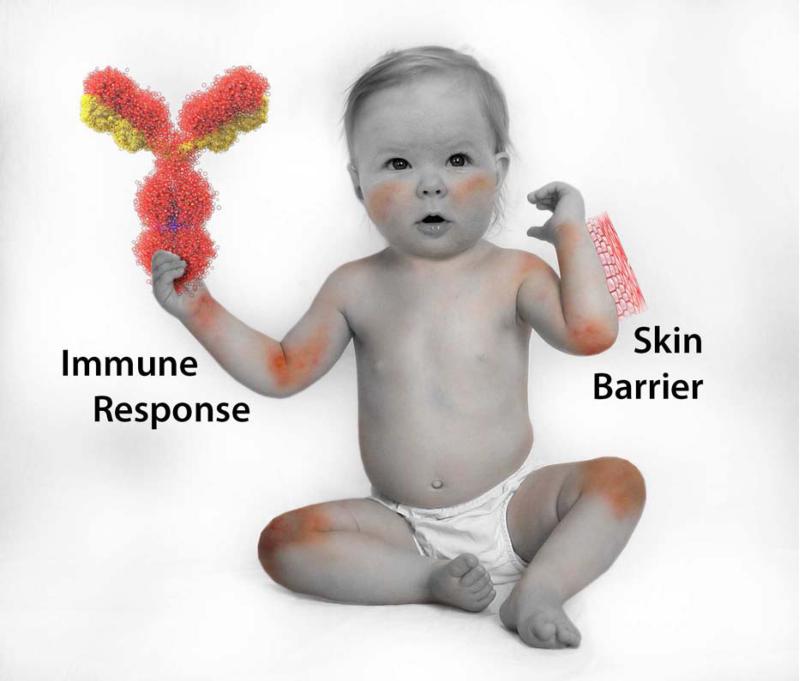
This month's JACI theme focuses particularly on the importance of both genetic and acquired causes of epithelial skin barrier dysfunction in driving the natural history of AD (22-24). Two original articles this month report that early emollient use to protect the skin barrier may prevent AD (25,26). While dermatologists and allergists often debate the relative importance of genetic defects in the skin barrier giving rise to a leaky skin epithelial barrier that allows the penetration of allergens and microbes into AD skin, i.e. the so-called “outside-in hypothesis” as opposed to the “inside-out hypothesis”, i.e. a polarized immune response giving rise to the defective skin barrier, this argument is moot in patients with established AD as both processes are equally important (Figure 1). The majority of AD patients constitute admixtures of genetic defects in their skin barrier and immune responses strongly influenced by environmental factors. The current review will highlight recent insights into the crosstalk between the skin barrier and immune dysfunction leading to AD. Effective prevention and treatment of AD requires a multi-pronged approach involving the maintenance of skin barrier integrity, control of skin inflammation, nutrition, identification and management of allergenic and microbial triggers (27).
Figure 1.
Is it clinically relevant whether skin barrier dysfunction or an immune response occurred first? Once AD is established, the physician needs to address both aspects of AD pathophysiology! However, prevention of AD may require identification of patients with primary defects in barrier vs immune dysfunction. Figure courtesy of Boyd Jacobson, National Jewish Health, Denver, CO.
Complex Causes of Epithelial Skin Barrier Dysfunction in AD
Multi-functional Role of Filaggrin
The robust association of loss of function mutations in the skin barrier gene encoding filaggrin (FLG) with risk of AD has focused attention on the important role of epithelial barrier dysfunction in this skin disease (28, 29). Patients with filaggrin mutations have been found to have dry skin, early onset AD that is more persistent and often associated with asthma, food allergy and microbial infection (30-32). Recent studies suggest that stratification of patients with vs without filaggrin mutations identifies patients with different mechanistic pathways of inflammation (Table 1). Patients with filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in their stratum corneum and type 1 interferon–mediated stress response (33, 34). AD children with normal filaggrin genes have been reported to have dysregulation of lipid metabolic processes (34). Filaggrin dependent secretion of sphingomyelinase has also been found to protect against Staphylococcal alpha-toxin-induced keratinocyte death (35). This strongly suggests that patients with filaggrin mutations are a distinct endotype of AD with different mechanistic outcomes (Table 1), which could be used to identify one subset of AD, particularly for the development of new therapies targeting skin barrier function.
Table 1.
Comparison of clinical and biophysical features of atopic dermatitis patients with (ADFLG) and without (ADNON-FLG) filaggrin mutations.
| Clinical Features | Biophysical Features | |
|---|---|---|
| ADFLG | Palmar Hyperlinearity More Persistent ↑Allergic Sensitization ↑Risk of Asthma ↑Severity of AD ↑Eczema Herpeticum |
Severe Decrease in Natural Moisturizing Factor (NMF) pH IL-1β Type 1 interferon–mediated stress response |
| ADNON-FLG | No Palmar Hyperlinearity Less Persistent Less Allergic Sensitization Lower Risk of Asthma |
Mild Decrease in Natural Moisturizing Factor (NMF) pH Lower Compared to ADFLG IL-1β Low Compared to ADFLG Dysregulation of lipid metabolic processes |
*Modified with permission from: McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol 2013;131:280-91.
Clinical expression of AD is dependent on gene-environment and gene-gene interactions. Gene-environmental interactions is best illustrated in filaggrin deficient mice where exposure of the skin to allergens or microbes predictably leads to the development of eczema (36). Three recent articles in JACI demonstrate that environmental peanut may drive sensitization to peanut allergy in AD patients particularly those with filaggrin mutations is a clinically relevant example of the importance that environmental exposures in house dust may contribute to allergen sensitization in AD (6-8).
The importance of gene-gene interactions is illustrated in Flaky Tail mice, an animal model of AD that spontaneously develops eczema under pathogen-free conditions. These mice carry a double mutation involving the matted (ma) gene giving them a matted hair phenotype as well as a deletion in FLG. It was originally thought that the filaggrin deficiency in Flaky Tail mice explained the propensity of these mice to develop AD. Surprisingly, the derivation of genetically engineered filaggrin-deficient mice that were free of the ma gene mutation, were found to display impaired barrier function but to lack the propensity to spontaneously develop eczema (37, 38). The matted phenotype in flaky tail mice was found to be due to a loss-of-function mutation in the Tmem79 gene. Unexpectedly, the Tmem79 mutation rather than the deletion in FLG was found to be associated with the development of dermatitis in mice. Interestingly, Tmem79 encodes lamellar granules that are required for processing of filaggin, lipids, proteases and antimicrobial peptides (22). Saunders et al (37) has also found that a single nucleotide polymorphism in the human TMEM79 gene confers a significant risk for AD in humans, even when controlling for the effect of FLG mutations, suggesting both genes are involved in AD and the need for gene-gene interactions.
Depending on the population, FLG mutations are found in up to 40% of patients with severe AD, but less than 20% of these severe patients are homozygous or compound heterozygotes for FLG mutations (39). Furthermore, only a minority of European American and Asian, and none of the African American patients with AD have FLG mutations (28,29, 40, 41). Reduction in filaggrin expression are also pronounced in the skin of AD patients who have no detectable FLG null mutations but are most profound when combined with FLG mutations (42). Thus, there are multiple causes for the low FLG expression in the skin. The most common reason is likely to be immune activation (42-44). Intragenic copy number variation within the filaggrin gene also contributes to the risk of AD with a dose-dependent effect (45). The expression of FLG gene expression can also be reduced by epigenetic modification (46).
Skin Barrier Dysfunction- beyond filaggrin
Aside from FLG, AD has been associated with variants in other genes that encode a cluster of proteins in the epidermal differentiation complex located on chromosome 1q21 (47). These include filaggrin-2 (48), hornerin (49) and the cornified envelope precursor, SPRR3 (50). It is noteworthy, however, that unlike FLG, the biologic function of these EDC gene variants as it relates to AD are not well understood. A substantial amount of information, however, indicates that loss of function mutations in serine protease inhibitors (e.g. SPINK5), augments protease activated pathways that enhance Th2 responses supporting the argument that epidermal barrier dysfunction can induce allergic skin diseases (23). This complexity in epidermal gene variants is further modified by variants in genes that control innate and adaptive immune responses (reviewed in references 9 and 51).
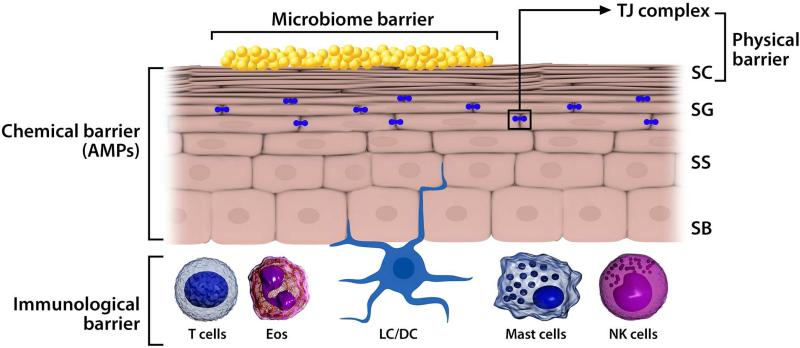
The normal skin can be viewed as containing a series of interrelated barriers whose function is retention of moisture and to repel penetration of the skin with allergens and microbial invasion. Once the stratum corneum is breached, e.g. due to a deficiency of structural proteins such as filaggrin, involucrin, loricrin and/or lipids such as ceramides, other barrier structures are engaged (Figure 2). These includes tight junction proteins, such as the claudins, which are found on opposing membranes of stratum granulosum keratinocytes directly below the stratum corneum and thereby form a second physical barrier in the epidermis (51). Gene profiling in AD epidermis have revealed downregulation of claudin protein and function in AD. Once these 2 physical barriers (filaggrin, tight junctions) are breached, a rapid, innate immune response must be initiated to prevent further microbial invasion. Keratinocytes and antigen presenting cells in the skin express innate pattern recognition receptors such as Toll like receptors (TLRs). Stimulation of TLRs by microbes or tissue injury leads to the release of antimicrobial peptides, and enhanced strength of TJs to limit penetration of allergens and microbes (51). Patients with AD have reduced TLR function.
Figure 2.
The skin as a multi-tiered barrier. The stratum corneum (SC) is the first physical barrier protecting the skin from the environment. Gene mutations, e.g. filaggrin null mutations) or cytokines (e.g. IL-4, Il-13, IL-25, IL-33, etc) downregulating epidermal proteins including filaggrin, leads to allergen or microbial penetration through this barrier. Tight junctions (TJs) found at the level of the stratum granulosum (SG) provide an additional barrier. Disruption of both physical barriers enables the uptake of allergens, irritants, and microbes by Langerhans cells (LC)/DCs. Keratinocytes produce AMPs as a chemical barrier in response to pathogen colonization/infection. The skin surface is colonized with a diverse array of microorganisms (microbiome barrier), which iregulates local immune responses and inhibits pathologic microbes. Infiltration of a number of cells into the AD skin lesion, including T cells, eosinophils (Eos), DCs, NK cells, and mast cells/basophils. Collectively, these cells constitute the cutaneous immunologic barrier. Pattern recognition receptors (PRRs) regulate the function of all of these barriers (physical, chemical, microbiome, and immunologic). SB, Stratum basale; SG, stratum granulosum; SS, stratum spinosum. This figure is modified from Kuo I, et al. J Allergy Clin Immunol 2013;131:266-78.
Loss of skin barrier function and increased severity of AD predisposes to microbial colonization and chronic skin inflammation. This is due to increased expression of tissue receptors for Staphylococcus aureus that leads to colonization of S. aureus in atopic skin (52, 53). Keratinocytes from AD skin have also been found to be deficient in their ability to produce antimicrobial peptides that are needed to control S. aureus and viral replication (54,55). Interestingly commensal bacteria also produce antimicrobial peptides capable of controlling S. aureus growth (56). S. aureus produce high levels of serine proteases that can degrade skin barrier (57). Therefore an overabundance of S. aureus in poorly controlled AD can reduce barrier function via multiple mechanistic pathways.
Immune mediated barrier dysfunction
Although there are strong arguments for the “outside-in” hypothesis suggesting that AD is fundamentally a disease of fixed (genetic) epidermal barrier defects (22, 23), there are equally compelling arguments that some forms of AD are primarily driven by polarized immune pathways that downregulate keratinocyte terminal differentiation thereby creating a secondary skin barrier defect. The arguments against a primary role of the barrier defect in triggering keratinocyte hyperplasia and secondary immune activation include: 1) The FLG mutation is absent in most AD patients (28, 29, 58); 2) The majority of children with AD outgrow their disease even in the presence of a FLG mutation (59); 3) Unlike ichthyosis vulgaris where the entire skin is affected at birth, in the same genetic background AD patients with FLG mutations have both lesional and non-lesional skin, and the disease develops at some later time-point and does not start at birth; 4) Both lesional and non-lesional AD skin exhibit a broad range of differentiation abnormalities beyond filaggrin (loricrin, involcucrin, corneodesmosin, claudins, etc), suggesting reactive epidermal differentiation/cornification alterations (60, 71); 5) treatment of keratinocytes with IL-4, IL-13, IL-22, IL-25 and IL-31 directly downregulate filaggrin expression and increases kallikrein function which can directly cause barrier dysfunction (21, 23, 42-44, 61, 62). IL-22 directly induces keratinocyte hyperplasia, and down-regulation of filaggrin expression (63, 79); 6) mice that are genetically engineered to overexpress Th2 cytokines in their skin spontaneously develop AD and in vivo skin barrier defects (64-67); 7) Filaggrin expression is restored using anti-inflammatory regimens with either topical calcineurin inhibitors or topical corticosteroids (68); 8) Finally, the strongest argument is resolution of clinical AD disease activity in moderate to severe patients with broad based immunosuppressive therapies such as cyclosporine or narrow band UV phototherapy (69, 70), and immune targeted therapeutics (dupilumab), that is coupled with resolution of the abnormal epidermal responses (20).
It is noteworthy that AD skin lesions are always associated with underlying immune activation (71-73). In chronic AD, several underlying features are invariably present: 1) increased skin infiltration by T-cells (approx. 10-fold increase over background T-cell levels in normal skin); 2) increased skin infiltration by myeloid (CD11c+) dendritic cells (DCs) (also approx.10-fold increase over normal skin levels), with most DCs having an “inflammatory phenotype” (BDCA1-/CD11c+) (74); 3) increased production of cytokines and chemokines by activated T-cells and DCs within skin lesions, as measured by quantitative mRNA measures for individual molecules, and by immunohistochemical detection of associated protein products in skin lesions; and 4) reactive epidermal hyperplasia or “regenerative maturation,” showing an unusual hyperplasia response in which mRNAs and proteins of epidermal cornification are highly suppressed in the associated epidermis (21, 75-77).
Immune Pathways Driving AD Skin Lesions
In AD patients with elevated IgE levels, non-lesional AD is associated with a selective expansion of Th2 cells in a dermal perivascular distribution (72). The acute initiation of AD skin lesions is associated with Th2, Th22, and also Th17 cytokine activation (see Figure 3). In parallel, there are epidermal (S100) responses marked by an extremely high increase in the expression of the pro-inflammatory epidermal differentiation complex (EDC) cluster-encoded S100A (S100A7-9) genes, known to be regulated by IL-22 and IL-17 cytokines (78, 79). In chronic AD, intensification of Th2 and Th22 activation occurs with the appearance of a significant Th1 component, but not a complete switch to Th1 (21). Although Th2 cytokine effects remain during chronic AD, the rise in interferon gamma contributes to the inflammatory response and causes keratinocyte apoptosis (80).
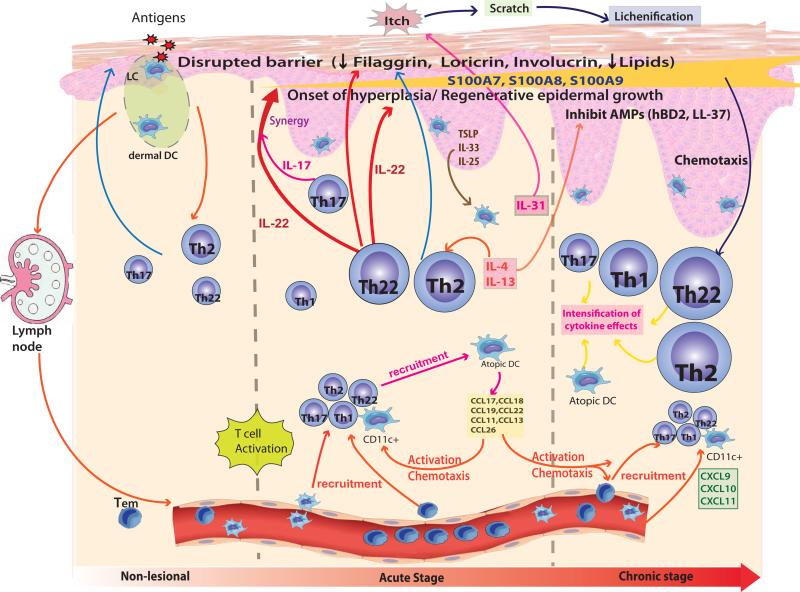
Figure 3.
Immunologic Pathways involved in different phases of Atopic Dermatitis. Nonlesional AD skin lesions contain immune infiltrates that produce cytokines, e.g. IL-4 and IL-13, which contribute to a defective epidermal barrier. Barrier defects lead to penetration by epicutaneous allergens that encounter Langerhans cells in the epidermis and dermal DCs in the dermis to activate TH2 and TH22 cells involved in acute disease onset. Smaller increases in TH1 and TH17 immune axes are also found in acute lesions. A progressive activation of TH2 and TH22, as well as TH1, pathways is characteristic of chronic AD. IL-22 induces epidermal hyperplasia and, synergistically with the TH17 cytokine IL-17, drives an abrupt increase in a subset of terminal differentiation genes, specifically S100A7, S100A8, and S100A9 proteins. The increases in these barrier proteins contrast with the uniformly disrupted epidermal differentiation gene products (eg, filaggrin, loricrin, and corneodesmosin) throughout nonlesional, acute, and chronic AD skin. The TH2 and TH22 cytokines contribute to inhibition of the terminal differentiation proteins. IL-31 is thought to contribute to the itch in acute AD. Updated with permission from Gittler JK, et al. J Allergy Clin Immunol 2012;130:1344-54.
In the last decade, Th2 and Th22 cytokines have been reported to modulate the epidermal barrier, including suppression of keratinocyte differentiation, hyperplasia, apoptosis of keratinocytes, and production of antimicrobial peptides (AMPs) (42-44, 54, 81-84). The cytokine effects include: 1) Suppression of terminal differentiation genes such as filaggrin (FLG), loricrin (LOR) and involucrin (IVL) by Th2 cytokines (IL-4, IL-13, IL-31) and Th22/IL-22 cytokines; 2) Inhibition of AMP production by the Th2 cytokines (IL-4, IL-13); 3) Upregulation of S100As by IL-17 and IL-22 (21, 83-85); and 4) Induction of epidermal hyperplasia by the Th22 IL-22 cytokine (78).
AD disease activity (quantified by SCORAD) has also been shown to positively correlate with lesional and non-lesional skin expression of Th2 and Th22 mediators (i.e IL-13, IL-22), and negatively with expression of terminal differentiation markers (71, 81-84). Although immune activation is significantly higher in lesional than in non-lesional skin, impressive reductions in expression of a broad array of epidermal differentiation genes (i.e., loricrin, periplakin, and involucrin, in addition to filaggrin) characterize both lesional and non-lesional AD skin. Since Th2 (IL-4/IL-13) and Th22 (IL-22) cytokines have been shown to inhibit expression of terminal differentiation products in keratinocytes, increased circulating levels of these cytokines may cause this global suppression of barrier proteins as well as the increases in S100As detected in onset of acute lesions (21).
Implications of AD Pathobiology for General Management Approaches
Patients with established AD, wlll have a combination of skin barrier dysfunction and skin inflammation driving their skin disease. Therefore keys to successful management of AD should include skin hydration and skin barrier repair, topical anti-inflammatory medications (topical corticosteroids or calcineurin inhibitors), control of infection and elimination of exacerbating factors (including allergens, irritants and emotional triggers) that may exacerbate the scratch-itch cycle. Treatment should utilize a stepwise approach that is dependent on the severity of skin disease. The reader is referred to several recent excellent reviews on the management of AD (27, 85-87).
In managing patients with chronic AD, it is important to recognize that gene profiling and immunohistology studies reveal subclinical inflammation and downregulation of terminal epithelial differentiation with reduced skin barrier proteins and increased trans epidermal water loss (TEWL) even in non-lesional AD skin (47, 71, 73, 87, 88). Thus, it is important to maintain skin barrier therapy in the form of emollient therapy even during periods of remission. In AD patients who are prone to frequent relapses, the subclinical inflammation can be managed with intermittent (2 times per week) corticosteroids or alternate day topical calcineurin inhibitors as maintenance therapy (89). For acute AD exacerbations, medium and high-potency corticosteroids can be used short periods of time to control the disease. Oral or systemic corticosteroids should be avoided due to rebound flares when patients are being weaned for oral corticosteroids.
In AD patients who are refractory or do not clinically respond to conventional treatment approaches, a number of alternative strategies may be used including cyclosporine, methotrexate, azathioprine, IL-6 blockade, dust mite immunotherapy when indicated, Wet Wrap therapy and ultraviolet light (90-94). Recent studies with broad based targeting therapeutics (69-70) have used disease-related cellular and molecular biomarkers to: i) show that the chronic AD phenotype can be reversed to a non-lesional state, as has been shown for psoriasis patients treated with effective therapeutics and ii) map inflammatory disease-related pathways. The narrowband (NB) UVB phototherapy and CsA trials showed elimination of the pathologic epidermal hyperplasia (suprabasal K16 expression immunohistochemically and increased K16 and Ki67 mRNA expression) after 12 wks of treatments. The improvement in disease activity as identified by Scoring of AD (SCORAD) and epidermal pathology were highly linked to clearance of excess T-cell and dendritic-cell (DC) infiltrates, as well as decreased expression of inflammatory markers (69-70, 95, 96).
Prevention of Atopic Dermatitis by Early Intervention
Since current treatment approaches are not curative, there is considerable interest in studying approaches to prevent AD (97). The use of probiotic therapy or bacterial lysates early in the course of illness to prevent AD remains an area of active investigation (98-100) but results have been inconsistent. This may be due to lack of standardization of the bacterial preparations, or lack of biomarkers to identify which AD phenotype would benefit from this approach.
The potential contribution of vitamin D deficiency to allergic inflammation, corticosteroid insensitivity and downregulation of innate immune responses has also been an active area of study (101-106). Reproducible well controlled studies of oral vitamin D supplementation are lacking and when done have yielded confusing results. The greatest benefit are likely in populations who have extremely low levels such as people living in upper latitudes during the winter or darkly pigmented individuals. In the current issue of JACI, Camargo and co-workers present results from a randomized, placebo-controlled trial demonstrating that winter related AD can be improved with vitamin D oral supplementation (13).
The importance of AD skin barrier dysfunction in driving allergen sensitization is highlighted by 3 papers in the current issue suggesting that severe AD drives sensitization with environmental peanut (6-8). In 2 of these papers, filaggrin mutations predicted increased association of AD with peanut allergy. These papers suggest the possibility that controlling environmental peanut in the household may reduce peanut allergen sensitization. Alternatively studies are needed to determine whether effective control of AD with barrier therapy and anti-inflammatory treatment to reduce AD skin severity will reduce absorption of environmental allergens and decrease onset of food allergies or respiratory allergy.
Considering the important role that skin barrier dysfunction plays in the initiation of AD, the current issue of JACI contains two different international investigations assessing early intervention with skin emollient therapy to prevent AD and allergic sensitization during infancy (see references 25 and 26). Simpson et al (25) performed a randomized controlled trial of 124 neonates at high-risk for developing AD. Parents in the intervention arm were instructed to apply full-body emollient therapy at least once per day starting within three weeks of birth. Parents in the control arm were asked to use no emollients. The primary outcome was the cumulative incidence of atopic dermatitis at six months. Their results demonstrated a statistically significant protective effect with the use of daily emollient on the cumulative incidence of AD with a relative risk reduction of 50%.
Horimukai et al (26) did a randomized, controlled trial with early moisteurizer intervention conducted in 116 neonatal participants at high familial risk for AD. The primary outcome was the cumulative incidence of AD, as of week 32, as evaluated by a dermatologist. The intervention with the moisturizer significantly lowered (by approximately 40%) the risk of AD compared to the controls (P=0.002) as of week 32. The two groups showed similar rates of allergic sensitization. However, the rate of allergic sensitization of infants with AD, was significantly higher than the rest of infants.
These 2 studies suggest that early intervention with emollient therapy from birth represents a safe and effective approach for AD prevention. If confirmed to be effective in future studies, emollient therapy from birth would be a simple and low-cost intervention that could reduce the global burden of allergic diseases. Whether or not, this form of intervention can prevent The Atopic March is unresolved and may require combination with intermittent anti-inflammatory therapy and environmental control.
Clinical Phenotypes of AD
AD is primarily defined by clinical criteria (107). There is, however, increasing recognition that AD is a complex syndrome with multiple etiologies and mechanistic pathways that clinically can be distinguished by age of onset, severity of illness, racial modifiers, response to therapy, and triggers (including infections, allergens, stress and irritant threshold). Table 2 lists some of the major clinical phenotypes of AD (15,16). These phenotypes often have overlapping features, but contain dominant characteristics that distinguish them from each other (Figure 4). The majority of infants who present with mild AD will outgrow their skin disease in later childhood. However, a group of difficult to manage patients exist who have early onset eczema, with severe life long AD. Adult onset AD has also been increasingly reported although it is unclear whether these may be patients that had eczema during infancy, then went into a prolonged remission only to have relapse of eczema later in life since recall history can be unreliable. Up to 50% but certainly not all AD have associated asthma, allergic rhinitis or food allergy. Identification of genetic and biomarkers of patients likely to undergo the atopic march would allow early intervention for prevention of mucosal allergy including food allergy and asthma.
Table 2.
Clinical Phenotypes of AD
| • Onset in infancy, outgrown in childhood |
| • Onset in infancy, persistent, severe eczema |
| • Adolescent-Adult onset, mild-moderate eczema |
| • Adolescent-Adult onset, persistent, severe eczema |
| • Increased IgE with food or aeroallergen sensitization (Extrinsic) |
| • Non-IgE mediated (Intrinsic) |
| • AD with S. aureus infection/colonization |
| • AD with history of disseminated viral infections e.g. eczema herpeticum |
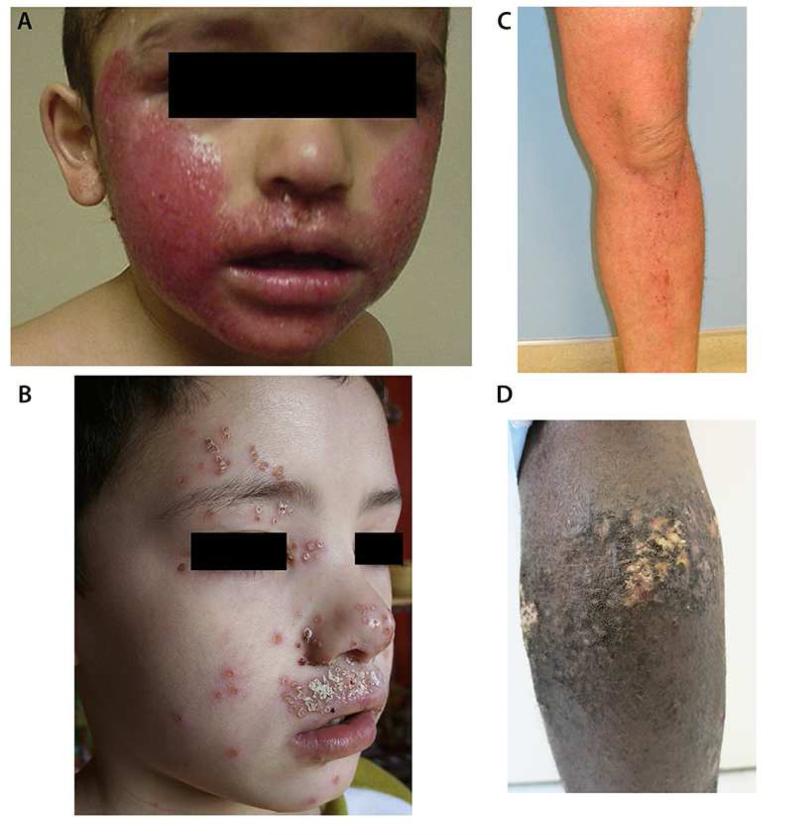
Figure 4.
Clinical Phenotypes in Atopic Dermatitis: Eczema herpeticum (panel A), S. aureus colonized AD (panel B), Mild AD (panel C), Severe AD (panel D). Panels A and B are from: Boguniewicz M and Leung DYM. J Allergy Clin Immunol. 2010; 125: 4-13; Panels C and D are contributed by Dr Emma Guttman- Yassky at The Icahn School of Medicine at Mt Sinai, NYC.
Approximately 80% of AD patients have elevated serum IgE and/or immediate skin test reactivity to allergens but 20% of AD have no IgE to food or inhalant allergens. However, it is possible that such intrinsic or non-atopic patients may have IgE or autoreactive T cells to autoallergens or microbial antigens, which are not routinely measured (108-110). Other AD subsets exist including those who are prone to skin infection such as Staphylococcus aureus skin infections or eczema herpeticum (111-115). Although up to 90% of AD may have problems with S. aureus skin colonization, actual overt skin infections requiring systemic antibiotic treatment affect less than 50%. Less than 3% of AD are predisposed to eczema herpeticum. These different phenotypes likely arise from a complex combination of mutations and epigenetic effects on genes controlling protein expression in the skin barrier, innate and adaptive immune response controlled by environmental influences (9. 116).
Defining Endotypes in Atopic Dermatitis
The importance of eventually defining endotypes in AD is that these new subtypes can be used in clinical study design and drug development to target therapies to patients most likely to benefit from a mechanism-based treatment. In the future, AD may be stratified by genotype, and biomarkers reflecting immune polarization to complement their clinical phenotype. As noted in Table 1, filaggrin genotyping defines AD subsets with different mechanistic pathways. Importantly the severity of AD is related to filaggrin expression with a dose-dependent effect (45).
AD patients with homozygous filaggrin null mutations or compound heterozygotes, as compared to patients with normal filaggrin gene expression, have early onset of skin disease, more persistent, severe eczema. They often have palmar hyperlinearity, greater risk of allergen sensitization, and a history of food allergy and asthma. As well, they have increased pH in their stratum corneum, which may predispose them to S.aureus colonization. Patients who have heterozygous FLG mutations have an intermediate phenotype and can outgrow their AD in adolescence whereas homozygotes or compound heterozygotes can have lifelong disease (59). These patients may also serve as a target for filaggrin therapy (117).
Given the complex genetic milieu of AD, the development of biomarkers is important to assess the final immune polarized pathways that may exist in various AD subsets. The best biomarkers for AD currently define patients who are Th2 polarized vs those who are not. In the future a combination of epidermal proteomics, genomics, gene transcriptomes, blood biomarkers in combination with the clinical phenotype will offer more precision in defining endotypes of AD (118-120). Approximately 80% of AD patients have elevated serum IgE levels, often with increased eosinophilia and serum levels of the Th2 chemokine thymus and activation regulated chemokine (TARC). Additional markers are needed to better monitor patients with so-called intrinsic AD. It is noteworthy, however, that studies of so-called intrinsic AD patients who lacked IgE to conventional inhalant and food allergens did have detectable serum IgE to autoantigens in the skin and microbial antigens from bacterial and fungi that colonize the skin (109, 110). Therefore a wider range of IgE screens to various exogenous and endogenous antigens is warranted to determine potential triggers of AD as it may have an important impact on pathways triggering allergic skin inflammation.
The mechanisms underlying S. aureus and disseminated viral infections in AD is an active area of investigation. These patients are generally very atopic with increased serum IgE, and eosinophilia. This may reflect high level Th2 cytokine pathway activation which is known to reduce skin barrier function, enhance S. aureus skin colonization, reduce antimicrobial peptide (AMP) production and impair innate immune responses. However, since eczema herpeticum is extremely rare and HSV exposure is very common, it is likely that additional immunologic and genetic factors contribute to AD associated with a history of eczema herpeticum (ADEH+). To identify novel gene signatures, in the current issue of JACI, Bin et al (121) used a RNA-sequencing (RNA-seq) approach to evaluate global transcriptional changes in peripheral blood mononuclear cells (PBMCs) from ADEH+ and AD without a history of EH (ADEH-). They found that PBMCs from ADEH+, stimulated with HSV-1, were deficient in their anti-viral immune response involving interferon regulatory factor (IRF) 3 and IRF7 innate immune pathways in ADEH+. This likely contributes to the reduced interferon response in ADEH+ that predisposes to increased susceptibility to disseminated viral infection. Interestingly, DOCK8 deficiency which presents with eczema and recurrent herpetic skin infections has recently been found to respond well to treatment of IFN-alpha 2b (122).
Disease severity appears to be related to the magnitude and polarity of immune activation as well as effects of immune cytokines on epidermal responses (21, 71, 95, 96, 123) (Table 3). Severe AD may thus be associated with systemic immune activation, including significant pathology in non-lesional (NL) skin (71), explaining the frequent need for systemic immune-suppressants and the inadequacy of treating only lesional (LS) skin with topical agents. In contrast, due to minimal systemic involvement, it may be most appropriate to treat mild disease with topical agents directed only to LS sites.
Table 3.
Summary of cytokine effects on epidermis in AD
| • Induce epidermal hyperplasia (IL-22) |
| • Induce spongiosis (Th2 cytokines IL-4/IL-13 and TNF) |
| • Inhibit keratinocyte terminal differentiation (IL-4, IL-13, IL-31, IL-25/Th2, IL-22/Th22, and TNF) with potential for feedback hyperplasia |
| • Inhibit Synthesis of Antimicrobial Peptides (Th2 cytokines IL-4, IL-13, IL-33) |
| • Inhibit Lipid Synthesis (Th2 cytokines IL-4/IL-13, IL-31, and TNF) |
| • Increase expression of S100A7, 8, 9 (IL-22+IL-17) |
| • Induce TSLP production in KCs (IL-4/IL-13, and TNF) |
| • Promote itch (IL-31, TSLP) |
| • Promote anti-viral responses (IFN gamma, IFN alpha, IL-29) |
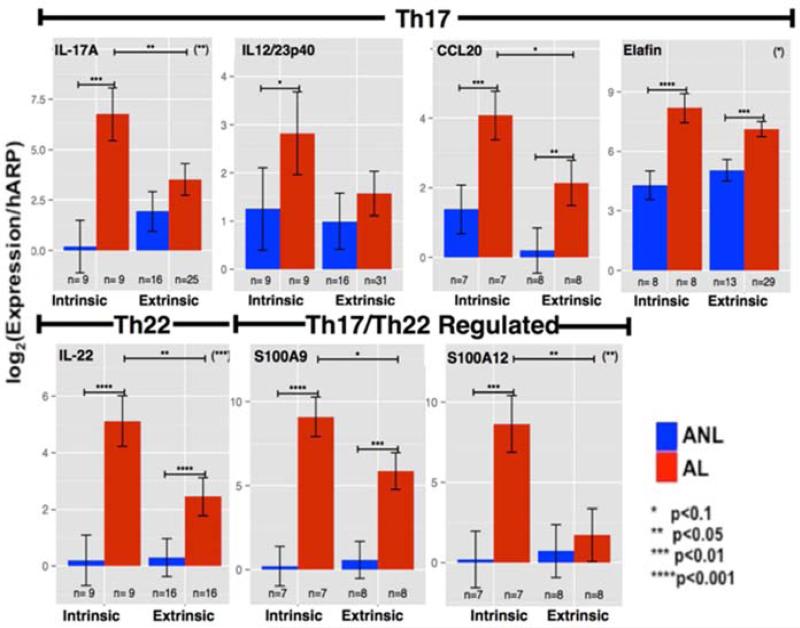
Immune studies suggest that different AD phenotypes are associated with distinct patterns of activation (or suppression) of polar immune axes and corresponding tissue responses. There are distinct differences in cytokine production in patients with intrinsic versus extrinsic AD (123). In intrinsic AD, which has normal IgE levels, there is significantly increased expression of IL-17, IL-23, IL-22 and their respective keratinocyte-induced products (i.e S100As, elafin/PI3, CCL20), suggesting a subset with potential of greater responsiveness to suppression of the IL-17/IL-23/IL-22 axes (Figure 5). Despite, high levels of IgE in extrinsic AD, similar expression levels of Th2-related products were observed in both extrinsic and intrinsic AD, suggesting that these phenotypes might have similar responses to IL-4R antagonism. Indeed, Beck LA and collaborators showed that responses to dupilumab treatment were similar in intrinsic and extrinsic AD patients (20).
Figure 5.
Measures of mRNA levels (normalized to hARP mRNA) for specific Th17/IL-23 and Th22 cytokines and inflammatory products in non-lesional (ANL) and lesional AD (AL) skin from patients with intrinsic versus extrinsic disease. From: Suarez-Farinas M, et al. J Allergy Clin Immunol 2013;132:361-70.
Strong support for the changing pathogenic and therapeutic AD paradigm comes from a recently report that IL-4R alpha antagonist/dupilumab conducted in moderate-to-severe AD patients (20). This study showed major improvement (~70%) in disease activity in the higher dose (compared with only ~20% in the placebo group), coupled with large improvement in the AD genomic phenotype and reversal of the epidermal hyperplasia (as quantified by larger reductions in K16 expression) (20). In fact the reduction in hyperplasia was much higher in the 4-week trial than with 5 mg/kg CsA given for 12 weeks to patients with similar disease activity (95).
Concluding Comments: The translational revolution in AD
AD poses a large unmet need for more effective topical and systemic therapeutics. In addition to Th2 antagonists (i.e anti IL-4R/dupilumab), the key role of TSLP-receptor signaling (124, 125) and IL-22 (126) that clinical trials with agents targeting TSLP, Th22, and Th17/IL-23 will be of interest. Selection of immune targeted therapeutics for patients with different degrees of disease severity or recognized AD phenotypes should not be done by serendipity but should be guided by defining the extent of activation of polar immune circuits in skin and blood (Table 4). For example, anti IL-23/IL-17 might provide beneficial responses in AD, and particularly in intrinsic AD patients. The individual contributions of the Th22, Th17, and Th2 immune pathways to the disease phenotype will be clarified through clinical trials coupled with mechanistic studies that are currently in progress.
Table 4.
Phenotypes of Severity and Treatment Response
| • Pre-clinical: use skin barrier cream for prevention of AD |
| • Chronic AD in remission: use barrier cream in combination with maintenance topical corticosteroid or calcineurin inhibitors to prevent relapse |
| • Relapse of Mild-Moderate AD: use topical corticosteroids or calcineurin inhibitors for control of inflammation, identify and avoid triggers (irritants, allergens, infection), immunotherapy for allergen-driven AD |
| • Persistent Moderate-Severe AD not controlled on topical corticosteroids or calcineurin inhibitors: Wet Wrap Therapy, Allergen Immunotherapy, Non-specific immunosuppressives (cyclosporine, methotrexate, narrow band UV phototherapy, mycophenolate) |
| • Future targeted therapies for Moderate-Severe AD (anti-IL-4 receptor alpha, anti-IL-22, anti-IL-23/IL-17 or other biologics that interrupt polarized immune pathways) |
Acknowledgements
This work was funded in part by The NIH/NIAID Atopic Dermatitis Research Network contract HHSN272201000020C, NIAMS grant AR41256 and the Colorado CTSA/CCTSI grant UL1 RR025780 from NCRR/NIH and UL1 TR000154 from NIH/NCATS. Additionally, DYML wishes to acknowledge The Edelstein Family Foundation for their generous support of his work.
Abbreviations
- AD
Atopic Dermatitis
- ADEH
Atopic Dermatitis with a history of Eczema Herpeticum
- AMP
Anti Microbial Peptides
- CsA
Cyclosporin
- DC
Dendritic Cells
- EDC
Epidermal Differentation Complex
- Eos
Eosinophils
- FLG
Filaggrin
- HOME
Harmonising Outcome Measures for Eczema
- HSV
Herpes Simplex Virus
- IL
Interleukin
- IRF
Interferon Regulatory Factor
- IVL
Involucrin
- LC
Langerhans Cells
- LOR
Loricrin
- LS
Lesional skin
- MA
Matted
- NB
Narrowband
- NL
Non-Lesional
- NMF
Natural Moisturizing Factor
- PBMC
Peripheral Blood Mononuclear Cells
- PRR
Pattern Recognition Receptor
- SC
Stratum Corneum
- SG
Stratum Granulosum
- TARC
Thymus and Activation Regulated Chemokine
- TEWL
Trans Epidermal Water Loss
- TJ
Tight Junction
- TLR
Toll Like Receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013;132:1337–47. doi: 10.1016/j.jaci.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428–33. doi: 10.1016/j.jaci.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boguniewicz M, Leung DYM. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean WH, Palmer CN, Henderson J, Kabesch M, Weidinger S, Irvine AD. Filaggrin variants confer susceptibility to asthma. J Allergy Clin Immunol. 2008;121:1294–5. doi: 10.1016/j.jaci.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Venkataraman D, Soto-Ramirez N, Kurukulaaratcy R, Holloway J, Karmaus W, Ewart S, et al. Loss of function mutations in the filaggrin gene and their association with food allergy: path analysis of direct and indirect effects in a longitudinal cohort. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.07.033. (THIS ISSUE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: impact of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.08.011. Article In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown S, et al. Atopic dermatitis increases the impact of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2014;134 doi: 10.1016/j.jaci.2014.10.007. Article In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes KC. An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010;125:16–29. doi: 10.1016/j.jaci.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roduit C, Frei R, Loss G, Büchele G, Weber J, Depner M, et al. Development of atopic dermatitis according to age of onset and association with early-life exposures. J Allergy Clin Immunol. 2012;130:130–6. doi: 10.1016/j.jaci.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129:1470–7. doi: 10.1016/j.jaci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim EH, Ohm I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–8. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Camargo CA, Jr., Ganma D, Sidbury R, Erdenedelger KH, Radnaakhand N, Khandsuren B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.08.002. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 14.Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.07.043. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 15.Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67:1475–82. doi: 10.1111/all.12049. [DOI] [PubMed] [Google Scholar]
- 17.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–42. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Carolan BJ, Sutherland ER. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J Allergy Clin Immunol. 2013;131:627–34. doi: 10.1016/j.jaci.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. New Engl J Med. 2014;371:130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 21.Gittler J, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQF, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias P. Diverse mechanisms converge on lamellar body secretion, producing the barrier abnormality in atopic dermatitis. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.05.048. (THIS ISSUE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelov L, Sprecher E. Peeling off the genetics of atopic dermatitis-like congenital disorders. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.07.061. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 24.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.06.014. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 25.Simpson E, Chalmers J, Hanifin JM, Thomas KS, Cork MJ, McLean WHI, et al. A randomized trial of emollients to prevent atopic dermatitis - could it really be that simple? J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.08.005. (THIS ISSUE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. A randomized, controlled intervention trial of early emollient use in prevention of atopic dermatitis and allergic sensitization during infancy. J Allergy Clin Immunol. 2014;134:xxxx. (THIS ISSUE) [Google Scholar]
- 27.Schneider L, Lio P, Boguniewicz M, Beck L, LeBovidge J, Novak N, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295–9. doi: 10.1016/j.jaci.2012.12.672. [DOI] [PubMed] [Google Scholar]
- 28.McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013;131:280–91. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- 29.Irvine AD, McLean WHI, Leung DYM. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 30.Margolis DV, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912–7. doi: 10.1016/j.jaci.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böhme M, Söderhöll C, Kull I, Bergström A, van Hage M, Wahlgren C. Filaggrin mutations increase the risk for persistent dry skin and eczema independent of sensitization. J Allergy Clin Immunol. 2012;129:1153–5. doi: 10.1016/j.jaci.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J Allergy Clin Immunol. 2012;129:1538–46. doi: 10.1016/j.jaci.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 33.Kezic S, O'Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–9. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole C, Kroboth K, Schurch NJ, Sandilands A, Sherstnev A, O'Regan GM, et al. Filaggrin-stratified transcriptome analysis of paediatric skin identifies mechanistic pathways in atopic dermatitis. J Allergy Clin Immunol. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brauweiler AM, Bin L, Kim BE, Oyoshi MK, Geha RS, Goleva E, et al. Filaggrin dependent secretion of sphingomyelinase protects against Staphylococcal alpha-toxin-induced keratinocyte death. J Allergy Clin Immunol. 2013;131:421–7. doi: 10.1016/j.jaci.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit Th17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–93. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132:1121–9. doi: 10.1016/j.jaci.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, et al. A homozygous nonsense mutation in the gene for Tmem79, a component for lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013;132:1111–20. doi: 10.1016/j.jaci.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 39.Mohiuddin M, Ramamoorthy P, Reynolds PR, Curran-Everett D, Leung DYM. Increased compound heterozygous filaggrin mutations in severe atopic dermatitis in the United States. J Allergy Clin Immunol-In practice. 2013;1:534–6. doi: 10.1016/j.jaip.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thawer-Esmail F, Jakasa I, Todd G, Wen YR, Brown SJ, Kroboth K, et al. South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss-of-function mutations in filaggrin. J Allergy Clin Immunol. 2014;133:280–82. doi: 10.1016/j.jaci.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrett JPD, Hoffstad O, Apter AJ, Margolis DJ. Racial comparison of filaggrin null mutations in asthmatic patients with atopic dermatitis in a US population. J Allergy Clin Immnunol. 2013;132:1232–4. doi: 10.1016/j.jaci.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BE, Bin L, Ye YM, Ramamoorthy P, Leung DYM. IL-25 Enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with TH2 cytokines to enhance HSV-1 replication. J Invest Dermatol. 2013;133:2678–85. doi: 10.1038/jid.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Lüscher-Firzlaff J, Lüscher B, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2012;129:426–33. doi: 10.1016/j.jaci.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 45.Brown SJ, Kroboth K, Sandilands A, Campbell LE, Pohler E, Kezic S, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol. 2012;132:98–104. doi: 10.1038/jid.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez E, Baurecht H, Wahn AF, Kretschmer A, Hotze M, Zeilinger S, et al. An integrated epigenetic and transcriptomic analysis reveals distinct tissue-specific patterns of DNA methylation associated with atopic dermatitis. J Invest Dermatol. 2014;134:1873–83. doi: 10.1038/jid.2014.87. [DOI] [PubMed] [Google Scholar]
- 47.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Mechin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094–102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 48.Margolis DJ, Gupta J, Apter AJ, Ganguly T, Hoffstad O, Papadopoulos M, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2014;133:784–89. doi: 10.1016/j.jaci.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry J, Hsu CY, Haftek M, Nachat R, de Koning HD, Gardinal-Galera I, et al. Hornerin is a component of the epidermal cornified cell envelopes. Faseb J. 2011;25:1567–76. doi: 10.1096/fj.10-168658. [DOI] [PubMed] [Google Scholar]
- 50.Marenholz I, Rivera VA, Esparza-Gordillo J, Bauerfeind A, Lee-Kirsch MA, Ciechanowicz A, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644–9. doi: 10.1038/jid.2011.90. [DOI] [PubMed] [Google Scholar]
- 51.Kuo I, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266–78. doi: 10.1016/j.jaci.2012.12.1563. [DOI] [PubMed] [Google Scholar]
- 52.Cho S-H, Strickland I, Tomkinson A, Gelfand EW, Leung DYM. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001;116:658–63. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 53.Cho SH, Strickland I, Boguniewicz M, Leung DYM. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol. 2001;108:269–74. doi: 10.1067/mai.2001.117455. [DOI] [PubMed] [Google Scholar]
- 54.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 55.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, Leung DY. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–21. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlievert PM, Strandberg KL, Lin Y-C, Peterson ML, Leung DYM. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010;125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371–9. doi: 10.1016/j.jaip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872–7. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–44. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 61.Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morizane S, Yamasaki K, Kajita A, Ikeda K, Zhan M, Aoyama Y, et al. TH2 cytokines increase kallikrein 7 expression and function in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;130:259–61. doi: 10.1016/j.jaci.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 64.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–83. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 65.Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DYM, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition towards allergic skin inflammation. J Immunol. 2010;184:3186–90. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–52. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:19–28. doi: 10.1016/j.jaci.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Tintle S, Shemer A, Suarez-Farinas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khattri S, Shemer A, Rozenblit M, Dhingra N, Czarnowicki T, Finney R, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626–34. doi: 10.1016/j.jaci.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung DYM, Bhan AK, Schneeberger EE, Geha RS. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol. 1983;71:47–56. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- 73.Hamid Q, Boguniewicz M, Leung DYM. Differential In Situ Cytokine Gene Expression in Acute versus Chronic Atopic Dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novak N. An update on the role of human dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:879–86. doi: 10.1016/j.jaci.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 75.Dhingra N, Suarez-Farinas M, Fuentes-Duculan J, Gittler JK, Shemer A, Raz A, et al. Attenuated neutrophil axis in atopic dermatitis compared to psoriasis reflects T(H)17 pathway differences between these diseases. J Allergy Clin Immunol. 2013;132:498–501. doi: 10.1016/j.jaci.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyman O, Werfel T, Akdis CA. The suppressive role of IL-10 in contact and atopic dermatitis. J Allergy Clin Immunol. 2012;129:160–1. doi: 10.1016/j.jaci.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 77.Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: Implications for contact dermatitis. J Allergy Clin Immunol. 2013;131:300–13. doi: 10.1016/j.jaci.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nukui T, Ehama R, Sakaguchi M, Sonegawa H, Katagiri C, Hibino T, et al. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem. 2008;104:453–64. doi: 10.1002/jcb.21639. [DOI] [PubMed] [Google Scholar]
- 79.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–42. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 81.Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, et al. Mechanisms of IFN-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129:1297–306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 82.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 83.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 85.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 86.Boguniewicz M, Leung DYM. The ABC's of managing patients with severe atopic dermatitis. J Allergy Clin Immunol. 2013;132:511–12. doi: 10.1016/j.jaci.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arkwright PD, Stafford JC, Sharma Atopic dermatitis in children. J Allergy Clin Immunol Pract. 2014;2:388–95. doi: 10.1016/j.jaip.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 88.Tang TS, Bieber T, Williams HC. Are the concepts of induction of remission and treatment of subclinical inflammation in atopic dermatitis clinically useful? J Allergy Clin Immunol. 2014;133:1615–25. doi: 10.1016/j.jaci.2013.12.1079. [DOI] [PubMed] [Google Scholar]
- 89.Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415–28. doi: 10.1111/j.1365-2133.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- 90.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429–38. doi: 10.1016/j.jaci.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 91.Flohr C, Irvine AD. Systemic therapies for severe atopic dermatitis in children and adults. J Allergy Clin Immunol. 2013;132:774. doi: 10.1016/j.jaci.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Nicol NH, Boguniewicz M, Strand M, Klinnert MD. Wet wrap therapy in children with moderate to severe atopic dermatitis in a multidisciplinary treatment program. J Allergy Clin Immunol Pract. 2014;2:400–6. doi: 10.1016/j.jaip.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 93.Novak N, Bieber T, Hoffmann M, Fölster-Holst R, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012;130:925–31. doi: 10.1016/j.jaci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Bae JM, Choi YY, Park CO, Chung KY, Lee K. Efficacy of allergen-specific immunotherapy for atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–7. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 95.Rozenblit M, Suárez-Fariñas M, Shemer A, Khattri S, Gilleaudeau P, Sullivan-Whalen M, et al. Residual genomic profile after cyclosporine may offer insights into atopic dermatitis reoccurrence. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.05.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suarez-Farinas M, Gittler JK, Shemer A, Cardinale I, Krueger JG, Guttman-Yassky E. Residual genomic signature of atopic dermatitis despite clinical resolution with narrowband UVB. J Allergy Clin Immunol. 2013;131:577–9. doi: 10.1016/j.jaci.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 97.Simpson EL, Keck LE, Chalmers JR, Williams HC. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. J Allergy Clin Immunol. 2012;130:137–44. doi: 10.1016/j.jaci.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long term outcomes. J Allergy Clin Immunol. 2012;130:1209–11. doi: 10.1016/j.jaci.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 99.Lau S, Gerhold K, Zimmermann K, Ockeloen CW, Rossberg S, Wagner P, et al. Oral application of bacterial lysate in infancy decreases the risk of atopic dermatitis in children with 1 atopic parent in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:1040–7. doi: 10.1016/j.jaci.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132:601–7. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 101.Muehleisen B, Gallo R. Vitamin D in allergic disease: shedding light on a complex problem. J Allergy Clin Immunol. 2013;131:324–9. doi: 10.1016/j.jaci.2012.12.1562. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Leung DYM, Goleva E. Anti-inflammatory and corticosteroid-enhancing actions of vitamin D in the monocytes of patients with steroid-resistant and those with steroid-sensitive asthma. J Allergy Clin Immunol. 2014;133:1744–52. doi: 10.1016/j.jaci.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132:297–304. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 104.Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014;133:1048–55. doi: 10.1016/j.jaci.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 105.Mohiuddin MS, Curran-Everett D, Leung DYM. Vitamin D and food allergy in patients with severe atopic dermatitis. J Allergy Clin Immunol. 2013;132:1011. doi: 10.1016/j.jaci.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 106.Allen KJ, Koplin J, Ponsonby AL, Vuillermin P, Dharmage S. Vitamin D and food allergy in patients with severe atopic dermatitis. J Allergy Clin Immunol. 2013;132:1011–2. [Google Scholar]
- 107.Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:33–51. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang TS, Bieber T, Williams H. Does “autoreactivity” play a role in eczema? J Allergy Clin Immunol. 2012;129:1209–15. doi: 10.1016/j.jaci.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Novak N, Allam J-P, Bieber T. Allergic hyperreactivity to microbial components: A trigger factor of “intrinsic” atopic dermatitis? J Allergy Clin Immunol. 2003;112:215–6. doi: 10.1067/mai.2003.1590. [DOI] [PubMed] [Google Scholar]
- 110.Hiragun T, Ishii K, Hiragun M, Suzuki H, Kan T, Mihara S, et al. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J Allergy Clin Immunol. 2013;132:608–15. doi: 10.1016/j.jaci.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 111.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bin L, Kim BE, Brauweiler A, Goleva E, Streib J, Ji Y, et al. Staphylococcus aureus a-toxin modulates skin host response to viral infection. J Allergy Clin Immunol. 2012;130:683–91. doi: 10.1016/j.jaci.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-g response. J Allergy Clin Immunol. 2011;127:965–73. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beck LA, Boguniewicz M, Hata TR, Schneider LC, Hanifin JM, Gallo RL, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–9. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041–7. doi: 10.1016/j.jaci.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Rebane A, Runnel T, Aab A, Maslovskaja J, Rückert B, Zimmermann M, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.05.022. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 117.Otsuka A, Doi H, Egawa G, Maekawa A, Fujita T, Nakamizo S, et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J Allergy Clin Immunol. 2014;133:139–46. doi: 10.1016/j.jaci.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 118.Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, et al. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011;127:186–93. doi: 10.1016/j.jaci.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakabe J, Kamiya K, Yamaguchi H, Ikeya S, Suzuki T, Aoshima M, et al. Proteome analysis of stratum corneum from atopic dermatitis patients by hybrid quadrupole-orbitrap mass spectrometer. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.07.054. (THIS ISSUE) [DOI] [PubMed] [Google Scholar]
- 120.Choy DF, Hsu DK, Seshasayee D, Fung MA, Modrusan Z, Martin F, et al. Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J Allergy Clin Immunol. 2012;130:1335–43. doi: 10.1016/j.jaci.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bin L, Edwards MG, Heiser R, Streib J, Richers B, Hall C, et al. Identification of novel gene signatures in atopic dermatitis complicated by eczema herpeticum. J Allergy Clin Immunol. 2014;134:xxxx. doi: 10.1016/j.jaci.2014.07.018. (THIS ISSUE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keles S, Jabara H, Reisli I, McDonald D, Barlan I, Hanna-Wakim R, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: Rescue of severe herpetic infections with IFN-α 2b therapy. J Allergy Clin Immunol. 2014;133:1753–5. doi: 10.1016/j.jaci.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suarez-Farinas M, Dhingra N, Gittler J, Shemer A, Cardinale I, Strong CD, et al. Intrinsic atopic dermatitis shows similar T(H)2 and higher T(H)17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132:361–70. doi: 10.1016/j.jaci.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakajima S, Igyártó BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–55. doi: 10.1016/j.jaci.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Landheer J, Giovannone B, Mattson JD, Tjabringa S, Bruijnzeel-Koomen CAFM, McClanahan T, et al. Epicutaneous application of house dust mite induces thymic stromal lymphopoietin in non-lesional skin of patients with atopic dermatitis. J Allergy Clin Immunol. 2013;132:1252–4. doi: 10.1016/j.jaci.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 126.Teraki Y, Sakurai A, Izaki S. IL-13/IL-22-coproducing T cells, a novel subset, are increased in atopic dermatitis. J Allergy Clin Immunol. 2013;132:971–4. doi: 10.1016/j.jaci.2013.07.029. [DOI] [PubMed] [Google Scholar]