Abstract
Background
Fecal immunochemical tests (FITs) are recommended to screen average-risk adults for colorectal cancer (CRC). Little research has examined whether a two-sample FIT affects participant uptake, compared with a one-sample FIT. Examining participant uptake is important, as evidence suggests that a two-sample FIT may increase the sensitivity to detect CRC.
Objective
This study had two objectives: (i) to evaluate FIT completion in a population that received either a one-sample FIT kit (1-FIT) or a two-sample FIT kit (2-FIT) and (ii) to understand whether uptake varies by age, sex, or receipt of prior CRC screening.
Methods
We conducted a randomized controlled trial in which 3081 participants who were aged between 50 and 75 years and were at an average risk for CRC, and who had requested FITs, randomly received 1-FIT (n=1540) or 2-FIT (n=1541) kits. FIT completion was defined as the completion and return of a one-sample test by the patients in the 1-FIT group or of both sample tests by those in the 2-FIT group. Cox proportional hazard regression models were used to determine the independent effect of group type (2-FIT vs. 1-FIT) on the completion of the FIT, adjusting for age, sex, and receipt of prior CRC screening.
Results
The 2-FIT group had lower test completion rates (hazard ratio=0.87; 95% confidence interval=0.78–0.97; P=0.01) after adjusting for age, sex, and receipt of prior CRC screening. Participant uptake did not vary by age, sex, or receipt of prior CRC screening.
Conclusion
This unique, rigorous randomized controlled trial found that the 2-FIT regimen decreases completion of FIT. Further research is needed to understand whether decreases in participant uptake are offset by increased gains in test sensitivity.
Keywords: colorectal cancer, fecal immunochemical tests, participant uptake
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, with nearly 1.4 million new cases diagnosed in 2012 and with expected growth to an annual incidence of more than 2.4 million cases by 2035. Moreover, ∼700 000 deaths are attributed to CRC each year worldwide (Ferlay et al., 2013).
The early detection of advanced neoplasia or CRC through appropriate screening is associated with decreased incidence of and mortality from CRC (Hewitson et al., 2008; Atkin et al., 2010; Doubeni et al., 2013; Nishihara et al., 2013). Advanced neoplasia is defined as high-risk lesions that have a high likelihood of becoming cancerous (Lieberman et al., 2007). In 2008, the United States Preventive Services Task Force recommended that men and women of average risk begin screening for CRC at the age 50 years (Levin et al., 2008; Whitlock et al., 2008) through fecal testing, colonoscopy, or sigmoidoscopy. In 2010, a large multidisciplinary group supported by the European Commission and the WHO recommended fecal testing and sigmoidoscopy as primary methods of screening in Europe (Von Karsa et al., 2013). Fecal testing and sigmoidoscopy are the primary screening modalities in most European countries (Zavoral et al., 2009; Riemann, 2011), with some countries (Poland, Austria, Germany) favoring colonoscopy for screening average-risk participants.
Randomized controlled trials have shown that annual or biennial guaiac-based fecal blood testing (gFOBT) is associated with a 15–33% reduction in CRC mortality (Mandel et al., 1993; Hewitson et al., 2008). Multiple European and US professional societies have endorsed the use of the fecal immunochemical test (FIT) to replace gFOBT because of FIT’s improved performance characteristics and potential for higher participant uptake rates (Levin et al., 2008; Whitlock et al., 2008). gFOBT detects only about 13–50% of cancers over one round of screening in asymptomatic patients (Lieberman and Weiss, 2001; Imperiale et al., 2004; Park et al., 2010), whereas FIT detects ∼79% of cancers over one round of screening (Lee et al., 2014). In addition, adherence to repeated rounds of gFOBT testing in real-world screening programs is often low (partly because of requisite dietary and medication changes), raising concerns about its effectiveness as a screening test (Fenton et al., 2010; Gellad et al., 2011). Many FITs require only one or two stool samples, and none require dietary or medication restrictions, increasing the ease of use; several studies have demonstrated a 6–16% increase in one-time completion of FIT by participants, compared with gFOBT (Cole et al., 2003; Federici et al., 2005; Van Rossum et al., 2008; Hol et al., 2009; Hoffman et al., 2010; Digby et al., 2013). The improved accuracy and participant uptake of FIT in comparison with gFOBT have led several health organizations to adopt FIT in centralized screening outreach programs (Denters et al., 2009; Parente et al., 2009; Liles et al., 2012; Australian Government Department of Health and Aging, 2013; Cole et al., 2013).
Efforts to optimize the use of FIT focus on improving diagnostic sensitivity without detrimentally affecting test completion. Two studies on FIT screening accuracy (Nakama et al., 1999; Park et al., 2010) have indicated that increasing the number of fecal samples tested from one to two, or from two to three, increases the sensitivity, albeit with a decrease in specificity. Prior small or nonrandomized studies specifically focusing on gFOBT and FIT uptake have indicated that uptake for a two-sample test is either similar to or lower than that for a one-sample test (Cole et al., 2003; Mysliwiec et al., 2008), and that it declines further with an increase from a two-sample to a three-sample test. Factors affecting participant uptake are important both for determining population-level test effectiveness and for tailoring screening outreach efforts.
The objectives of this study, therefore, were to: (i) rigorously evaluate participant uptake of FIT testing in a population that received either a one-sample FIT kit (1-FIT) or a two-sample FIT kit (2-FIT) and (ii) understand how participant uptake of FIT screening (1-FIT vs. 2-FIT) may vary by age, sex, and receipt of prior CRC screening.
Methods
The protocol for this study was approved by the Institutional Review Board of the study Health Maintenance Organization (HMO). The need for individually signed consent forms was waived.
Study setting and data sources
The study was conducted at Kaiser Permanente Northwest, a nonprofit HMO with about 485 000 members in southwest Washington and the Portland, Oregon, metropolitan area. The demographic characteristics (age, sex, race/ethnicity) of the members are similar to those of the population in the area. Kaiser Permanente Northwest regional electronic databases provided data on patient membership, demographics, primary-care assignment, and clinical data (including weight and height, laboratory results, and other healthcare utilization, such as CRC screening). These data capture more than 95% of all medical and pharmacy services that members receive, and data are linked through each member’s health record number.
Participant selection
We included HMO members aged between 50 and 75 years who were at an average risk for CRC and were overdue for screening, and who (i) received an automated telephone call (ATC) as part of a CRC screening outreach campaign and (ii) requested that an FIT kit be sent directly to their home (through mail) at the end of the automated call. The specific details of the automated call, including the definition of ‘average risk’, have been described previously (Mosen et al., 2010). Briefly, the average-risk population includes those who are overdue for screening and do not meet any of the following criteria: (i) have risk factors that would indicate need for nonroutine screening (e.g. presence of inflammatory bowel disease), (ii) have been diagnosed with adenomatous polyps or CRC, (iii) have been referred for colonoscopy or sigmoidoscopy in the previous 3 months, (iv) have received clopidogrel, warfarin, or other anticoagulant medications in the previous 4 months, which may increase the risk for false-positive results on FIT, or (v) have a condition that would make screening inappropriate (e.g. end-stage renal disease, receipt of current hospice care). The brief ATCs included information about the benefits of CRC screening, encouraged the use of FIT as a relatively low-risk method of screening, and allowed patients to request an FIT kit by pressing a number on their telephone (Mosen et al., 2010).
Fecal sampling and fecal immunochemical test analysis
For this study, we used the OC-Micro (Polymedco, Cortland Manor, New York, USA), a latex agglutination FIT with analytical characteristics similar to the discontinued OC-Hemodia. The OC-Auto Micro 80 instrument processed and quantified the FIT results at the manufacturer-recommended concentration cutoff value of 100 ng hemoglobin (Hb)/1 ml of buffer (20 μgHb/g feces) for a positive test result. Invitees received illustrated, English-language instructions on sampling feces from one bowel movement by briefly sweeping the tip of a probe several times though the feces, while the feces was suspended on a paper ‘raft’ (provided) that kept the feces clear of toilet water. After sampling, they were to insert the probe into the collection tube, enter the date of collection on the side of the tube, place the tube in a plastic bag, and insert the bag in a prestamped cardstock envelope that was preaddressed to a central laboratory. Participants also had the option of bringing the completed kit with them to a local laboratory at their own clinic site, although this was not expressly outlined in the instructions. Participants in the 2-FIT group had the same instructions, except for an additional line in the instructions advising them to go through the collection process twice and send both kits in one envelope. No dietary or medication restrictions were recommended.
Study design and randomization
Figure 1 shows the process flow for population selection and randomization. A total of 3971 members received an ATC and requested an FIT between 29 August 2012 and 27 February 2013. Of these 3971 members, we excluded 850; 94% of these members were excluded because they lacked continuous health plan membership in the year before enrollment.
Fig. 1.
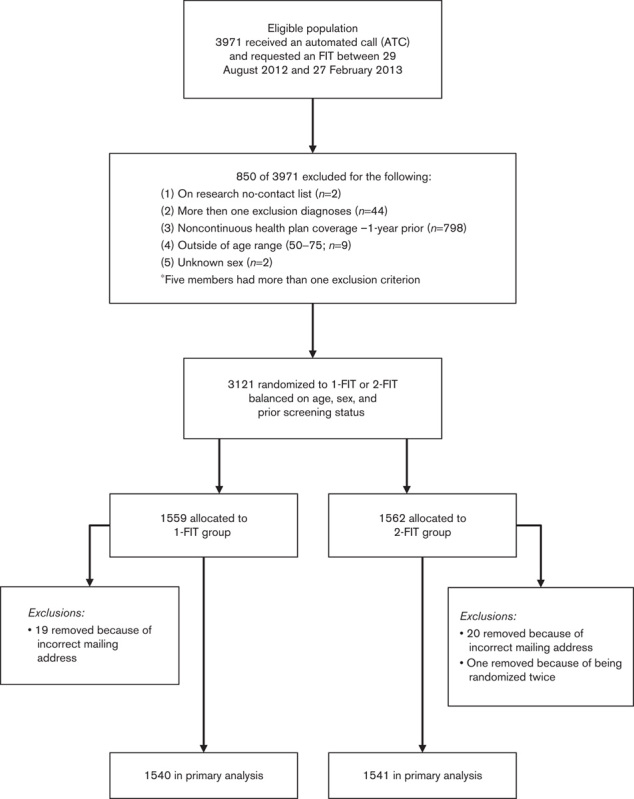
Study design and population selection.
Of the 3121 members eligible for randomization, 1559 were assigned to the 1-FIT group (receiving one FIT kit), whereas 1562 were assigned to the 2-FIT group (receiving two FIT kits). We stratified randomization by age, sex, and history of prior screening. After randomization, but before mailing, 19 members in the 1-FIT group and 20 members in the 2-FIT group were excluded because of an incorrect address. An additional member in 2-FIT group was excluded because of being randomized twice (total excluded=21). Of the remaining population, we mailed FIT kits to the homes of 1540 members of the 1-FIT group and 1541 members of the 2-FIT group.
Study variables
The primary outcome measure was completion of the FIT within 180 days of the index date (mailing date). FIT completion was defined as completion and return of a one-sample test (1-FIT group) or both sample tests (2-FIT group).
The primary independent variable was FIT group (1-FIT vs. 2-FIT). Other explanatory variables included age (<60 and >60 years), sex, and prior screening status (whether the patient had been previously screened by any method). Age was assessed at the index date, before randomization. Prior screening status was assessed through automated extraction from the electronic medical record (EMR) of information on any previously completed CRC screening test (colonoscopy, flexible sigmoidoscopy, or fecal testing), before randomization. Prior screening was assessed for the following time intervals before the index date: colonoscopy, 10 years; sigmoidoscopy, 5 years; and FIT, 1 year.
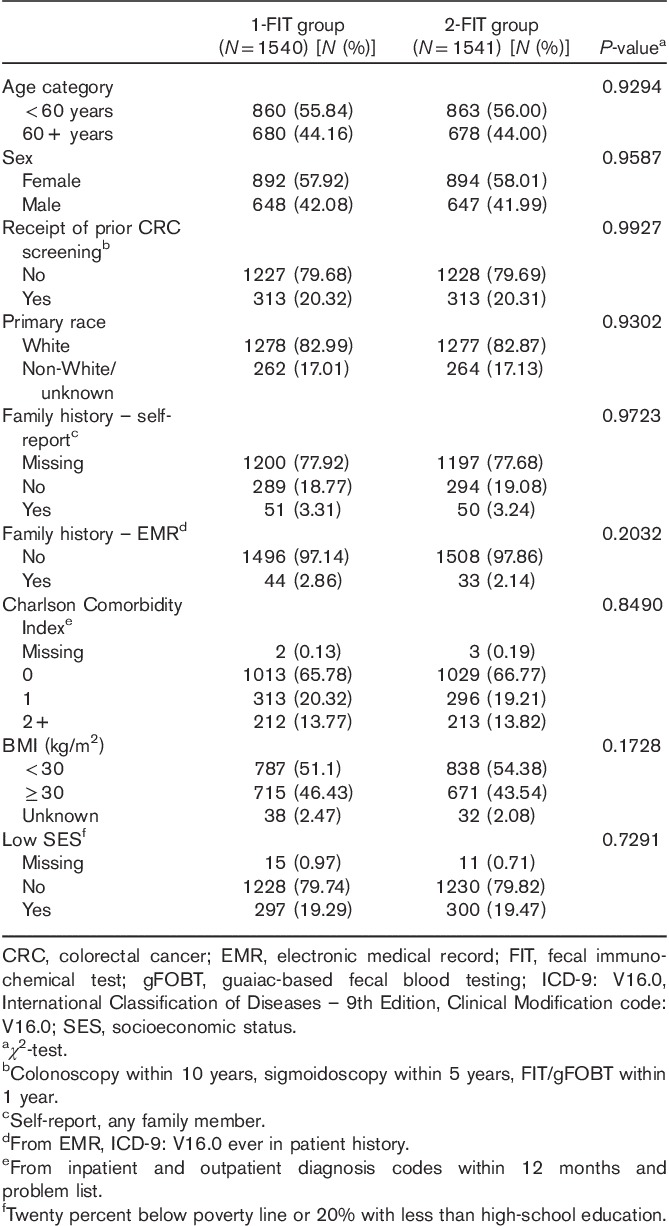
For descriptive purposes, we compared the 1-FIT and 2-FIT groups in terms of race or ethnicity (White, non-White, unknown), family history of CRC, Charlson Comorbidity Index (CCI; Charlson et al., 1987; Deyo et al., 1992; a score of 0, 1, 2+), BMI (kg/m2; <30, >30, and unknown), and a proxy measure for low socioeconomic status (SES; yes, no). Race/ethnicity was available through automated extraction from the EMR (White, non-White, and unknown). We ascertained a family history of CRC using two separate methods: we searched for the presence of a diagnosis code as far back as membership existed for International Classification of Diseases – 9th Edition, Clinical Modification code: V16.0, or for a family history of malignant neoplasm of the gastrointestinal tract. We also asked the patients to return the survey enclosed with the FIT kit(s) they received, which asked about a family history of CRC in any relative.
The CCI is a well-established measure for assessing comorbidity; we extracted all relevant diagnosis codes to inform this index from the EMR, drawing on the prior year of inpatient and outpatient visits and from the permanent problem list. We assessed SES through census block (demographic data are available through the EMR). Low SES was defined as either: (i) more than 20% of the individual’s census block with less than a 12th-grade education, or (ii) more than 20% of the individual’s census block below the federal family poverty level (Kuntz et al., 2012).
Statistical analysis
First, we used χ2-tests to compare the 1-FIT and 2-FIT groups for variables that had not been considered in the stratified randomization, namely, family history of CRC, CCI, race/ethnicity, SES, and BMI.
We used Cox proportional hazard regression models for the primary analysis of time before return of the FIT kit(s), in days, during the 6-month follow-up period. FIT group (1-FIT vs. 2-FIT) was the primary independent variable. In addition, we examined whether test uptake differed on the basis of age, sex, and prior CRC screening by testing interaction terms with group assignment (1-FIT or 2-FIT group). The final Cox regression model included: (i) FIT group [1-FIT (reference group) vs. 2-FIT], (ii) age [<60 years (reference group) vs. >60 years], (iii) sex [male (reference group) vs. female], and (iv) prior CRC screening [no (reference group) vs. yes].
Results
Table 1 presents the baseline characteristics of the 1-FIT and 2-FIT groups. We found no statistically significant differences between the two groups for any of the baseline characteristics. Slightly more than 40% of the participants were 60 years or older. Nearly 60% were women, and more than 80% were White. About 20% in each group had received some prior CRC screening, whereas less than 5% had a family history of CRC, as determined by self-report or EMR. Indicative of a population with low severity of illness, about 65% of the participants in both groups had a CCI score of 0. Finally, more than 40% of the population in both groups were categorized as obese, and about 20% were of low SES.
Table 1.
Baseline population characteristics: 1-FIT versus 2-FIT
Six months after FIT mailing, 43.3% of the 1-FIT group had completed and returned the 1-FIT kit compared with 39.6% (P=0.012) of the 2-FIT group (Table 2). Figure 2 shows the unadjusted time to completion of the FIT kits for both groups, measured in days. In both groups, more kits were completed during the first 30–45 days after mailing, with more 1-FIT kits being completed and returned during the initial period. However, from 46 days until the end of the observation period, the two study group lines remained parallel, suggesting no additional gain with time in patient adherence for the 1-FIT group compared with the 2-FIT group.
Table 2.
FIT completion – 6 months after FIT mailing
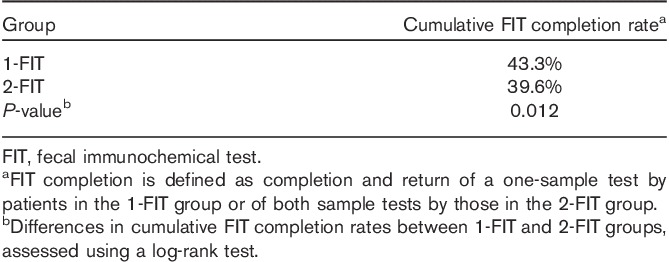
Fig. 2.
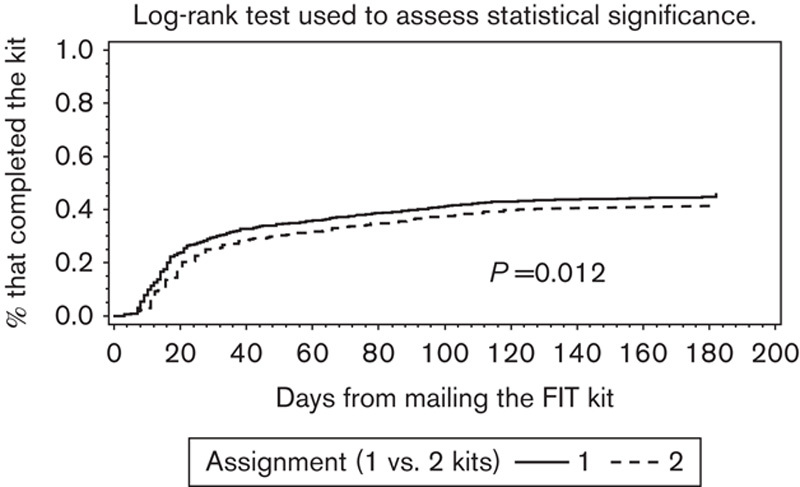
Kaplan–Meier curve: time to completion of the FIT. Log-rank test used to assess statistical significance. FIT, fecal immunochemical test.
Table 3 presents unadjusted and adjusted Cox regression results. Compared with the 1-FIT group, the 2-FIT group was found to be less likely to complete the FIT on both unadjusted [hazard ratio (HR)=0.87; 95% confidence interval (CI)=0.78–0.97; P=0.012] and adjusted (HR=0.87; 95% CI=0.78–0.97; P=0.010) analyses. In addition, there was no significant interaction among group types (2-FIT vs. 1-FIT) by age, sex, or receipt of prior screening (results not shown).
Table 3.
Cox regression results: completion of FIT 6 months after FIT mailinga
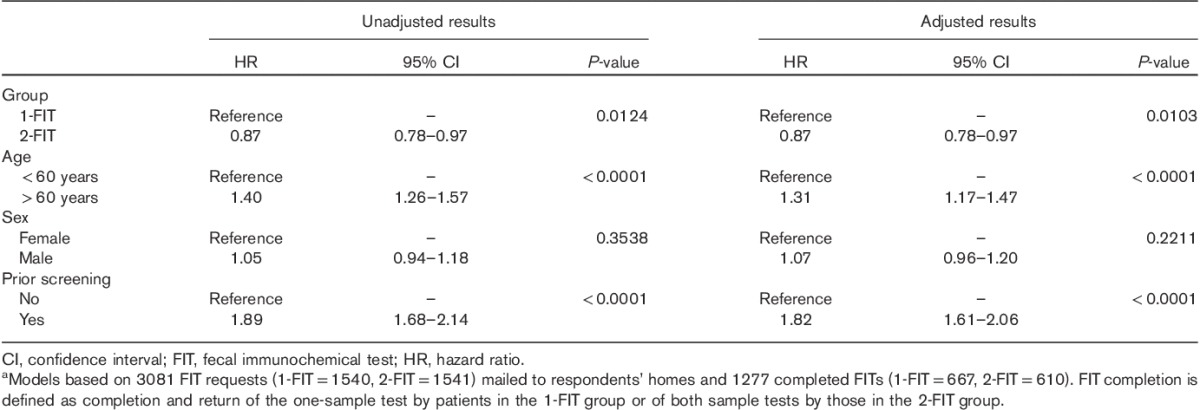
Age and receipt of prior CRC screening were also significantly associated with completion of FIT. Adults aged 60 years or older (vs. <60 years) were found to be more likely to complete the FIT on unadjusted (HR=1.40; 95% CI=1.26–1.57; P<0.001) and adjusted (HR=1.31; 95% CI=1.17–1.47; P<0.001) analyses. Finally, those who had received prior CRC screening (vs. nonreceipt of screening) were found to be more likely to complete the FIT on both unadjusted (HR=1.89; 95% CI=1.68–2.14; P<0.001) and adjusted analyses (HR=1.82; 95% CI=1.61–2.06; P<0.001).
Discussion
This randomized controlled trial found that a two-sample FIT regimen resulted in a relative reduction in test completion (13%; absolute reduction, 3.7%) at 6 months after FIT mailing, compared with the one-sample FIT regimen, in a population of adults aged between 50 and 75 years at an average risk for CRC. We found this difference in test completion rates even after adjusting for age, sex, and prior screening. Older age and receipt of prior CRC screening were associated with FIT completion in both groups, a finding consistent with previous research (Mosen et al., 2010). However, there was no interaction between adherence and age, sex, or receipt of prior screening; lower adherence in the 2-FIT group was consistent across these subgroups.
The current study design was able to isolate the effect of requiring a second fecal sample on uptake of a mailed FIT screening program. A previous small randomized adherence trial (Cole et al., 2003) comparing participant uptake among three groups, two-sample FIT, three-sample FIT, and three-sample gFOBT, used two different FITs with unique packaging and sampling techniques. They found increased uptake of the two-sample FIT (39.6%) as compared with the three-sample FIT (23.4%), but it was unclear how the differences in test instructions and sampling techniques among brands may have affected completion. A prior nonrandomized study of mailed FITs by Mysliwiec et al. (2008) that found little difference in test completion between the 1-FIT and 2-FIT groups (1-FIT=43.5%, 2-FIT=42%) had also compared two separate brands of FIT.
Our findings indicate that there is a 3.7% absolute decrease in uptake of the OC-Micro FIT when a second sample is required. This difference may be offset by the added sensitivity afforded by the second sample. A prior study (N=770) on OC-Micro in an asymptomatic population found a 14–15% increase in the sensitivity for CRC detection with the addition of a second sample (Park et al., 2010). A prior study on OC-Micro in a high-risk (symptomatic) population found an 8–14% increase in the sensitivity for detection of advanced adenomas with the addition of a second sample (Rozen et al., 2009). Although Magstream, another quantitative FIT that uses a similar sampling technique (Morikawa et al., 2005; Launoy et al., 2005), would likely have a similar reduction in uptake with the addition of a second sample, no published studies have evaluated this. However, studies do suggest that lowering the cutoff concentration of hemoglobin for a single-sample test (which is a capability of quantitative FITs) may achieve similar gains in sensitivity – 8–13% for OC-Micro (Park et al., 2010; De Wijkerslooth et al., 2012) and 8–25% for Magstream (Launoy et al., 2005) – with mild decreases in specificity. The use of OC-Micro in key studies makes optimization of FIT performance important. The two ongoing randomized trials comparing the effectiveness of FIT and colonoscopy use a single-sample version of OC-Micro, although with different cutoff concentrations (15 μgHb/g feces and 20 μgHb/g feces; international units; Fraser et al., 2012) for signifying a positive test. The results of these studies are likely to impact recommendations on the relative effectiveness of primary screening with both FIT and colonoscopy.
Although we found a statistically significant difference in test completion rates between one-sample FIT and two-sample FIT, the 3.7% absolute difference could potentially be offset by investment of system resources to increase participant test completion. Our study did not utilize reminder calls or letters after the initial automated phone call to prompt participants to complete the FIT that they had received. Prior studies have demonstrated that repeated use of automated phone calls (Mosen et al., 2010; Hendren et al., 2014) and tailored navigation (Myers et al., 2007, 2008; Green et al., 2013) increase completion rates in CRC screening, with tailored navigation producing the highest completion rates (Myers et al., 2007, 2008; Green et al., 2013). In addition, although we did find lower participant uptake in the 2-FIT group, 2.2% (n=34) of the 2-FIT group completed one of the two sampling procedures in the FIT kit, suggesting an interest in test completion. If this population had completed both FIT sampling procedures, the differences in participant uptake between the 1-FIT and 2-FIT groups would have been reduced (43.3 vs. 41.8%). Further outreach and education efforts (e.g. tailored navigation) may reduce differences in 1-FIT and 2-FIT uptake. Further research (including that underway by the authors) will better illuminate the relative effects of sample number and cutoff concentration of hemoglobin on the effectiveness (and cost-effectiveness) of this and similar FITs. Cost-effectiveness research is important as only a few rigorous studies have been completed (Goede et al., 2013).
This study has several limitations. First, the findings may not be generalizable beyond a group-model HMO setting. However, notwithstanding this limitation, study results are likely applicable to other delivery systems, given that the mailing of FIT kits is a simple direct-to-participant intervention. Second, the study included few racial or ethnic minorities, limiting our ability to determine whether the main study findings would be similar in more diverse populations.
Conclusion
This randomized controlled trial found slightly lower completion rates of FIT among those assigned two-sample FIT kits, compared with those assigned one-sample FIT kits, after adjusting for age, sex, and receipt of prior CRC screening. Indicative of a nonsignificant interaction effect, lower participant uptake in the 2-FIT group did not vary by age, sex, or receipt of prior CRC screening.
Acknowledgements
The authors thank the following individuals, whose contributions made this research possible: Lucy Fulton, Matthew Hornbrook, Heather Block, and Andre Smith. This project was supported by grant #R01CA154982-04 from the National Cancer Institute.
Conflicts of interest
There are no conflicts of interest.
References
- Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. (2010). UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375:1624–1633 [DOI] [PubMed] [Google Scholar]
- Australian Government Department of Health and Aging. 2013National Bowel Cancer Screening Program
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383 [DOI] [PubMed] [Google Scholar]
- Cole SR, Young GP, Esterman A, Cadd B, Morcom J. (2003). A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen 10:117–122 [DOI] [PubMed] [Google Scholar]
- Cole SR, Tucker GR, Osborne JM, Byrne SE, Bampton PA, Fraser RJ, Young GP. (2013). Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust 198:327–330 [DOI] [PubMed] [Google Scholar]
- De Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, et al. (2012). Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 107:1570–1578 [DOI] [PubMed] [Google Scholar]
- Denters MJ, Deutekom M, Fockens P, Bossuyt PM, Dekker E. (2009). Implementation of population screening for colorectal cancer by repeated fecal occult blood test in the Netherlands. BMC Gastroenterol 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619 [DOI] [PubMed] [Google Scholar]
- Digby J, McDonald PJ, Strachan JA, Libby G, Steele RJ, Fraser CG. (2013). Use of a faecal immunochemical test narrows current gaps in uptake for sex, age and deprivation in a bowel cancer screening programme. J Med Screen 20:80–85 [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Weinmann S, Adams K, Kamineni A, Buist DS, Ash AS, et al. (2013). Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med 158:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici A, Giorgi Rossi P, Borgia P, Bartolozzi F, Farchi S, Gausticchi G. (2005). The immunochemical faecal occult blood test leads to higher compliance than the guaiac for colorectal cancer screening programmes: a cluster randomized controlled trial. J Med Screen 12:83–88 [DOI] [PubMed] [Google Scholar]
- Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. (2010). Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med 8:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available at: http://globocan.iarc.fr.
- Fraser CG, Allison JE, Halloran SP, Young GP. Expert Working Group on Fecal Immunochemical Tests for Hemoglobin, Colorectal Cancer Screening Committee, World Endoscopy Organization. (2012). A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst 104:810–814 [DOI] [PubMed] [Google Scholar]
- Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, Yancy WS., Jr (2011). Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol 106:1125–1134 [DOI] [PubMed] [Google Scholar]
- Goede SL, van Roon AH, Reijerink JC, van Vuuren AJ, Lansdorp-Vogelaar I, Habbema JD, et al. (2013). Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 62:727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. (2013). An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med 158:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren S, Winters P, Humiston S, Idris A, Li SX, Ford P, et al. (2014). Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med 29:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. (2008). Am J Gastroenterol 103:1541–1549 [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Steel S, Yee EF, Massie L, Schrader RM, Murata GH. (2010). Colorectal cancer screening adherence is higher with fecal immunochemical tests than guaiac-based fecal occult blood tests: a randomized, controlled trial. Prev Med 50:297–299 [DOI] [PubMed] [Google Scholar]
- Hol L, Wilschut JA, van Ballegooijen M, van Vuuren AJ, van der Valk H, Reijerink JC, et al. (2009). Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer 100:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. (2004). Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 351:2704–2714 [DOI] [PubMed] [Google Scholar]
- Kuntz J, Johnson E, Raebel M, Petrik A, Yang X, Thorp M, et al. (2012). Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infect Control Hosp Epidemiol 33:1031–1038 [DOI] [PubMed] [Google Scholar]
- Launoy GD, Bertrand HJ, Berchi C, Talbourdet VY, Guizard AV, Bouvier VM, Caces ER. (2005). Evaluation of an immunochemical fecal occult blood test with automated reading in screening for colorectal cancer in a general average-risk population. Int J Cancer 115:493–496 [DOI] [PubMed] [Google Scholar]
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. (2014). Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 160:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. (2008). Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 58:130–160 [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Weiss DG. (2001). Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 345:555–560 [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, et al. (2007). Five-year colon surveillance after screening colonoscopy. Gastroenterology 133:1077–1085 [DOI] [PubMed] [Google Scholar]
- Liles EG, Perrin N, Rosales AG, Feldstein AC, Smith DH, Mosen DM, Schneider JL. (2012). Change to FIT increased CRC screening rates: evaluation of a US screening outreach program. Am J Manag Care 18:588–595 [PMC free article] [PubMed] [Google Scholar]
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. (1993). Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 328:1365–1371 [DOI] [PubMed] [Google Scholar]
- Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. (2005). A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 129:422–428 [DOI] [PubMed] [Google Scholar]
- Mosen DM, Feldstein AC, Perrin N, Rosales AG, Smith DH, Liles EG, et al. (2010). Automated telephone calls improved completion of fecal occult blood testing. Med Care 48:604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. (2007). A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer 110:2083–2091 [DOI] [PubMed] [Google Scholar]
- Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Cocroft J, et al. (2008). Tailored navigation in colorectal cancer screening. Med Care 46:S123–S131 [DOI] [PubMed] [Google Scholar]
- Mysliwiec P, Courteau S, Zhao W, Chung E, Maurer D, Levin T. (2008). T1110, a colorectal cancer screening outreach using fecal immunochemical tests. Gastroenterology 134:A-485–A-486 [Google Scholar]
- Nakama H, Yamamoto M, Kamijo N, Li T, Wei N, Fattah AS, Zhang B. (1999). Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterology 46:228–231 [PubMed] [Google Scholar]
- Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. (2013). Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 369:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente F, Marino B, DeVecchi N, Moretti R, Ucci G, et al. (2009). Lecco Colorectal Cancer Screening Group. Faecal occult blood test-based screening programme with high compliance for colonoscopy has a strong clinical impact on colorectal cancer. Br J Surg 96:533–540 [DOI] [PubMed] [Google Scholar]
- Park DI, Ryu S, Kim YH, Lee SH, Lee CK, Eun CS, Han DS. (2010). Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 105:2017–2025 [DOI] [PubMed] [Google Scholar]
- Riemann JF. (2011). Colonoscopy screening: status in Europe. Dig Dis 29Suppl 153–55 [DOI] [PubMed] [Google Scholar]
- Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, et al. (2009). Quantitative colonoscopic evaluation of relative efficiencies of an immunochemical faecal occult blood test and a sensitive guaiac test for detecting significant colorectal neoplasms. Aliment Pharmacol Ther 29:450–457 [DOI] [PubMed] [Google Scholar]
- Van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, et al. (2008). Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 135:82–90 [DOI] [PubMed] [Google Scholar]
- Von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp-Vogelaar I, et al. (2013). European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 45:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. (2008). Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 149:638–658 [DOI] [PubMed] [Google Scholar]
- Zavoral M, Suchanek S, Zavada F, Dusek L, Muzik J, Seifert B, Fric P. (2009). Colorectal cancer screening in Europe. World J Gastroenterol 15:5907–5915 [DOI] [PMC free article] [PubMed] [Google Scholar]


