Abstract
Oocytes of nonhuman primates such as rhesus monkeys are excellent models for diverse studies on developmental biology, epigenetics, human reproduction and assisted reproductive technologies, as well as on transgenics. Such studies require numerous oocytes that can be retrieved after controlled ovarian stimulation. Currently, most primate centers use laparoscopic aspiration or laparotomy followed by aspiration to collect rhesus oocytes although the ultrasound-guided needle aspiration is more advantageous due to reduced infection risk, less injury, and a shorter recovery period. Yet, some initial difficulties associated with the ultrasound-guided needle aspiration limit its broader application. The objective of the present study was to address these obstacles. By presenting practical solutions to the initial difficulties, results from our study show that it is possible to collect a mean number of 38±10 rhesus oocytes per hormonally stimulated female. These results compare favorably to the average number of rhesus oocytes collected using the laparoscopic approach and suggest that when initial obstacles are overcome, the ultrasound-guided oocyte retrieval represents a good alternative to more invasive approaches.
Keywords: Rhesus monkey, Macaca mulatta, Ultrasound, Oocyte, Retrieval
INTRODUCTION
As the closest phylogenetic relatives to humans, nonhuman primates such as rhesus monkeys (Macaca mulatta) are extremely useful research models for human development, a variety of human diseases, as well as for human assisted reproductive technologies (ARTs) (Barratt-Boyes et al. 2006; Brenner et al. 2004; Hendrickx and Peterson 1997; Makori et al. 2001; Sutovsky et al. 1996). The ARTs are in turn important for conservation of endangered nonhuman primate species and efficient breeding of transgenic lines. For the success of the ARTs, it is critical to harvest an adequate number of developmentally competent oocytes after hormonal stimulation of follicle growth. Typically, the oocytes are harvested shortly before ovulation using one of three methods: (i) aspiration by laparotomy (Wolf et al. 1996); (ii) laparoscopic aspiration (Hewitson et al. 1998; Wolf et al. 1996; Yang et al. 2007); and (iii) ultrasound-guided aspiration (Cseh et al. 2002; Schuler et al. 2007; VandeVoort et al. 2003; VandeVoort and Tarantal 1991).
The laparotomy approach requires a ventral midline incision to directly visualize the ovaries. Although laparotomy has the advantage of direct access to the ovaries, it poses a risk of infection due to a relatively large incision and may lead to post-operative pain and extended care. Further, laparotomy is considered major surgery by institutional animal care committees, and this limits the number of procedures that may be done on each animal. The laparoscopic oocyte retrieval consists of standard laparoscopy and needle aspiration of the ovarian surface follicles. Compared to laparotomy, the laparoscopic technique is less invasive due to a smaller incision on the abdominal wall, and thus has a reduced infection risk and a shorter recovery period. Also, laparoscopy is not considered as major surgery and multiple procedures are usually allowed. However, the laparoscopic method is a tedious and time consuming procedure due to the complicated experimental setup (Bavister 2004; Flood et al. 1989). Furthermore, laparoscopic oocyte retrieval is only possible when the ovarian cortex is partly exposed and follicles are visible under the surface (Wiseman et al. 1989). The ultrasound-guided follicle aspiration procedure represents a better alternative to the other two methods in terms of reduced invasiveness, infection risk and other potential detrimental effects, work involved, and post-operative recovery (Flood et al. 1989; Seifer et al. 1988; Wiseman et al. 1989). The ultrasound-guided follicle aspiration was first introduced in 1981 (Lenz et al. 1981) as a trans-abdominal procedure for retrieval of human oocytes. In 1987, trans-vaginal ultrasound-guided egg aspiration was introduced (Wikland et al. 1987) and became the method of choice for the retrieval of human oocytes in the meantime. In contrast to human fertility clinics, ultrasound-guided oocyte aspiration technique is practiced only in a few primate centers around the world despite its many advantages. This is probably due to initial difficulties associated with ultrasound-guided aspiration such as acquiring technical competence with ultrasonography and assembling an appropriate needle aspiration devise. A wider application of this advantageous method to nonhuman primates requires addressing the associated difficulties.
The objective of the present study was to determine common obstacles associated with ultrasound-guided oocyte aspiration and then to develop solutions that will make possible a wider use of this less invasive technique.
RESULTS
A total of 11 egg retrieval attempts were carried out in three experimental groups after hormonal stimulation of 7 rhesus females as a part of our ongoing oocyte cryopreservation studies. The experimental groups were designed to compare combinations of different custom-made aspiration assemblies and ultrasound systems (see Materials and Methods for further details). In group 1, a non-echogenic coated aspiration needle assembly was combined with a low resolution ultrasound system while group 2 had a combination of an echogenic-coated needle assembly with the low resolution ultrasound system. In group 3, the echogenic-coated needle assembly was combined with a high resolution ultrasound. The combination of a high resolution ultrasound system with a non-coated needle as an additional group was not tested due to associated high costs. Two of the females used in the present study had ovaries that were dislocated to a deeper position in the abdominal cavity probably due to previous surgeries. However, we did not have a complete history of these rhesus females for definitive confirmation. Further, we did not have any information whether these two females were previously stimulated and used for oocyte retrieval. Unlike other rhesus females, these two females with dislocated ovaries did not properly respond to hormonal stimulations. Nevertheless, we assigned these females to group 2 and 3, and were able to retrieve 6 to 10 oocytes from each female. The other rhesus females responded well to the hormonal stimulation and displayed at least 5 follicles with a diameter ≥ 3 mm before hCG injection (Fig. 2 and 3). The number of oocytes retrieved in each group and their meiotic status are summarized in Table 1. We were not able to retrieve any oocyte when we used a non-echogenic coated aspiration needle and a low resolution ultrasound system (group 1). In contrast, we retrieved an acceptable number of oocytes (18±11 per retrieval per female) when we combined an echogenic-coated needle assembly with the low resolution ultrasound (group 2). An additional dramatic increase in the mean number of retrieved oocytes (38±10 per retrieval per female) was obtained when we combined the echogenic-coated needle assembly with the high resolution ultrasound (group 3). The differences between group 1 and group 3 were significant with respect to the mean number of retrieved oocytes in all categories. The mean number of GV oocytes per retrieval in group 3 was also significantly higher than that in group 2. Otherwise, there was no statistically significant difference between groups 2 and group 3, as well as between groups 1 and group 2, probably due to relatively small number of retrievals in each group.
Figure 2.
Abdominal ultrasound of rhesus monkey ovaries before aspiration. Arrows indicate left and right ovaries with follicles. The size of ovaries is about 15.5 mm in diameter.
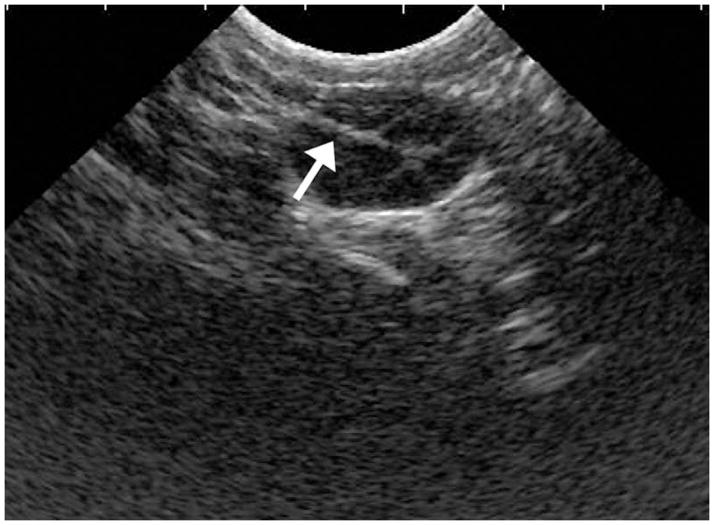
Figure 3.
Abdominal ultrasound image of rhesus monkey ovary during follicular aspiration. Arrow indicates the echogenic tip of aspiration needle.
Table 1.
Number of oocytes collected with different aspiration assemblies and ultrasound systems (mean±sem).
| Group | No. of Retrievals | Ultrasound System (US) and Aspiration Assembly | No. of oocytes per retrieval | Meiotic status of oocytes per retrieval | ||
|---|---|---|---|---|---|---|
| MII | MI | GV | ||||
| 1 | 3 | Low resolution US with non-coated needle | 0 a | 0 a | 0 | 0 a |
| 2 | 4 | Low resolution US with echogenic-coated needle | 18±11 a,b | 12±5 a,b (64%) | 2±3 (15%) | 4±3 a (21%) |
| 3 | 4 | High resolution US with echogenic-coated needle | 38±10 b | 21±7 b (54%) | 2±2 (6%) | 15±3 b (40%) |
Different superscripts within each column indicate statistically significant differences (p < 0.05)
DISCUSSION
Oocytes from nonhuman primates such as rhesus monkeys represent invaluable models for developmental biology, epigenetics, human ARTs, as well as for generation of genetically manipulated mutants to address a variety of human diseases. Such studies require collection of numerous oocytes, which can be accomplished using ultrasound-guided oocyte retrieval. Compared to laparotomic and laparoscopic techniques, the ultrasound-guided oocyte retrieval offers several advantages such as reduced infection risk and injury, a shorter recovery period, and less labor- and time consumption. However, its adaptation is associated with some obstacles that limit its broader use. The findings from the present study reveal common obstacles associated with the ultrasound-guided oocyte retrieval and present practical solutions to further facilitate the adaptation of this advantageous oocyte retrieval technique in more primate centers.
Currently, the laparoscopic approach is the method of choice for retrieval of rhesus oocytes (Bavister 2004). Using this approach, 18 to 38 oocytes can be retrieved from a hormonally stimulated rhesus female on average (Wolf et al. 2004; Zelinski-Wooten et al. 1997). In the present study, we were able to collect a comparable number (38±10) of oocytes using ultrasound-guided retrieval. Our results are also consistent with those previously obtained using ultrasound-guided retrieval (VandeVoort et al. 2003; VandeVoort and Tarantal 1991). Taken together, these results suggest that the ultrasound-guided retrieval of rhesus oocytes is a good alternative to the laparoscopic approach.
During the course of the present study, we found that several factors affect the outcome of ultrasound-guided follicle aspiration. These include the size, beveling angle, and ultrasonic visibility of the aspiration needle, image quality of ultrasound system, design of the aspiration assembly, obstruction of the aspiration assembly by blood coagulation and ultrasound gel, aspiration pressure, and the location of ovaries. Our results suggest that the features of the aspiration needle dramatically influence the success of ultrasound-guided egg retrievals. Initially, we tried a single-lumen human oocyte aspiration needle to retrieve rhesus oocytes because it was commercially available with appropriately fitted tubing and such a human oocyte aspiration assembly was successfully used for ultrasound-guided retrieval of baboon oocytes by other investigators (Cseh et al. 2002). However, we found that commercially available human oocyte aspiration needles are inappropriate for retrieval of rhesus oocytes for several reasons. Since they are designed for trans-vaginal oocyte aspiration by taking human anatomy and larger follicle size (17 to 25 mm) into account, such needles are long (~ 40 cm including the hub) and have a large diameter (16 to 17-gauge), long tip opening (5 mm) due to small beveling angle, and long tubing (60 cm) between the hub and stopper. Consequently, they are too large for the majority of rhesus follicles, which have a diameter ranging from3 to 5 mm before aspiration. Furthermore, the extra length of the needle and tubing represents a large dead volume for small amounts of rhesus follicle fluid/content. This means that aspirated rhesus oocytes will remain for a long period within the aspiration needle and tubing before arriving into the collection tube containing warm medium with heparin (Fig. 1). This also increases the possibility for the coagulation of blood, and thereby obstruction of the aspiration assembly as discussed later. For these reasons and based upon our initial experience with the human aspiration needles, we decided to build our own aspiration assembly specific for rhesus oocyte retrieval. To this end, we used an echogenic-coated needle and a non-coated needle and reduced the dead volume by decreasing the length of the needles and tubing (11.5 cm and 15 cm, respectively). As shown in Table 1, we were able to retrieve a large number of oocytes using echogenic polymer-coated aspiration needles, which we were not able to accomplish with the use of non-coated needles and the low resolution ultrasound. These results suggest that in addition to its appropriate size and shape, an aspiration needle should have good visualization by ultrasound. This is particularly important for an operator having limited experience with the rhesus model. Alternatively, the ultrasound’s resolution should be good enough to visualize a non-coated aspiration needle for successful oocyte retrieval. Indeed, this has been demonstrated in an earlier study where investigators retrieved 34 oocytes on average(VandeVoort et al. 2003). In the present study, we have not tested the combination of a high resolution ultrasound system with a non-coated needle due to associated high costs. Nevertheless, our results obtained using combinations of high and low resolution ultrasound systems with echogenic polymer-coated needles demonstrate the considerable impact of the ultrasound system upon the number of retrieved oocytes. As shown in Table 1, the combination of the high resolution ultrasound system with the echogenic needle (group 3) doubled the mean number of retrieved oocytes (38±10) compared to that (18±11) obtained using the low resolution ultrasound with the echogenic needle (group 2). It should be noted that the mean number of the GV oocytes in group 3 was significantly higher than that in group 2, probably due to aspiration of the GV oocytes from smaller follicles as a result of better ultrasound resolution and thus better visibility of such small follicles. It is noteworthy to mention that two of the retrievals in group 3 were performed in May during the non-breeding season. This might also have contributed to the higher number of GV oocytes because maturational competence of GV oocytes decreases during non-breeding season (Yeoman et al. 1994; Zheng et al. 2001). In addition, the relatively early collection timing (27h to 30h after hCG injection) might be another contributing factor to a higher number of immature oocytes. Indeed, many immature oocytes progressed to the M II stage during the subsequent culture resulting in a significantly higher proportion of the M II oocytes in group 3 (i.e., 74%).
Figure 1.
The aspiration assembly for oocyte retrieval consisting of an echogenic needle and two pieces of teflon tubing. The short piece is attached to the aspiration needle while the longer tubing is to connect to a vacuum pump (not show in the figure).
Another factor that influences the outcome of an ultrasound-guided oocyte retrieval is frequent obstruction of the aspiration assembly, particularly by coagulated blood present in the aspiration fluid. To minimize this complication, we decreased the dead volume of the aspiration assembly by reducing the length of the aspiration needle and tubing, and flushed the aspiration assembly with heparin-containing medium immediately before the oocyte retrieval. We also noticed that residues of ultrasound gel entering into the aspiration needle during penetration of the abdominal wall contribute to the clogging of the aspiration assembly. Therefore, it is important to remove the ultrasound gel just before puncturing the abdominal wall where the aspiration needle will be inserted. Despite these efforts, clogging of the aspiration assembly may still occur. If unnoticed, this may result in leakage of oocytes from punctured follicles into the abdominal cavity. Hence, it is critical to carefully check the continuous flow of the aspiration fluid during oocyte retrieval. In case of obstruction, the aspiration should be stopped immediately. Next, the aspiration assembly can be back flushed from the stopper end of the tubing using a syringe containing warm medium with heparin, and then oocyte aspiration can be resumed.
The anatomic location of rhesus ovaries favors transabdominal ultrasound-guided oocyte retrieval as opposed to the transvaginal approach used in humans. Unlike humans, the ovaries in the macaque are usually located closer to the abdominal wall than to the cervix (VandeVoort and Tarantal 1991). Consequently, the transabdominal approach has been used for oocyte retrieval in different small nonhuman primates (Cseh et al. 2001; Schuler et al. 2007; VandeVoort and Tarantal 1991). However, abdominal surgeries may alter the location of the ovaries due to adhesions and thus complicate the transabdominal approach. In the present study, we had two rhesus females with ovaries displaced to a deeper location closer to cervix. These females did not respond to hormonal stimulation well. Nonetheless, we were able to retrieve 6 to 10 oocytes from both females using transabdominal ultrasound-guided needle aspiration..
The aspiration pressure is also an important parameter to consider when a vacuum pump is used. A wide range of aspiration pressures from 50 to 300 mm Hg was used for retrieval of oocytes from different species (Bols et al. 1996; Hashimoto et al. 2007; Morton et al. 2008; Muechler et al. 1989; VandeVoort et al. 2003). For immature rhesus oocytes, the optimal aspiration pressure seems to be around −20kPa (−150 mm Hg)(VandeVoort et al. 2003). Since the aspiration pressure at the tip of the needle is different than that set at the vacuum pump and changes depending upon features of a particular aspiration assembly (e.g., diameter and length of the needle and tubing) (Bols et al. 1996; Hashimoto et al. 2007; Horne et al. 1996), the optimal aspiration pressure for immature rhesus oocytes may require an adjustment for a different aspiration assembly. In the present study, the aspiration pressure of the vacuum pump was set to −100 mm Hg. When we increased the aspiration pressure to −120 mm Hg to reduce clogging in one case, we found a few empty zona pellucidae in the aspiration fluid. Similar observations and a negative effect on oocyte quality were reported by others as a result of increased aspiration pressures (Cohen et al. 1986; Fry et al. 1997; Hashimoto et al. 2007; Horne et al. 1996; Muechler et al. 1989). Therefore, it is important to set the aspiration pressure to the lowest effective level by taking into consideration the diameter and length of the aspiration needle and tubing, as well as the types of connections.
In conclusion, when performed properly, the ultrasound-guided retrieval of rhesus oocytes is as efficient as the laparoscopic approach in terms of average number of oocytes retrieved. Ultrasound-guided oocyte retrieval offers important advantages over more invasive methods once the initial difficulties mastering the technique have been overcome.
MATERIALS AND METHODS
Ovarian stimulation
All procedures involving rhesus monkeys were reviewed and approved by the Institutional Animal Care and Use Committees at Medical College of Georgia. The animal facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Seven adult rhesus females in the age range 4 to 9 years were individually caged and housed in rooms with a constant temperature of 23°C and controlled light cycle (12 h light:12 h dark). To induce growth of multiple follicles, rhesus females exhibiting regular menstrual cycles were given twice daily intramuscular injections of 30 IU of recombinant human follicle stimulating hormone (rhFSH; Follistim, Organon, Inc, Roseland, NJ, USA) for 8 to 10 days starting on day 1 to 3 of their menstrual cycle. From day 7 onward, 60 IU of human recombinant luteinizing hormone (rhLH; Luveris, Organon Inc, Roseland, NJ, USA) and 5 μg/kg of gonadotropin releasing hormone (GnRH) antagonist (Antagon, Organon Inc, Roseland, NJ, USA) were given twice and once daily, respectively. To monitor the follicle growth, ultrasonography was performed on day 8 and subsequent days of the stimulation. When at least five follicles reached a diameter ≥ 3 mm, the stimulation was stopped and a single intramuscular injection of 1000 IU of human chorionic gonadotropin (hCG; Serono Laboratories, Norwell, MA, USA) was administered to induce meiotic maturation. Twenty-seven to thirty hours after hCG injection, ultrasound-guided oocyte aspiration was carried out.
Aspiration assembly
Currently, there is no commercially available follicle aspiration assembly specifically designed for rhesus monkeys. Therefore, we initially tried a single-lumen 17-gauge disposable needle assembly manufactured for human follicle aspiration (Cook Echotip® ovum aspiration needle; K-J-ANC-17R-33, Cook OB/GYN, Spencer, IN, USA). Although such a human follicle aspiration assembly was successfully used in baboon (Cseh et al. 2002), the 17-gauge needle was too large for rhesus follicles. Consequently, we built two different needle assemblies for rhesus follicle aspiration. The first one was constructed according to previously published specifications except a few modifications (VandeVoort and Tarantal 1991). Briefly, an 18-gauge× 6-inch non-coated spinal needle with 18° bevel (408360, BD Medical, Franklin Lakers, NJ, USA) was cut to remove the hub and to reduce its length to ~11.5 cm. The end of the needle without the hub was inserted into a 15-cm length of Teflon Medical Micro tubing (BB311-18, Scientific Commodities Inc, Lake Havasu City, AZ, USA). The inner diameter of the teflon tubing was similar to the outside diameter of the spinal needle. When the cut end of the spinal needle was inserted into the teflon tubing, the tubing was slightly expanded, and the attachment between the needle and the connected tubing became air-tight. Therefore, there was no need to use plastic bonding cement or glue. The opposite end of the teflon tubing was passed through one of two holes drilled into a silicone stopper (097041F, Fisher Scientific). To complete the aspiration assembly, one end of another teflon tubing of 150-cm length was inserted through the second hole of the silicone stopper. The opposite end of this long teflon tubing was connected to a vacuum pump (2511B-75, Welch, Skokie, IL, USA) to apply negative pressure during follicle aspiration after tightly fitting the silicon stopper onto a 14-ml sterile tube (352057, Falcon, Franklin Lakes, NJ, USA), which served as an oocyte trap. The second follicle aspiration assembly (Figure 1) was built similar to the first one except for the aspiration needle. To better visualize the tip of the aspiration needle by ultrasonography, we used an echogenic polymer-coated 18-gauge × 6-inch quincke-type needle with an 18° block bevel (6PTC18, Havel’s, Cincinnati, OH, USA). The echogenic polymer-coated needles come with calibration marks that are useful to monitor how far the needle is inserted into the abdominal cavity. The echogenic polymer-coated needles were also cut to remove the hub and to reduce its length to ~11.5 cm. Several follicle aspiration assemblies of both types were built and sterilized by autoclaving before use.
Follicle aspiration and recovery of oocytes
Twenty-seven to thirty hours after hCG injection, rhesus females were anaesthetized with ketamine hydrochloride 10 mg/kg (Fort Dodge Laboratories Inc., Fort Dodge, IA, USA) and maintained on a mixture of isoflurane 1.0–2.0% and oxygen 1.5 liters while follicle contents were collected by an ultrasound-guided follicular aspiration technique. To do so, rhesus females were positioned in a dorsal recumbency position, and abdominal hair was clipped. Next, a triple betadine scrub with a 70% alcohol rinse was followed by the application of sterile ultrasound gel (0168–0205–37, Fougera, Melville, NY, USA) to the abdomen. In addition, ultrasound gel was applied directly onto the ultrasound transducer that was subsequently covered with a sterile surgical glove (Accutech 870, Fisher Scientific, Pittsburgh, PA, USA) to ensure the sterility of the area. The ovaries and uterus were visualized using two different ultrasound systems. The first one was an older system from Pie Medical (Pie Medical 200 Veterinary Ultrasound, Esaote Pie Medical, Maastricht, The Netherlands) with a dual frequency (5.0/7.5 MHz) transducer. The second one was a newer digital ultrasound system (i.e. DP-6600Vet, Mindray, Shenzhen, China) equipped with a multi-frequency (5.0/6.6/8.0 MHz) transducer. In the present study, the first ultrasound machine was called “low resolution ultrasound system” due to its smaller and lower resolution image while the second one was designated as a “high resolution ultrasound system” based on its larger images with better resolution. It should be noted that there are a variety of high resolution newer ultrasound systems available from several other companies including Pie Medical under a new name of Esaote. While ovaries and follicles were examined just before follicle aspiration, a sterile aspiration assembly was removed from an autoclaved bag and set up as described earlier. Next, approximately 2 ml of warm HEPES-buffered Hypermedium (Eroglu et al. 2009; Eroglu et al. 2003) containing 4 mg/ml BSA and 50 IU/ml heparin was aspirated into a collection tube to flush the injection needle and tubing with heparin and thus to minimize blood coagulation within the aspiration assembly and collection tube. If needed, a transrectal palpation was performed to better position the ovaries for ultrasound examination and follicle aspiration. Before puncturing the abdominal wall with the aspiration needle, the ultrasound gel was removed from the puncture area using sterile gauzes wetted with 70% alcohol. Then, the aspiration needle was inserted through the abdominal wall while continuously imaging the target ovary by ultrasound. Once the needle was inserted into a follicle, gentle aspiration mostly at −100 mm Hg was applied using the vacuum pump operated with a foot pedal. Contents of multiple follicles were aspirated into several collection tubes containing 2 ml warm HEPES-buffered Hypermedium without withdrawing the needle through the abdominal wall. Typically 2 to 3 ml follicular fluid containing some blood was aspirated into a collection tube before switching to a new one. All collection tubes were kept at 37°C on a dry bath (Talboys, Thorofare, NJ, USA) during the aspiration of follicles. To dissociate granulosa and cumulus cells, approximately 120 IU/ml hyaluronidase was added to each collection tube containing follicular aspirates. Next, the follicular aspirates were filtered through a cell strainer with 70-μm pore size (Becton-Dickenson, Franklin Lakes, NJ). Immediately thereafter, the strainer was back-flushed with Hypermedium into a sterile 90-mm petri dish (BD Falcon, Becton Lakes, NJ), and then oocytes were recovered using a stereomicroscope. After further removal of remaining cumulus cells by gentle pipetting and washing in Hypermedium, the collected oocytes were classified according to their meiotic status as metaphase II (M II; displaying one polar body), germinal vesicle (GV; displaying an intact GV), and metaphase I (M I; displaying no polar body and no GV) oocytes. Subsequently, cumulus-free oocytes were cultured in modified CMRL-1066 medium (Invitrogen, Carlsbad, CA, USA) containing 0.2 mM sodium pyruvate, 1 mM glutamine, 10 mM sodium lactate, and 10% fetal bovine serum (FBS).
Statistical analysis
All data are expressed as mean ± SEM. A one-way analysis of variance (ANOVA) followed by an LSD test was used to analyze the number of eggs retrieved using different ultrasound system and aspiration needles. Differences were considered significant when p value was ≤ 0.05.
Acknowledgments
This work was supported by a grant from the National Institute of Child Health and Human Development awarded to A.E. (Grant Number R01HD049537). The authors thank Ms. Edyta Szurek, Mr. Bobby Leverett, Ms. Jacqueline Moredock, Mr. Marvin Thomas, and Mr. John Cratic for technical assistance.
References
- Barratt-Boyes SM, Brown KN, Melhem N, Soloff AC, Gleason SM. Understanding and exploiting dendritic cells in human immunodeficiency virus infection using the nonhuman primate model. Immunol Res. 2006;36(1–3):265–274. doi: 10.1385/IR:36:1:265. [DOI] [PubMed] [Google Scholar]
- Bavister BD. ARTs in action in nonhuman primates: symposium summary--advances and remaining issues. Reprod Biol Endocrinol. 2004;2:43. doi: 10.1186/1477-7827-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bols PE, Van Soom A, Ysebaert MT, Vandenheede JM, de Kruif A. Effects of aspiration vacuum and needle diameter on cumulus oocyte complex morphology and developmental capacity of bovine oocytes. Theriogenology. 1996;45(5):1001–1014. doi: 10.1016/0093-691x(96)00028-3. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Nichols SM, Jacoby ES, Bavister BD. Non-human primates as a model for reproductive aging and human infertility. Gynecol Obstet Invest. 2004;57(1):21–23. [PubMed] [Google Scholar]
- Cohen J, Avery S, Campbell S, Mason BA, Riddle A, Sharma V. Follicular aspiration using a syringe suction system may damage the zona pellucida. J In Vitro Fert Embryo Transf. 1986;3(4):224–226. doi: 10.1007/BF01132808. [DOI] [PubMed] [Google Scholar]
- Cseh S, Corselli J, Chan P, Bailey L. Controlled ovarian stimulation and ultrasound guided follicular aspiration in the baboon (Papio cynocephalus anubis) Reprod Nutr Dev. 2001;41(6):531–534. doi: 10.1051/rnd:2001107. [DOI] [PubMed] [Google Scholar]
- Cseh S, Corselli J, Chan P, Bailey L. Superovulation using recombinant human FSH and ultrasound-guided transabdominal follicular aspiration in baboon (Papio anubis) Anim Reprod Sci. 2002;70(3–4):287–293. doi: 10.1016/s0378-4320(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Eroglu A, Bailey SE, Toner M, Toth TL. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80(1):70–78. doi: 10.1095/biolreprod.108.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu A, Lawitts JA, Toner M, Toth TL. Quantitative microinjection of trehalose into mouse oocytes and zygotes, and its effect on development. Cryobiology. 2003;46(2):121–134. doi: 10.1016/s0011-2240(03)00018-x. [DOI] [PubMed] [Google Scholar]
- Flood JT, Muasher SJ, Simonetti S, Kreiner D, Acosta AA, Rosenwaks Z. Comparison between laparoscopically and ultrasonographically guided transvaginal follicular aspiration methods in an in vitro fertilization program in the same patients using the same stimulation protocol. J In Vitro Fert Embryo Transf. 1989;6(3):180–185. doi: 10.1007/BF01130785. [DOI] [PubMed] [Google Scholar]
- Fry RC, Niall EM, Simpson TL, Squires TJ, Reynolds J. The collection of oocytes from bovine ovaries. Theriogenology. 1997;47(5):977–987. doi: 10.1016/s0093-691x(97)00054-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Fukuda A, Murata Y, Kikkawa M, Oku H, Kanaya H, Sonoda M, Sugihara K, Murata T, Nagata F, Nakaoaka Y, Morimoto Y. Effect of aspiration vacuum on the developmental competence of immature human oocytes retrieved using a 20-gauge needle. Reprod Biomed Online. 2007;14(4):444–449. doi: 10.1016/s1472-6483(10)60891-7. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Peterson PE. Perspectives on the use of the baboon in embryology and teratology research. Hum Reprod Update. 1997;3(6):575–592. doi: 10.1093/humupd/3.6.575. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Takahashi D, Dominko T, Simerly C, Schatten G. Fertilization and embryo development to blastocysts after intracytoplasmic sperm injection in the rhesus monkey. Hum Reprod. 1998;13(12):3449–3455. doi: 10.1093/humrep/13.12.3449. [DOI] [PubMed] [Google Scholar]
- Horne R, Bishop CJ, Reeves G, Wood C, Kovacs GT. Aspiration of oocytes for in-vitro fertilization. Hum Reprod Update. 1996;2(1):77–85. doi: 10.1093/humupd/2.1.77. [DOI] [PubMed] [Google Scholar]
- Lenz S, Lauritsen JG, Kjellow M. Collection of human oocytes for in vitro fertilisation by ultrasonically guided follicular puncture. Lancet. 1981;1(8230):1163–1164. doi: 10.1016/s0140-6736(81)92335-7. [DOI] [PubMed] [Google Scholar]
- Makori N, Peterson PE, Hendrickx AG. 13-cis-retinoic acid causes patterning defects in the early embryonic rostral hindbrain and abnormal development of the cerebellum in the macaque. Teratology. 2001;63(2):65–76. doi: 10.1002/1096-9926(200102)63:2<65::AID-TERA1011>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Morton KM, Maxwell WM, Evans G. Effect of aspiration pressure during oocyte harvesting on oocyte recovery and in vitro development of ovine oocytes. Reprod Domest Anim. 2008;43(1):106–110. doi: 10.1111/j.1439-0531.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- Muechler EK, Graham MC, Huang KE, Partridge AB, Jones K. Parthenogenesis of human oocytes as a function of vacuum pressure. J In Vitro Fert Embryo Transf. 1989;6(6):335–337. doi: 10.1007/BF01138772. [DOI] [PubMed] [Google Scholar]
- Schuler AM, Westberry JM, Parks VL, Kuehl TJ, Abee CR. Ultrasound-guided follicular aspiration in squirrel monkeys. J Med Primatol. 2007;36(2):113–117. doi: 10.1111/j.1600-0684.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Collins RL, Paushter DM, George CR, Quigley MM. Follicular aspiration: a comparison of an ultrasonic endovaginal transducer with fixed needle guide and other retrieval methods. Fertil Steril. 1988;49(3):462–467. doi: 10.1016/s0015-0282(16)59774-x. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Hewitson L, Simerly C, Schatten G. Molecular medical approaches for alleviating infertility and understanding assisted reproductive technologies. Proc Assoc Am Physicians. 1996;108(6):432–443. [PubMed] [Google Scholar]
- VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology. 2003;59(3–4):699–707. doi: 10.1016/s0093-691x(02)01129-9. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tarantal AF. The macaque model for in vitro fertilization: superovulation techniques and ultrasound-guided follicular aspiration. J Med Primatol. 1991;20(3):110–116. [PubMed] [Google Scholar]
- Wikland M, Enk L, Hammarberg K, Nilsson L. Use of a vaginal transducer for oocyte retrieval in an IVF/ET program. J Clin Ultrasound. 1987;15(4):245–251. doi: 10.1002/jcu.1870150405. [DOI] [PubMed] [Google Scholar]
- Wiseman DA, Short WB, Pattinson HA, Taylor PJ, Nicholson SF, Elliott PD, Fleetham JA, Mortimer ST. Oocyte retrieval in an in vitro fertilization-embryo transfer program: comparison of four methods. Radiology. 1989;173(1):99–102. doi: 10.1148/radiology.173.1.2528782. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Alexander M, Zelinski-Wooten M, Stouffer RL. Maturity and fertility of rhesus monkey oocytes collected at different intervals after an ovulatory stimulus (human chorionic gonadotropin) in in vitro fertilization cycles. Mol Reprod Dev. 1996;43(1):76–81. doi: 10.1002/(SICI)1098-2795(199601)43:1<76::AID-MRD10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod. 2004;71(2):486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- Yang S, He X, Hildebrandt TB, Jewgenow K, Goeritz F, Tang X, Zhou Q, Ji W. Effects of rhFSH dose on ovarian follicular response, oocyte recovery and embryo development in rhesus monkeys. Theriogenology. 2007;67(6):1194–1201. doi: 10.1016/j.theriogenology.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Yeoman RR, Helvacioglu A, Williams LE, Aksel S, Abee CR. Restoration of oocyte maturational competency during the nonbreeding season with follicle-stimulating hormone stimulation in squirrel monkeys (Saimiri boliviensis boliviensis) Biol Reprod. 1994;50(2):329–335. doi: 10.1095/biolreprod50.2.329. [DOI] [PubMed] [Google Scholar]
- Zelinski-Wooten MB, Hutchison JS, Trinchard-Lugan I, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in gonadotrophin-stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Hum Reprod. 1997;12(9):1877–1885. doi: 10.1093/humrep/12.9.1877. [DOI] [PubMed] [Google Scholar]
- Zheng P, Si W, Wang H, Zou R, Bavister BD, Ji W. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and follicle-stimulating hormone-stimulated rhesus monkeys. Biol Reprod. 2001;64(5):1417–1421. doi: 10.1095/biolreprod64.5.1417. [DOI] [PubMed] [Google Scholar]