Abstract
Little data on cutaneous squamous cell carcinoma [SCC] epidemiology within the United States are currently available. Prior studies have focused on populations outside of the United States or been limited to regions within the US. In this study, prospective data were collected via biennial questionnaires from a total of 261 609 participants, which included women in the Nurses’ Health Study [NHS, 1976–2008] and Nurses’ Health Study II [NHS II, 1989–2009], and men in the Health Professionals Follow-Up Study [HPFS, 1986–2008]. History of physician-diagnosed invasive SCC was confirmed by pathology record review. Over the entire follow up period for each cohort, there were 1265 invasive SCC cases per 100 000 persons in the NHS cohort, 389 cases per 100 000 persons in NHS II, and 2154 cases per 100 000 persons in HPFS. An 18-year follow-up of participants in these cohorts revealed increasing invasive SCC incidence rates over time, with rates for men being consistently higher than those for women. In women, a larger proportion of invasive SCC lesions occurred on the lower extremities compared to men (21% in NHS vs. 6% in HPFS, p<0.0001; 14% in NHS II vs. 6% in HPFS, p<0.0001), while in men, a larger proportion occurred on the head/neck (43% in NHS vs. 60% in HPFS, p<0.0001; 48% in NHS II vs. 60% in HPFS, p<0.0001). In summary, invasive SCC incidence rates among US men have been greater than those for women with distinct sites of common occurrence between men and women.
Keywords: squamous cell carcinoma, SCC, incidence
INTRODUCTION
Over the last few decades, there has been increasing recognition of the personal and economic burden of cutaneous squamous cell carcinoma [SCC] across the world. Our understanding of SCC epidemiology within the United States has been hampered by lack of data. Large national cancer registries, such as the Surveillance, Epidemiology, and End Results [SEER] program, do not collect data on SCC incidence and mortality data. Previous studies have provided a glimpse into SCC trends within the United States.
An estimate of SCC incidence for the United States in 1994 was 93 000 to 157 000 cases in men and 42 000 to 95 000 cases in women [10]. More recently, for the year 2012, Karia and others estimated invasive SCC incidence rates in the United States of 139.8 cases per 100 000 person-years for men and 49.6 cases per 100 000 person-years for women [9]. Unfortunately, other estimates of SCC incidence rates have been limited to specific states or regions within the United States. For the 1993–1994 period, a study of SCC cases in the New Hampshire population calculated incidence rates of 97.2 per 100 000 person-years for men and 32.4 per 100 000 person-years for women, increases of 235% and 350% from the 1979–1980 period, respectively [8]. Age-adjusted incidence rates for non-melanoma skin cancer, which includes SCC, approximately doubled from 1977–1978 to 1998–1999 in a study of northcentral New Mexico [1]. Among men, the rate increased from 187.5 to 356.2 cases per 100 000 person-years, while among women, the rate increased from 71.8 to 150.4 cases per 100 000 person-years [1]. Another analysis of non-melanoma skin cancer used claims data on the Medicare population to estimate a 4.2% annual average increase in non-melanoma skin cancer cases from 1992 to 2006 [12]. Unfortunately, since these studies, little work has emerged to clarify recent changes in SCC incidence in the US, especially to detect impact from increased awareness, public health interventions, and changing personal sun exposure habits. Of note, even less is known about invasive SCC, which, in comparison to the in situ form, generally carries a worse prognosis.
Cutaneous SCC merits additional attention as it is one of the non-melanoma skin cancers [NMSCs]. These cancers are a significant source of US healthcare costs, among the top five costliest cancers for Medicare [6]. Knowledge of SCC incidence and at-risk populations may aid in curbing these escalating costs.
Our goal was to analyze trends in invasive cutaneous SCC incidence trends over the last few decades in both women and men. Results for this study came from three large, prospectively followed cohort studies of health care workers: an older cohort of women in the Nurses’ Health Study [NHS], a younger cohort of women in the Nurses’ Health Study II [NHS II], and men in the Health Professionals Follow-Up Study [HPFS].
MATERIALS AND METHODS
Study populations
The Nurses’ Health Study [NHS] is a prospective, longitudinal cohort study in which 121 701 married, registered, female nurses aged 30–55 were enrolled in 1976 after they completed and returned a mailed questionnaire inquiring about their medical history and lifestyle risk factors. The Nurses’ Health Study II [NHS II] was established in 1989 when 116 686 female nurses aged 25–42 completed and returned a mailed baseline questionnaire. Participants were initially limited to 11 and 14 states, respectively, but now reside in every US state. The Health Professionals Follow-Up Study [HPFS] was established in 1986 with 51 529 men aged 40–75, employed in a health profession, and living in all 50 states. In all cohorts, a mailed questionnaire was administered to each participant biennially inquiring about disease history, exposures, and lifestyle habits. A response rate exceeding 90% has been achieved in each follow-up cycle. The cohorts are each approximately 97% Caucasian, reflecting the ethnic background of registered female nurses and male health professionals nationally at the time of cohort inception.
Ascertainment of SCC cases
Participants in the cohorts reported new cases of first-incident in situ or invasive SCC biennially. Thus, individuals who developed more than one SCC were counted only for their first lesion. Information on the cumulative number of SCC lesions was collected through 2008 in NHS, 2009 in NHS II, and 2008 in HPFS. All cases of SCC were verified from primary histopathology reports by study physicians and classified as in situ or invasive. Individuals with a prior history of any cancer were excluded from analysis to minimize detection bias. Additionally, individuals who died during the follow-up period were excluded from analysis. Since the cohorts were all greater than 95% Caucasian, non-white participants were excluded from analysis. The study was approved by the Institutional Review Board of Brigham and Women’s Hospital. We considered participants’ completion and return of the self-administered questionnaire as informed consent for the present study.
Ascertainment of exposure and phenotypic data
Across all three cohorts, the following data was collected through questionnaires: (1) family history of malignant melanoma in first-degree relatives, (2) the number of nevi measuring 3mm or greater on an extremity, (3) natural hair color at age 21, (4) skin burning reaction after 2 or more hours of bright sun exposure during childhood/adolescence, and (5) the number of lifetime severe or blistering sunburns. Family and personal disease history are updated with each questionnaire cycle. For the nevus count on an extremity, the left arm (shoulder to wrist) was used in NHS, the bilateral lower legs (knee to ankle) were used in NHS II, and the bilateral forearms (wrist to elbow) were used in HPFS.
Statistical analysis
In order to determine the statistical significance of differences in the proportion of SCC lesions occurring at particular body sites in men and women, we used the z-test for proportions to compare proportions from two independent samples. All P values were calculated from 2-sided tests, and a P value < 0.05 was considered significant.
RESULTS
Table 1 and Supplementary Table 1 list descriptive statistics for individuals who have been diagnosed with invasive or in situ SCC, respectively. For invasive SCC, there were 1265 cases per 100 000 persons in NHS, 389 cases per 100 000 persons in NHS II, and 2154 cases per 100 000 persons in HPFS (Table 1). For in situ SCC, there were 681 cases per 100 000 persons in NHS, 272 cases per 100 000 persons in NHS II, and 736 cases per 100 000 persons in HPFS (Supplementary Table 1). For individuals diagnosed with invasive or in situ SCC, the mean ages were similar for the NHS and HPFS cohorts, while the NHS II women were younger. Among the cohorts, there were similar proportions of individuals with phenotypic and exposure characteristics of interest. However, the NHS II cohort (composed of younger women) had a greater proportion of individuals with a large number of extremity moles compared to the NHS I and HPFS cohorts. Also, the HPFS cohort had a higher proportion of men with 6 or more sunburns.
Table 1.
Characteristics of US Women and Men With Invasive Squamous Cell Carcinoma in the Nurses’ Health Study (1976–2008), Nurses’ Health Study II (1989–2009), and Health Professionals Follow-Up Study (1986–2008)
| NHS n=1539 |
NHS II n=454 |
HPFS n=1110 |
|
|---|---|---|---|
| Incidence rate for total follow-up period, per 100 000 persons in cohort | 1265 | 389 | 2154 |
| Age, mean(SD)a, years | 65.5(8.4) | 48.3(6.2) | 67.6(8.8) |
| Family history of malignant melanomab, % | 7 | 9 | 5 |
| ≥6 moles on an extremityc,d, % | 6 | 20 | 6 |
| History of ≥6 sunburnsc,% | 11 | 17 | 43 |
| Painful or blistering reaction to the sun as child/adolescente, % | 19 | 38 | 35 |
| Natural red or blonde hairf, % | 22 | 26 | 18 |
Abbreviations: HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; SD, standard deviation
Mean age, in years, at the time of diagnosis of invasive SCC
Family history of malignant melanoma at the time of diagnosis of invasive SCC
≥5 for NHS II
Defined as moles ≥3 mm on the left arm in NHS, bilateral lower legs in NHS II, and bilateral forearms in HPFS
Skin reaction to 2 or more hours of bright sun exposure as a child/adolescent
Natural hair color at age 21 years
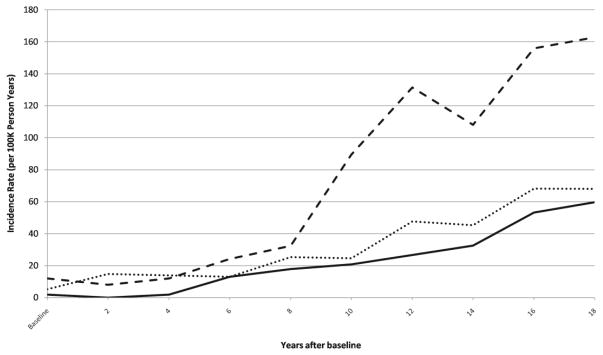
To enable comparisons in SCC incidence trends among the cohorts, each of which began in a different calendar year, participants aged 35–45 at the first year of data collection in each cohort were followed for 18 years. Invasive and in situ SCC incidence rates were calculated for these subsets of the three cohorts and are shown in Figure 1 and Supplementary Figure 1, respectively. To compare invasive and in situ SCC incidence rates, the ratio of invasive to in situ SCC incidence rates were calculated at 6-year intervals (Table 2). For all three cohorts, the ratio tended to be greater than 1, indicating a greater invasive SCC incidence rate compared to that for in situ SCC. For men, the ratio rose from 0.9 to above 1 during the follow-up period.
Figure 1.
Plot of invasive SCC incidence rates for US women and men aged 35–45 at baseline; Nurses’ Health Study (NHS, 1976–1994), Nurses’ Health Study II (NHS II, 1989–2007,), and Health Professionals Follow-Up Study (HPFS, 1986–2004). NHS: dark line, NHS II: dotted line, HPFS: dashed line.
Table 2.
Ratio of Invasive to In Situ SCC Incidence Rates among US Women and Men, Aged 35–45 at Baseline
| Years after Baseline
|
NHS
|
NHS II
|
HPFS
|
|---|---|---|---|
| 0 | 1 | Indeterminate* | 0.9 |
| 6 | 14 | 1.2 | 0.9 |
| 12 | 1.4 | 2.9 | 1.3 |
| 18 | 1.3 | 2.9 | 1.1 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study
The ratio could not be calculated due to a lack of in situ SCC cases in the baseline year of the NHS II cohort.
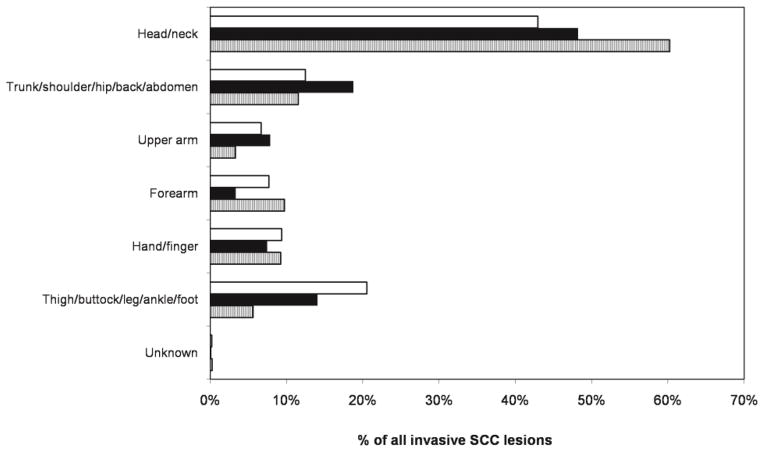
Figure 2 summarizes the percentages of all incident invasive lesions that occurred at each body site. The majority of lesions occurred in the head or neck region. There were two sites with striking differences between women and men. Head or neck SCC lesions accounted for a larger proportion of all invasive lesions for men than for women (60% in HPFS vs. 43% in NHS, p<0.0001; 60% in HPFS vs. 48% in NHS II, p<0.0001). In comparison, lower extremity invasive SCC lesions (thigh, buttock, leg, ankle, and foot distribution) accounted for a larger proportion of all invasive SCC lesions for women than for men (21% in NHS vs. 6% in HPFS, p<0.0001; 14% in NHS II vs. 6% in HPFS, p<0.0001). These body site distribution disparities were similar for incident in situ SCC lesions (Supplementary Figure 2).
Figure 2.
Body site distributions of invasive SCC lesions in US women and men; Nurses’ Health Study (NHS, 1976–2008), Nurses’ Health Study II (NHS II, 1989–2009), and Health Professionals Follow-Up Study (HPFS, 1986–2008). NHS: white bar, NHS II: dark bar, HPFS: striped bar.
DISCUSSION
A number of studies have provided glimpses into SCC trends at different points in time during the past few decades. None has provided a continuous examination of incidence rates over such a span of time. Furthermore, even less is known about the invasive and in situ forms of SCC individually. In this study, we followed cohorts of women and men over several decades, collecting information on first incident cases of invasive and in situ SCC.
To examine incidence trends, we studied participants who were aged 35–45 at the inception of their cohorts. In the three cohorts of women and men, the incidence rates of invasive SCC increased, consistent with an increased incidence as women or men age. Interestingly, the invasive SCC incidence rate for men was consistently greater than those for women in the NHS and NHS II cohorts, respectively. Men in our study developed invasive SCC at a higher rate than women, suggesting sexual differences in the development of advanced SCC. While biological differences may contribute to the greater occurrence of invasive SCC among men, differences in sun exposure habits between women and men may have predisposed men to these more advanced lesions. Furthermore, men may be less likely to perform skin exams and seek appropriate medical care for suspicious skin lesions, allowing any diagnosed SCC to be more advanced. Such appears to be the case with melanoma, in which men are less likely to conduct self-skin examinations and experience greater melanoma mortality rates than women [11,4]. Of note, a higher proportion of men who developed invasive SCC had a history of 6 or more sunburns compared to women who developed invasive SCC (43% in HPFS vs. 12% in NHS and 16% in NHS II), which may also have contributed to the higher incidence of invasive SCC in men.
Other studies have shown greater SCC incidence rates in men compared to women. In 1987, the age-adjusted incidence rate for SCC among men in British Columbia was 31.2 cases per 100 000 people compared to 16.9 cases per 100 000 people among women [3]. In a study of SCC cases in New Hampshire, the age-adjusted incidence rates for men was 97.2 cases per 100 000 person-years vs. 32.4 cases per 100 000 person-years among women [8].
Meanwhile, the invasive SCC incidence rates for women in the NHS and NHS II cohorts tracked similarly over time, despite the approximately one decade gap between the first years at which these cohorts were followed. From the 1980s until the present day, there has been increasing awareness of skin cancer and the need for sun protection. The similarity in incidence trends among two groups of women from different decades suggests that past exposures, such as ultraviolet radiation exposure during childhood, may play a large role in the occurrence of SCC years later.
In contrast to invasive SCC, the incidence rates for in situ lesions increased similarly for all three cohorts. When comparing the ratios of invasive to in situ SCC incidence rates in the three cohorts, we find varying trends. Among men, the ratio remained above 1, meaning invasive SCC incidence rates were above those for in situ SCC. Among women in the NHS cohort, the ratio decreased towards 1, which may be the result of the improved early detection of SCC lesions in their in situ form. Similar findings have been reported. A study of patients from Alberta, Canada showed that the incidence rate for invasive SCC lesions was greater than that for in situ SCC lesions [7]. Furthermore, the gap between incident invasive and in situ SCC narrowed from 1990–1994 to 2000–2004 [7], similar to the rise in our ratios toward 1 in the NHS cohort. These authors suggested that the gradual rise in the amounts of incident in situ lesions relative to invasive lesions may be the result of greater awareness and screening of skin cancer, leading to earlier detection of SCC [7].
Aside from increasing age, several reasons may fuel the rise in SCC incidence rates, including changes in sun exposure habits, artificial UV tanning bed use, ozone depletion, or increases in life expectancy [2]. With regards to our study, the migration of female participants in the NHS and NHS II cohorts from a smaller collection of states at the beginning of the follow-up period to all 50 states by the end may partly explain increases in SCC incidence rates if these participants migrated to states with increased sun exposure. It is important to note that there was no similar migration among male participants in the HPFS study, who were based in all 50 states at the outset. Yet, the incidence rates of SCC rose greater in the HPFS cohort than in the two female cohorts over time.
Other exposures associated with increased SCC, such as immunosuppression and chemical carcinogens, also may be fueling the rise in incidence rates. Despite public health efforts to decrease UV exposure by encouraging individuals to stay indoors during peak UV hours, increase sunscreen use, and reduce ultraviolet tanning bed use, our data emphasize the challenge of cutaneous SCC and the need for improved efforts to reduce its development. Outside of these presumed causative factors, we may also attribute a portion of these increases in incidence rates to improved surveillance and detection.
Results from this study also highlight the differences in SCC incidence between men and women. The disparate body site distributions of SCC lesions between women and men may reflect differences in sun exposure habits, such as clothing and recreational activities. Lesions of the head or neck accounted for a greater proportion of all lesions in men compared to older women, while lesions of the lower extremities were more common in older women. Nevertheless, SCC occurred most frequently in the head or neck region for both sexes, consistent with findings in other studies [5,1,7,3,8]. Though our cohorts were composed exclusively of health professionals, variations in occupational exposure to the sun also might explain differences in SCC distribution between the sexes in the general (i.e., non-health care worker) population.
A limitation of this study is that the cohorts examined may not be representative of the US population. These populations were composed of Caucasian female and male health professionals, who may have had different sun exposure habits and access to health care compared to the general population. Additionally, attempts to describe in situ SCC trends may be limited, as pathologic definitions of what constitutes in situ SCC vary. Certain lesions may be interpreted as benign, while others cross the threshold for interpretation as malignant and are consequently labeled in situ SCC. Furthermore, in practice, only some in situ SCC lesions are biopsied, and many are treated empirically without biopsy. Despite these complications, we believe our high quality data are still useful in providing an approximate sense of increases in diagnoses of in situ SCC over time.
A number of strengths accompanied this study. There were a large number of participants who had regular follow-up over several decades with a high response rate. With 3103 incident invasive SCC lesions and 1525 incident in situ lesions, this analysis is among the largest studies to date. Additionally, the similar occupations, socioeconomic status, education level, medical knowledge, and health care access of participants in these cohorts allowed for more homogeneous populations. The consistency of findings across the three cohorts increases the validity of the data. Lastly, all primary pathology reports were reviewed by an experienced dermatologist (A.A.Q.), minimizing misclassification of the outcome (invasive and in situ SCC).
Non-melanoma skin cancer, including squamous cell carcinoma, can result in substantial morbidity and account for a significant burden on the healthcare system. This analysis provides further evidence that the healthcare impact of this disease is ever-growing, with invasive SCC incidence rates in men outpacing those of women. SCC incidence may be impacted by addressing modifiable risk factors to lessen behaviors leading to SCC as well as improving screening of susceptible individuals.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers CA87969, CA50385, and CA055075] and the Doris Duke Charitable Foundation [grant to K.D.N.].
Abbreviations
- HPFS
Health Professionals Follow-Up Study
- NHS
Nurses’ Health Study
- NHS II
Nurses’ Health Study II
- SCC
squamous cell carcinoma
References
- 1.Athas WF, Hunt WC, Key CR. Changes in nonmelanoma skin cancer incidence between 1977–1978 and 1998–1999 in Northcentral New Mexico. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12 (10):1105–1108. [PubMed] [Google Scholar]
- 2.Diepgen TL, Mahler V. The epidemiology of skin cancer. The British journal of dermatology. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher RP, Ma B, McLean DI, Yang CP, Ho V, Carruthers JA, Warshawski LM. Trends in basal cell carcinoma, squamous cell carcinoma, and melanoma of the skin from 1973 through 1987. Journal of the American Academy of Dermatology. 1990;23 (3 Pt 1):413–421. doi: 10.1016/0190-9622(90)70234-9. [DOI] [PubMed] [Google Scholar]
- 4.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969–1999. JAMA : the journal of the American Medical Association. 2002;288 (14):1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 5.Harris RB, Griffith K, Moon TE. Trends in the incidence of nonmelanoma skin cancers in southeastern Arizona, 1985–1996. Journal of the American Academy of Dermatology. 2001;45 (4):528–536. doi: 10.1067/mjd.2001.114742. [DOI] [PubMed] [Google Scholar]
- 6.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, Chen GJ. Skin cancer is among the most costly of all cancers to treat for the Medicare population. Journal of the American Academy of Dermatology. 2003;48 (3):425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 7.Jung GW, Metelitsa AI, Dover DC, Salopek TG. Trends in incidence of nonmelanoma skin cancers in Alberta, Canada, 1988–2007. The British journal of dermatology. 2010;163 (1):146–154. doi: 10.1111/j.1365-2133.2010.09809.x. [DOI] [PubMed] [Google Scholar]
- 8.Karagas MR, Greenberg ER, Spencer SK, Stukel TA, Mott LA. Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. New Hampshire Skin Cancer Study Group. International journal of cancer Journal international du cancer. 1999;81 (4):555–559. doi: 10.1002/(sici)1097-0215(19990517)81:4<555::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. Journal of the American Academy of Dermatology. 2013;68 (6):957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. Journal of the American Academy of Dermatology. 1994;30 (5 Pt 1):774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 11.Miller DR, Geller AC, Wyatt SW, Halpern A, Howell JB, Cockerell C, Reilley BA, Bewerse BA, Rigel D, Rosenthal L, Amonette R, Sun T, Grossbart T, Lew RA, Koh HK. Melanoma awareness and self-examination practices: results of a United States survey. Journal of the American Academy of Dermatology. 1996;34 (6):962–970. doi: 10.1016/s0190-9622(96)90273-x. [DOI] [PubMed] [Google Scholar]
- 12.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Archives of dermatology. 2010;146 (3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.