Abstract
Using a comprehensive microarray database of human gene expression, we identified that in mammals, a secreted protein known as isthmin 1 (ISM1) is expressed in skin, mucosal tissues, and selected lymphocyte populations. ISM1 was originally identified in Xenopus brain during development, and it encodes a predicted ∼50-kDa protein containing a signal peptide, a thrombospondin domain, and an adhesion-associated domain. We confirmed the pattern of expression of ISM1 in both human and mouse tissues. ISM1 is expressed by DX5+ lung lymphocytes that include NK and NKT-like cells, and is also expressed by some CD4+ T cells upon activation but its expression increases significantly when CD4+ T cells were polarized to the Th17 lineage in vitro. The presence of IFN-γ during CD4+ T cell polarization inhibits ISM1 expression. Given that ISM1 has been reported to have anti-angiogenic properties, these observations suggest that ISM1 is a mediator of lymphocyte effector functions and may participate in both innate and acquired immune responses.
Introduction
Isthmin (ISM) is a secreted protein originally identified in the brain of Xenopus laevis (Pera and others 2002). There are 2 ISM genes in the genome of mammals, ISM1 and ISM2/Tail1, both of which encode secreted proteins that exhibit signal peptides, as well as thrombospondin (TSR) and adhesion-associated (AMOP) domains (Rossi and others 2004). ISM1 is located in human chromosome 20, and in mouse chromosome 2. ISM1 was identified in 2002 as a gene expressed in the midbrain-hindbrain boundary or isthmus organizer of the Xenopus brain during development and was therefore called isthmin (Pera and others 2002). Few reports exist on this molecule. However, ISM1 has been shown to have antiangiogenic, antitumorigenic, and proapoptotic properties (Xiang and others 2011; Zhang and others 2011; Yuan and others 2012). Importantly, ISM1 expression has only been described in the central nervous system (CNS) of Xenopus and no information exists on its expression in human or mouse tissues.
We analyzed a comprehensive human gene expression database [body index of gene expression (BIGE)] (Lee and others 2005; Roth and others 2006; Hevezi and others 2009), based on the Affymetrix U133 2.0 genearray. We searched the BIGE database for genes encoding secreted proteins expressed by cells of the immune system. This screen revealed that human ISM1 (hISM1) is expressed in the skin, mucosal tissues, and some lymphocyte populations. We sought to identify the lymphocytes that express ISM1 and found that it is expressed by human or mouse activated CD4+ T cells. ISM1 is also expressed by DX5+NKp46+ NK and NKT cells located in normal mouse lung. Further analysis of ISM1 expression by CD4+ T cells indicates that it is strongly expressed by CD4+ T helper (Th) cells polarized toward the Th17 lineage and that its expression is inhibited by IFN-γ. These observations indicate that in mammals, ISM1 is associated with the immune system. It may mediate some of the effector functions of Th17, NKT, and NK cells, and may be involved in innate and acquired immune responses.
Materials and Methods
BIGE database
The BIGE database has been described (Lee and others 2005; Roth and others 2006; Hevezi and others 2009). Briefly, samples from 105 different tissues and cell types of the human body were analyzed for gene expression using U133 2.0 genearrays (Affymetrix). The resulting data were normalized, and a probeset corresponding to ISM1/C20orf82 (235182_at) was used to determine the expression of ISM1 in the human body.
Mice
BALB/C and C57BL/6 mice were obtained from Charles River. The NSG (NOD Scid gamma) mouse strain was purchased from the Jackson Laboratory, JAX mouse stock name NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Production and detection of recombinant mouse ISM1
An ISM1 expression plasmid (pcDNA3.1+/mISM1) was constructed by cloning the full mouse ISM1 (mISM1) cDNA sequence into the plasmid pcDNA3.1+ (Invitrogen). Primers used for cDNA amplification by polymerase chain reaction (PCR) were designed based on the ISM1 sequence (accession NM_001126490) as follows: 5′-AATTGAATTCATGGTGCGCCTGGCTGCGGAAC-3′ and 5′-CTAGTCTAGAGTACTCTCTGGCTTCTTGGAAC-3′; restriction sites used to subclone the PCR product are underlined (EcoRI and XbaI). HEK293 cells were cultured in nonconfluent conditions and, after 48 h, they were transfected with the construct pcDNA3.1+/mISM1. After 5 days, supernatant was recovered to detect recombinant ISM1 by SDS-PAGE analysis (10% gel) with primary anti-mISM/ISM1 antibody (Biolegend).
Cell activation
Human peripheral blood mononuclear cells (hPBMCs) were purchased from Sanguine Biosciences and mouse lymphocytes were activated with solid-phase anti-CD3 (Icyt) and soluble anti-CD28 (Icyt) for 12 h. Jurkat cells were stimulated with 10 ng/mL of phorbol 12-myristate 13-acetate (PMA; Sigma) and 200 ng/mL of ionomycin (Sigma) for 12 h. RNA was then extracted for quantitative real-time PCR (qPCR) analysis of selected genes.
Isolation of naive CD4+ T cells and Th polarization conditions
Naive CD4+ T cells were purified from lymph nodes of BALB/C female mice by using the CD4+ T cell isolation kit, and CD62L MicroBeads (Miltenyi). Purified naive CD4+ T cells were cultured in RPMI 1640 with 10% fetal bovine serum, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2.5 μM β-mercaptoethanol at 37°C and in 5% CO2. Naive CD4+ T cells were stimulated with solid-phase anti-CD3 (3 μg) and 3 μg of soluble anti-CD28 for 4–5 days. Cytokines and antibodies used for the generation of polarized CD4+ T cells are as follows: Th1: IL-12 (10 ng/mL), IL-2 (5 ng/mL), and anti-IL-4 (10 ng/mL); Th2: IL-4 (4 ng/mL), IL-2 (5 ng/mL), anti-IFN-γ (10 μg/mL), and anti-IL-12 (10 μg/mL); iTreg: TGFβ (5 ng/mL) and IL-2 (5 ng/mL); and Th17: TGFβ (5 ng/mL), IL-6 (20 ng/mL), anti-IFN-γ (10 μg/mL), and anti-IL-4 (10 μg/mL). All reagents were obtained from Icyt or eBioscience.
qPCR analysis
qPCR data were generated with a Roche LightCycler 480 using a Universal Probe Library–based system. Briefly, total RNA was extracted from each mouse tissue sample using TRIzol (Invitrogen) followed by RNA purification and DNase digest using RNeasy columns (Qiagen). Human RNA samples were purchased from Clontech and did not require extra preparation. Two hundred fifty nanograms of total RNA was used to generate cDNA (Qiagen) and 12.5 ng of RNA equivalent was used in each qPCR. Gene-specific primers and corresponding reporter hydrolysis probes were used to quantify ISM1 and GAPDH (control gene) transcript levels in each tissue sample. All qPCR data are presented as relative expression normalized to GAPDH.
Isolation of lung cells and flow cytometry
Lungs from C57BL/6 mice were removed, and minced and incubated in 10 mL of Hanks buffer containing 1 mg of collagenase (Sigma) and 2 mg of DNase I (Sigma). Lungs were digested for 1 h at 37°C with constant horizontal shaking. The cell suspension was disaggregated, strained through a 40-μM mesh, and centrifuged to recover cells. Cells were then treated with ACK lysis buffer, and washed and finally resuspended in phosphate-buffered saline 1×. Flow cytometry was performed following surface staining by using PerCP anti-CD3, FITC anti-CD8, APC anti-CD4, FITC DX5, APC-anti-γδ TCR (clone GL3), and eFluor 660 anti-NKp46 antibodies (eBioscience), followed by intracellular staining with PE-conjugated anti-ISM1 (Biolegend). Data acquisition was performed on an FACScalibur Flow Cytometer followed by analysis using FlowJo software (TreeStar).
Results
ISM1 is a secreted molecule expressed by skin, mucosal tissues, and CD4+ T cells
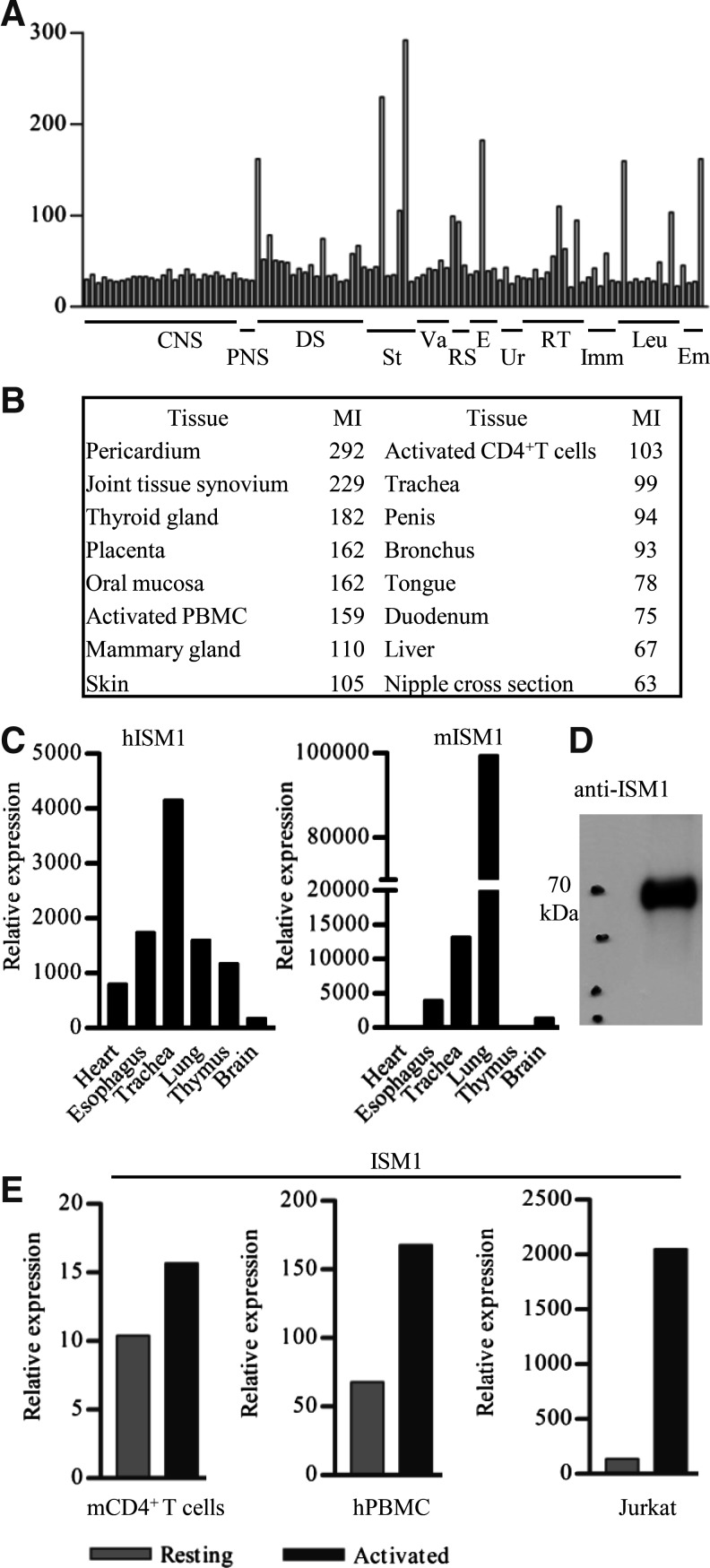
We sought to identify novel secreted proteins expressed in human lymphocytes. To this end, we screened the BIGE database for novel secreted proteins and identified ISM1 as a gene expressed by activated CD4+ T cells. There are 2 ISM genes (ISM1 and ISM2/Tail1) in the human genome. Expression data from the BIGE database indicate that ISM2 expression is restricted to the placenta, a result that we also confirmed by qPCR analysis (data not shown) (Rossi and others 2004). In contrast, ISM1 exhibits a broader expression pattern. Microarray data from the BIGE database indicate that ISM1 is expressed by activated peripheral blood CD4+ T cells, tissues containing skin, and mucosal tissues, including oral mucosa, mammary gland, trachea, bronchus, and duodenum (Fig. 1A, B). To confirm that ISM1 is a secreted protein, we performed western blot analysis of supernatants from HEK293 cells transfected with full-length mISM1 cDNA and observed that ISM1 runs as a ∼70-kDa protein (Fig. 1D).
FIG. 1.
ISM1 is selectively expressed in the human body and upregulated in activated CD4+ T cells. (A) Mean expression values (y-axis) from microarray data for 105 human tissues are displayed across the x-axis. CNS, central nervous system; PNS, peripheral nervous system; DS, digestive system and oral mucosa; St., muscle, adipose, skin; Va., heart and blood vessels; RS, respiratory system; E, endocrine organs; Ur., kidney, urethra; RT, reproductive tract (male and female); Imm., immune tissues; Leu., peripheral blood cells (monocytes, B and T cells), Em, embryonic (fetal kidney, brain, and placenta). (B) Tissues with the highest ISM1 expression are shown, based on the average signal intensity values of the corresponding probeset (235182_at) from the BIGE database. The MI signal is the average microarray signal from the replicates for each tissue included in the BIGE database. Values reflect (A). (C) Selected tissues were tested to confirm ISM1 expression by qPCR, both in hISM1 and mISM1. (D) mISM1 was detected by western blot in the supernatants of HEK293 cells transfected with the construct pcDNA3.1+/mISM1. (E) ISM1 expression was measured by qPCR in resting or activated mouse naive CD4+ lymph node T cells, human PBMCs, or Jurkat cells. A representative experiment (out of 3) is shown in (C) and (D). BIGE, body index of gene expression; hISM1, human isthmin 1; mISM1, mouse isthmin 1; MI, mean intensity; PBMCs; qPCR, quantitative real-time polymerase chain reaction.
We should note that in humans, ISM1 is either not expressed (or expressed at very low levels) in the CNS (Fig. 1A, B). This observation indicates that, while ISM1 was initially described as a molecule expressed in the Xenopus brain (Pera and others 2002), its expression in mammals is significantly different. To confirm the microarray data we performed qPCR assays, which showed similar results (Fig. 1C). Microarray and qPCR data indicate that ISM1 is also expressed by anti-CD3 and anti-CD28 activated human peripheral blood CD4+ T cells (Fig. 1A, E). Further, we also observed that Jurkat T cells activated with ionomycin and PMA produce ISM1 (Fig. 1E). However, qPCR analyses performed using naive mouse CD4+ lymph node T cells showed only a small upregulation of ISM1 upon activation with anti-CD3 and anti-CD28 (Fig. 1E). The latter observation suggests that there is either a difference between ISM1 expression between human and mouse, or, more likely, that ISM1 is expressed by subsets of CD4+ T cells that are present in higher numbers in PBMCs from adult human donors than in lymph nodes from laboratory mice. In either case, these results indicate that some CD4+ T cells can produce ISM1 upon activation.
ISM1 is expressed by lung NK and NKT cells
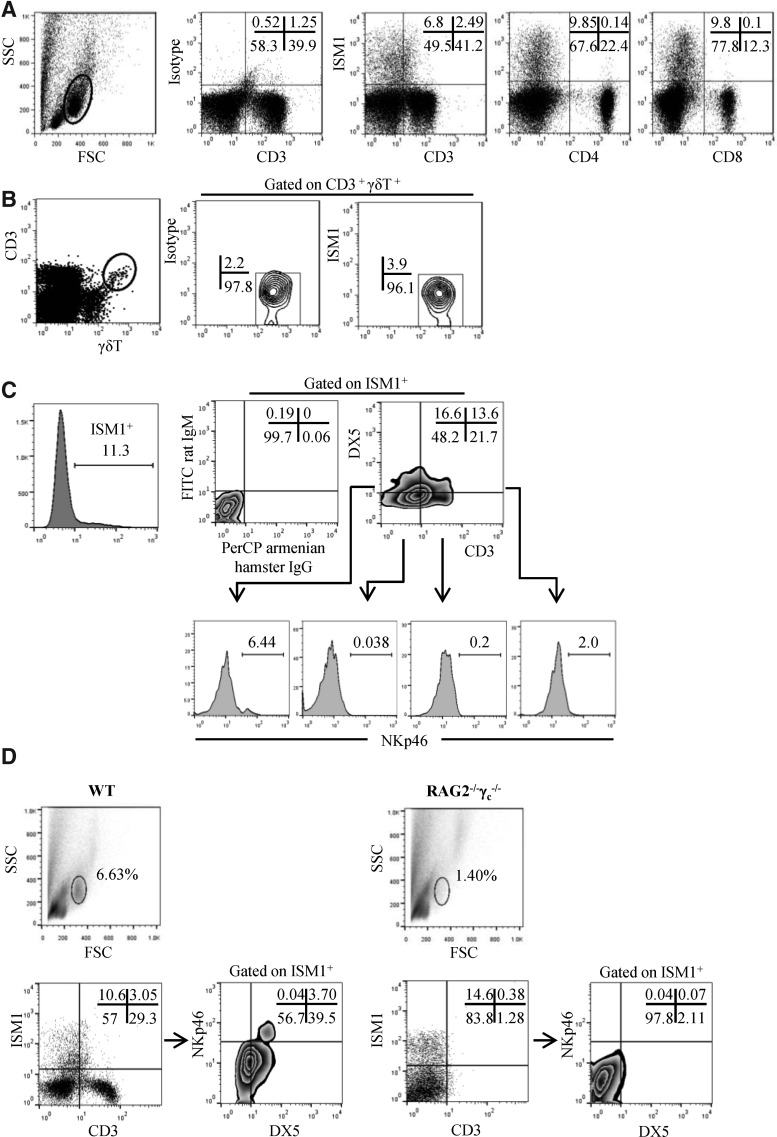
The expression of ISM1 in the BIGE database indicates that it is a constitutively expressed molecule in certain tissues, which can also be upregulated upon activation in a subset of CD4+ T cells. We sought to identify lymphoid cells that express ISM1. To this end, we analyzed the intracellular expression of ISM1 by FACS in various mouse organs, including spleen, lymph nodes, peripheral blood, and Peyer's patches. We did not detect ISM1 expression in cells within the lymphoid gate in cells derived from several lymphoid organs isolated from normal mice (data not shown). However, as shown in Fig. 1C, we detected ISM1 expression in lymphoid cells from normal mouse lung. FACS analyses of lung lymphocytes indicate that it is produced by double-negative (DN) T cells (CD3lowCD4−CD8−) and CD3−DN cells (Fig. 2A). ISM1 production was not observed in γδ T cells (Fig. 2B), macrophages (CD11b+), dendritic cells (CD11c+), or B cells (CD19+) (data not shown).
FIG. 2.
ISM1 is produced by DX5+CD4−CD8−CD3low and DX5+CD4−CD8−CD3− lung cells. (A) Fresh lung cells from C57BL/6 mice were obtained following collagenase digestion and stained for CD3, CD8, and CD4, followed by intracellular staining for ISM1. (B) Lung cells were stained for CD3, γδ TCR, and ISM1. (C) Lung cells were assayed for surface staining with anti-CD3, DX5, and anti-NKP46 antibodies followed by intracellular ISM1 staining. Corresponding isotype controls for anti-CD3 (PerCP hamster IgG) and DX-5 (FITC rat IgM) antibodies were used to define the CD3- or DX5-positive cells. (D) Fresh lung cells from RAG−/− γc−/− mice were stained as in (C). The percentage of cells corresponding to each FACS quadrant is shown. All FACS analyses were performed on the gated lymphocyte population defined by forward versus side scatter (A). Representative experiments are shown (out of 3).
To further characterize the ISM1-producing lung cells, we stained with DX5 antibodies, which recognize CD49b, a specific marker of NK cells (Arase and others 2001), as well as some populations of NKT cells (Ortaldo and others 1998; Werner and others 2011). We also stained lung cells with anti-NKp46, another NK cell marker that defines a subpopulation of NKT cells (Walzer and others 2007; Spits and Di Santo 2010; Yu and others 2011). The ISM1-producing lung cells include CD3lowDX5+, CD3−DX5+, and CD3+DX5− (Fig. 2C). We also observed significant NKp46 expression in the DX5+ISM1+ subpopulation and in a small percentage of DX5+CD3lowISM1+ cells, but no expression in CD3+ISM1+ cells. Interestingly, we also identified a subpopulation of ISM1+ cells lacking all the markers used for this staining (Fig. 2C). To verify the lymphoid nature of the cells expressing ISM1, we analyzed the lung cells obtained from SCID/cγ chain−/− mice (which do not have mature T, NK, or NKT cells) and observed that ISM1+ cells were significantly reduced in lungs from these mice, confirming that some of the ISM1-expressing cells belong to these lineages. However, there was still a small population of lung cells expressing ISM1, but lacking lineage markers (Fig. 2D) within the lymphoid gate of the FACS. Taken together, these results indicate that ISM1 is expressed by some NK and NKT-like cells in the normal mouse lung.
ISM1 expression is associated with the Th17 lineage
The low levels of ISM1 observed in activated naive mouse lymph node CD4+ T cells (Fig. 1E) led us to hypothesize that ISM1 expression could be associated with a particular stage of differentiation, for example, a specific CD4+ T lineage. We therefore decided to explore whether ISM1 production was associated with a particular subset of CD4+ Th cells (Th1, Th2, iTreg, and Th17) (Zhu and Paul 2010). To this end, we polarized mouse CD4+ T cells in vitro to obtain various Th cell subsets.
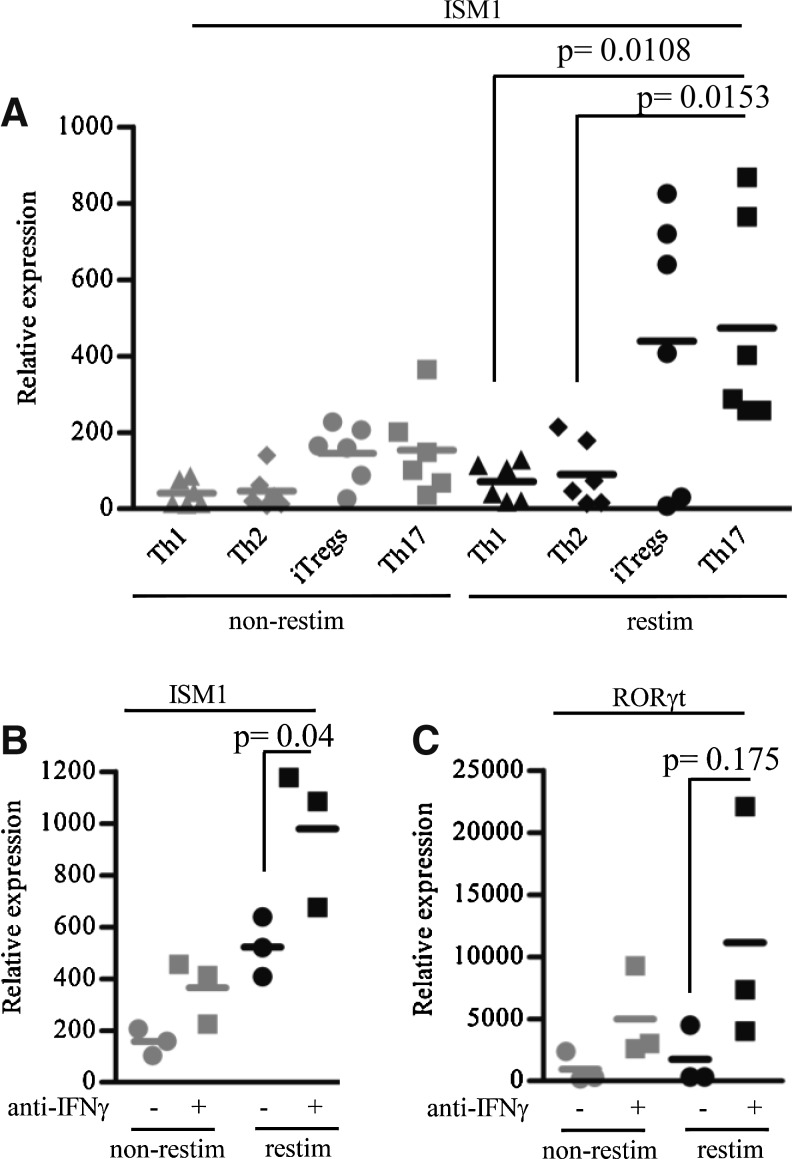
We successfully polarized CD4+ T cells into Th1, Th2, Th17, and iTreg subsets based on the expression of specific cytokines and transcription factors that define each subpopulation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jir) (Zhu and Paul 2010). We measured the expression of ISM1 in these subsets by qPCR and observed that it is overexpressed in Th17 cells but not in Th1 or Th2 cells (Fig. 3A). We also observed lower levels of ISM1 expression by iTreg cells (Fig. 3A).
FIG. 3.
ISM1 expression is associated with Th17 cells and negatively regulated by IFN-γ. (A) Mouse lymph node naive CD4+ T cells were cultured under CD4+ Th polarizing conditions for 5 days. ISM1 expression was measured under non-restimulated (non-restim) or restimulated (restim) conditions by qPCR. Significance was calculated using the mean and standard deviation of 6 independent experiments. (B) Mouse lymph node naive CD4+ T cells were cultured with TGFβ+IL-2 or TGFβ+IL-2+anti-IFN-γ for 4 days. ISM1 expression was measured by qPCR from RNA of non-restimulated or restimulated cells. (C) Analysis of RORγt expression was performed by qPCR as described in (B). Statistics were calculated using Student's t-test from 3 independent experiments. Th, T helper.
IFN-γ inhibits ISM1 expression in polarized CD4+ T cell cultures
We sought to further explore the expression of ISM1 observed between Th17 and iTreg cells. The polarizing conditions that give rise to these subsets are similar because they both require TGFβ. However, IFN-γ is known to regulate the plasticity of the T cells that differentiate toward these subsets (Weaver and Hatton 2009). We therefore hypothesized that variations in endogenous IFN-γ in these cultures could regulate the expression of ISM1 in Th17 and iTregs. We then repeated the polarization of naive CD4+ T cells under iTreg conditions in the presence or absence of neutralizing anti-IFN-γ antibodies, and measured ISM1 expression. As shown in Fig. 3B, the neutralization of IFN-γ resulted in higher ISM1 production levels than when cells were polarized in the absence of anti-IFN-γ. Moreover, the level of expression of the transcription factor RORγt, which controls the commitment toward the Th17 lineage, correlated with the observed ISM1 levels (Fig. 3C). These results strongly suggest that ISM1 expression in CD4+ T cells is associated with the Th17 lineage.
Discussion
In the present study we report that a relatively uncharacterized secreted protein (ISM1) is produced by various leukocytes and therefore has links to the immune system. We initially performed a comprehensive analysis of a human gene expression database (BIGE) looking for genes associated with the immune system. Our survey revealed that ISM1 is expressed in skin, various mucosal sites, and selected populations of lymphocytes. This expression pattern suggests that ISM1 has a barrier function. ISM1 is a secreted protein of an estimated ∼50 kDa that contains TSR and AMOP domains. ISM1 was initially reported as a molecule expressed in the isthmus in Xenopus during development (Pera and others 2002). It has been reported to have antiangiogenic activity (Xiang and others 2011; Zhang and others 2011; Yuan and others 2012). Importantly, there are no previous reports that describe its expression in mammalian tissues. The expression of ISM1 in the BIGE database, which contains more than 20 sites of the human CNS, does not show significant ISM1 expression in any of the CNS sites (Fig. 1A). Further, the BIGE database also contains human fetal brain, which shows no significant ISM1 expression. We therefore conclude that while ISM1 is present in the genomes of many species, including birds (Gallus gallus) and amphibians (X. laevis), its expression in mammals, including humans, is significantly different that in those species. Specifically, in mammals, ISM1 is not expressed in the CNS and is instead strongly associated with barrier tissues (ie, skin and mucosa) as well as selected lymphocyte populations, including activated human peripheral blood CD4+ T cells (Fig. 1C, E). The strong expression of ISM1 in skin and certain mucosal tissues suggests that ISM1 is also expressed by nonlymphoid cells in these tissues, possibly in a homeostatic manner; in support of this, we have obtained preliminary data that indicate that ISM1 is produced by keratinocytes and we have also detected a small population of ISM1-producing lymphoid cells in the intestinal lamina propria (unpublished observations).
We then sought to obtain more information on the lymphoid cells that express ISM1. Based on the BIGE database (Fig. 1A) we initially focused on the lung. Our results indicate that ISM1 is produced by some NK (DX5+NKp46+CD3−ISM1+) or NKT-like (DX5+NKp46+CD3+ISM1+) cells that reside in the normal mouse lung. This suggests a potential role for ISM1 in the homeostasis or in the barrier function of this organ (Holt and others 2008). The small lymphoid populations that still express ISM1 in the lungs of the SCIDγ-chain-knockout mice that do not have T, NK, or NKT cells could represent some of the recently reported innate populations of lymphocytes (Spits and Di Santo 2010).
The difference in ISM1 expression between human and mouse activated CD4+ T cells (Fig. 1E) led us to hypothesize that its production may be linked to subsets of differentiated CD4+ T cells since laboratory mice have more naive CD4 T cells than PBMCs from adult humans. To investigate this possibility, we polarized naive mouse CD4+ T cells toward the Th1, Th2, Tregs, and Th17 lineages and measured ISM1 expression in the polarized cells. We observed that activated Th17 cells produce ISM1 as well as iTreg cells (Fig. 3A) although the production by the latter was lower. The development of Th17 and Treg subsets is closely linked (Zhou and others 2008; Weaver and Hatton 2009), reflecting common in vitro conditions used to generate iTreg and Th17 (ie, stimuli like TGFβ) (Li and others 2006; Liu and others 2008). While TGFβ favors the differentiation of Th17, IFN-γ inhibits their development, and therefore antibodies against IFN-γ are often used to achieve optimal Th17 generation (Basso and others 2009). We hypothesized that endogenously produced IFN-γ was affecting ISM1 expression in Tregs versus Th17 subsets. As shown in Fig. 3B, neutralization of endogenous IFN-γ resulted in upregulation of ISM1 by Th17 cells, indicating that IFN-γ is a negative regulator of ISM1 expression. Given that Th17 development is known to be inhibited by IFN-γ (Ivanov and others 2006), this result strongly suggests that Th17 and not iTregs cells are the main ISM1 producers. In support of this, the levels of RORγt, a transcription factor that controls the development of Th17 cells (Ivanov and others 2006), increased along with ISM1 levels (Fig. 3C). We should note that the latter observation suggests that it is also possible that ISM1 expression may be controlled by RORγt rather than directly by IFN-γ.
ISM1 has antiangiogenic and antitumorigenic properties. Xiang and others (2011) showed that B16 melanoma cells that express ISM1 generated smaller tumors in mice than parental B16 cells. Further, ISM1 has also been reported to inhibit cell proliferation through the induction of apoptosis mediated by the activation of caspases 3 and 8 (Zhang and others 2011; Yuan and others 2012). These observations suggest that ISM1 may be part of the effector activity of NKT, NK, and Th17 cells, and may contribute to the reported antitumor activities of these cells (Kim and others 2007; Wilke and others 2011).
Taken together, our results indicate that human and mouse ISM1 is produced in barrier tissues, including the skin and mucosa, and by some lung lymphocytes that may be related to the NK, NKT, and Th17 cell lineages. These observations strongly suggest that ISM1 is a novel player in both innate and acquired immune responses.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health NIAID Grant 1R21AI096278-01 (to A.Z.), National Institutes of Health Immunology Research Training Program Grant T32AI60573 (to A.M.B.), and 2 University of California Institute for Mexico and the U.S. Postdoctoral grants (to R.V.-R. and J.L.M.-M.). The NSG mice were a kind gift of Dr. David Fruman (UCI).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Arase H, Saito T, Phillips JH, Lanier LL. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-2). J Immunol 167(3):1141–1144 [DOI] [PubMed] [Google Scholar]
- Basso AS, Cheroutre H, Mucida D. 2009. More stories on Th17 cells. Cell Res 19(4):399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, Yeh SA, Zoller M, Zlotnik A. 2009. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One 4(7):e6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. 2008. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 8(2):142–152 [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126(6):1121–1133 [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K. 2007. Cancer immunoediting from immune surveillance to immune escape. Immunology 121(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. 2005. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J 19(10):1356–1358 [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25(3):455–471 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. 2008. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 9(6):632–640 [DOI] [PubMed] [Google Scholar]
- Ortaldo JR, Winkler-Pickett R, Mason AT, Mason LH. 1998. The Ly-49 family: regulation of cytotoxicity and cytokine production in murine CD3+ cells. J Immunol 160(3):1158–1165 [PubMed] [Google Scholar]
- Pera EM, Kim JI, Martinez SL, Brechner M, Li SY, Wessely O, De Robertis EM. 2002. Isthmin is a novel secreted protein expressed as part of the Fgf-8 synexpression group in the Xenopus midbrain-hindbrain organizer. Mech Dev 116(1–2):169–172 [DOI] [PubMed] [Google Scholar]
- Rossi V, Beffagna G, Rampazzo A, Bauce B, Danieli GA. 2004. TAIL1: an isthmin-like gene, containing type 1 thrombospondin-repeat and AMOP domain, mapped to ARVD1 critical region. Gene 335:101–108 [DOI] [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. 2006. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7(2):67–80 [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. 2010. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12(1):21–27 [DOI] [PubMed] [Google Scholar]
- Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. 2007. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A 104(9):3384–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD. 2009. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol 9(12):883–889 [DOI] [PubMed] [Google Scholar]
- Werner JM, Busl E, Farkas SA, Schlitt HJ, Geissler EK, Hornung M. 2011. DX5+NKT cells display phenotypical and functional differences between spleen and liver as well as NK1.1-Balb/c and NK1.1+ C57Bl/6 mice. BMC Immunol 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. 2011. Th17 cells in cancer: help or hindrance? Carcinogenesis 32(5):643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Ke Z, Zhang Y, Cheng GH, Irwan ID, Sulochana KN, Potturi P, Wang Z, Yang H, Wang J, Zhuo L, Kini RM, Ge R. 2011. Isthmin is a novel secreted angiogenesis inhibitor that inhibits tumour growth in mice. J Cell Mol Med 15(2):359–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Mitsui T, Wei M, Mao H, Butchar JP, Shah MV, Zhang J, Mishra A, Alvarez-Breckenridge C, Liu X, Liu S, Yokohama A, Trotta R, Marcucci G, Jr., Benson DM, Loughran TP, Jr., Tridandapani S, Caligiuri MA. 2011. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J Clin Invest 121(4):1456–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Xian R, Ma J, Chen Y, Lin C, Song Y. 2012. Isthmin inhibits glioma growth through antiangiogenesis in vivo. J Neurooncol 109(2):245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen M, Venugopal S, Zhou Y, Xiang W, Li YH, Lin Q, Kini RM, Chong YS, Ge R. 2011. Isthmin exerts pro-survival and death-promoting effect on endothelial cells through alphavbeta5 integrin depending on its physical state. Cell Death Dis 2:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. 2008. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453(7192):236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. 2010. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 238(1):247–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.