Abstract
Alcohols and inhaled anesthetics modulate GABAA receptor (GABAAR) function via putative binding sites within the transmembrane regions (TMs). The relative position of the amino acids lining these sites could be either inter- or intra-subunit. We introduced cysteines in relevant TM locations and tested the proximity of cysteine pairs using oxidizing and reducing agents to induce or break disulfide bridges between cysteines, and thus change GABA-mediated currents in wild-type and mutant α1β2γ2 GABAARs expressed in Xenopus laevis oocytes. We tested for: (1) inter-subunit crosslinking: a cysteine located in α1TM1 [either α1(Q229C) or α1(L232C)] was paired with a cysteine in different positions of β2TM2 or TM3; (2) intra-subunit crosslinking: a cysteine located either in β2TM1 [β2(T225C)] or TM2 [β2(N265C)] was paired with a cysteine in different locations along β2TM3. Three inter-subunit cysteine pairs and four intra-subunits crosslinked. In three intra-subunit cysteine combinations, the alcohol effect was reduced by oxidizing agents, suggesting intra-subunit alcohol binding. We conclude that the structure of the alcohol binding site changes during activation and that potentiation or inhibition by binding at inter- or intra-subunit sites is determined by the specific receptor and ligand.
Keywords: GABAA receptor, binding site, ethanol, crosslink, methanethiosulfonate, homology model
1. Introduction
The γ-aminobutyric acid type A receptor (GABAAR) is a heteromeric ligand-gated ion channel (LGIC) and a major inhibitory receptor in the mammalian brain. Alcohols and anesthetics are positive modulators of the GABAAR (Yamakura et al. 2001, Lobo & Harris 2008). The GABAAR transmembrane regions (TMs) provide amino acid residues that line putative binding sites for these positive modulators (Jenkins et al. 2001, Mihic et al. 1997, McCracken et al. 2010, Jenkins et al. 2002). However, the relative position of these amino acids is still uncertain, as the pocket they line could be located between (Fig. 1A) or within subunits (Fig. 1B) (Bertaccini et al. 2010).
Figure 1.
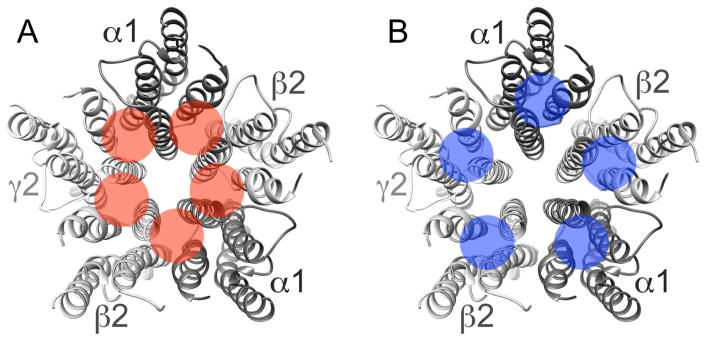
Inter- and intra-subunit putative binding sites. The numbered α-helices represent the transmembrane domains in the α1β2γ2 GABAAR, viewed from the extracellular domain. The shaded circles indicate the approximate location of the inter-(A) and intra- (B) subunit pockets where alcohol and inhaled anesthetics could bind.
Our early models of the related glycine receptor were based on the nicotinic acetylcholine receptor (Lobo et al. 2008). The crosslinking in the glycine receptor suggested that the amino acids critical for alcohol and inhaled anesthetics were located within each subunit. More recently, high-resolution crystals of channels that also belong to the Cys-loop LGIC family have provided new approaches to the modeling of GABAARs (McCracken et al. 2010). The C. elegans glutamate-gated chloride channel GluCl (Hibbs & Gouaux 2011), and Gloeobacter violaceus ligand-gated ion channel GLIC (Bocquet et al. 2009), are channels with high homology with the anionic GABAARs. GLIC is a bacterial cationic channel that is activated by protons. Several high-resolution X-ray structures have been obtained with ligands bound in GLIC crystals. The most relevant to our interests are ethanol (Sauguet et al. 2013) and desflurane (Nury et al. 2011). Desflurane (a negative modulator in GLIC) was found in an intra-subunit cavity within the wild-type GLIC subunit, while ethanol (a positive modulator) was located between subunits in a mutated GLIC that is more sensitive to ethanol due to a mutation in TM2 (Howard et al. 2011, Murail et al. 2012).
Our objective was to determine the relative location of amino acids critical for alcohol modulation and to update structural models based on recent high-resolution structures of related channels. We introduced cysteines in key TM locations of α1 and β2 subunits (Fig. 2): a cysteine located in either α1 TM1 (L232C) or β2 TM2 (N265C) was paired with a cysteine in different positions along β2 TM3 (positions 283–289), and a cysteine in α1 TM1 (Q229C) was paired with either β2 TM2 (N265C) or β2 TM3 (M286C), and a cysteine in β2 TM1 [β2(T225C)] was paired with cysteines in either β2 TM2 or TM3. We tested the proximity of cysteine pairs by applying reducing (dithiothreitol, DTT) and oxidizing (Cu++:phenanthroline) agents that may break or form disulfide bridges (crosslink) between cysteines that are close enough, such that, after crosslinking, the Cα- Cα distance is approximately 6.5 Å (Bass et al. 2007); usually these crosslinks alter GABA-induced currents. We also tested alcohol and inhaled anesthetics in GABAARs containing these cysteine combinations, after application of reducing or oxidizing agents. We expected reduced drug effects when cysteines are close enough to form a crosslink at the drug-binding site. In several cases, it was not possible to measure the effect of the drugs before and after establishing a disulfide bridge between cysteine pairs because the occurrence of spontaneous crosslinking introduced unstable conditions.
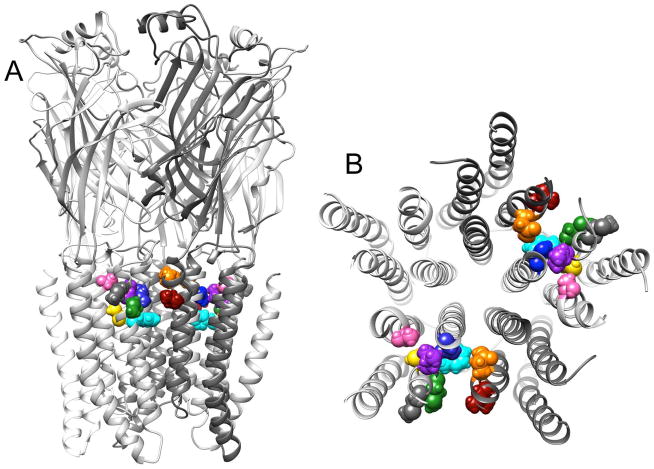
Figure 2.
Homology model of the α1β2γ2 GABAAR based on the GluCl structure. A. View from the side. B. View from the extracellular side, only of the transmembrane regions. The side chains of the amino acids studied are shown:
 , orange;
, orange;
 , dark red;
, dark red;
 , pink;
, pink;
 , blue;
, blue;
 , grey;
, grey;
 , purple;
, purple;
 , green;
, green;
 , yellow;
, yellow;
 , turquoise. β2G287 has no side chain, and β2Y284 did not express.
, turquoise. β2G287 has no side chain, and β2Y284 did not express.
We then tested single cysteine mutants in the positions that had participated in crosslinking using an alkyl methanethiosulfonate (MTS) reagent. We hypothesized that, if the cysteine was located in a water-filled cavity, the MTS group would react with the cysteine sulfhydryl group, leaving the alkyl thiol covalently bound to the cysteine, mimicking an irreversibly bound alcohol (Mascia et al. 2000). If the cavity was part of the alcohol binding site, then the GABA and the alcohol responses would be modified after the labeling.
2. Materials and Methods
We have reviewed the ARRIVE guidelines and we are in compliance with these guidelines. Xenopus laevis frogs were obtained from Nasco (Fort Atkinson, WI, USA). Dithiothreitol (DTT), Cu++, phenanthroline, ethanol, butanol and flunitrazepam were obtained from Sigma-Aldrich (St. Louis, MO). Isoflurane was obtained from Marsam Pharmaceuticals Inc. (Cherry Hill, NJ).
2.1. Clones
The complementary DNAs encoding the GABAA subunits rat α1 and γ2s and human β2 were provided by Dr. M. H. Akabas and Dr. Paul Whiting, respectively. Mutations in the α1, β2, and γ2s cDNAs were made through site-directed mutagenesis using a QuikChange kit (Agilent Technologies, Santa Clara, CA). Endogenous cysteines within the TM of all the subunits were mutated into alanines to be used as the wild-type control throughout the experiments (called Cys-less in the figures).
2.2. Transcription and Oocyte Injection
The in vitro transcription of wild-type and mutant α1, β2, and γ2s subunits was performed using mMessage mMachine (Life Technologies, Grand Island, NY). Extraction of ovarian tissue from Xenopus laevis frogs was in accordance with the National Institutes of Health guide for the care and use of laboratory animals. After isolation of Xenopus laevis oocytes, they were injected with capped complementary RNAs encoding wild-type or mutant α1, β2, and γ2s subunits in a ratio 2:2:20 ng. The injected oocytes were incubated at 15°C in sterilized Barth’s solution for 3–5 days before recording (McCracken et al. 2010). All surgery was performed according to an approved institutional protocol.
2.3. Electrophysiological recordings
The responses of GABAARs expressed in oocytes were studied through two-electrode voltage clamp (Oocyte Clamp OC-725C, Warner Instruments, Hamden, CT) (McCracken et al. 2010), recording through a PowerLab 4/30 system (ADInstruments, Colorado Springs, CO). The oocyte was placed in a chamber perfused with ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.5), and voltage-clamped at −70 mV. GABA applications lasted for 20–30 s and the interval between them was 5–15 min. DTT (2 mM) and Cu++:phenanthroline (100 and 200 μM, respectively) were applied for 2 min, after unclamping the oocyte. In order to test modulation with alcohols and inhaled anesthetics, the agents were first pre-applied for 1 min and then co-applied with GABA. Flunitrazepam was co-applied with GABA, with no pre-application.
2.4. Concentration-response curves
Increasing concentrations of GABA were applied (0.1–30,000 μM) and responses were expressed as percentages of the maximal current.
2.5. Redox protocol
The cross-linking application protocol was: EC50 GABA until a stable response was achieved or else four applications of EC50 GABA (whatever happened first), DTT, EC50 GABA, EC50 GABA, Cu++:phenanthroline, EC50 GABA, EC50 GABA. The response to GABA was expressed as a percent change of the initial response before the redox reagents. See Suppl. Fig. 1 for a representative tracing.
2.6. Allosteric modulator-redox protocol
The application sequence was as follows: Maximal GABA, DTT, EC10 GABA, EC10 GABA, pre-application of the drug immediately followed by a co-application with EC10 GABA, EC10 GABA, repeat after application of Cu++:phenanthroline. In the case of α1(Q229C)β2(N265C)γ2, we modified the application sequence, as the oxidation with Cu++:phenanthroline did not completely restore the GABA response to basal levels, and our concern was that after reduction, the cysteines were too far apart for Cu++:phenanthroline to crosslink them again. Therefore the application sequence was: Cu++:phenanthroline, Maximal GABA, EC10 GABA, EC10 GABA, pre-application of the drug immediately followed by a co-application with EC10 GABA, EC10 GABA, repeat after application of DTT. The response to GABA in the presence of a modulator was expressed as a percentage change compared to the mean of the previous and subsequent GABA responses.
2.7. Labeling protocol
The application sequence was as follows: Maximal GABA, EC10 GABA, EC10 GABA, application of butyl-MTS (BMTS, 0.5mM) in the absence or presence of maximal GABA, EC10 GABA, EC10 GABA. The responses to EC10 GABA were averaged (before and after-BMTS), and the change in the GABA response was expressed as a percent of the initial response before the labeling (Borghese et al. 2003).
2.8. Allosteric modulator-labeling protocol
The application sequence was as follows: Maximal GABA, EC10 GABA, EC10 GABA, pre-application of the drug immediately followed by a co-application with EC10 GABA, EC10 GABA, application of BMTS in the absence or presence of maximal GABA, repeat the pre-labeling sequence. The response to GABA in the presence of a modulator was expressed as a percentage change compared to the mean of the previous and subsequent GABA responses.
2.9. Statistical Analysis
Statistical analysis was performed in Prism 6.0 (GraphPad Software, La Jolla, CA). Pooled data are represented as mean ± S.E.M. (standard error of the mean). Statistical significance was determined using Student’s t-test, one- or two-way ANOVA, as indicated.
Nonlinear regression analysis was performed with Prism 6.0 by fitting the concentration-response curves to the following equation:
where I/IMAX is the fraction of the maximally-obtained GABA response, EC50 is the concentration of GABA producing a half-maximal response, [GABA] is GABA concentration and nH is the Hill coefficient. Agonist responses in each cell were normalized to the maximal current that could be elicited by GABA.
2.10. GABAA Model
We built a homology model of α1β2γ2 GABAARs by threading the primary sequence of these subunits onto the X-ray structure of GluCl (Protein Data Bank ID code 3RHW) (Hibbs & Gouaux 2011), as previously described for the case of a GLIC-based model (McCracken et al. 2010). We used the Modeler module of Discovery Studio 2.5 (Accelrys, San Diego, CA) to build models and chose the “best” model based on the force field potential energy measured by the Accelrys version of CHARMm. We used the “side-chain refinement” module of Discovery Studio 2.5 to optimize the side chain rotamers in the model. We tethered all backbone atoms with a quadratic restraint of 10 kcal/Å2 and optimized the resulting model to a gradient of 0.001 kcal/(mol × Å). Molecular graphics and distance analyses were performed with the UCSF Chimera package version 1.7 (Pettersen et al. 2004).
3. Results
There are several endogenous cysteines in the transmembrane domains of α1β2γ2 GABAARs: two in α1 (C234 and C293), one in β2 (C288), and two in γ2 (C244 and C303). We mutated them to alanines to eliminate the possibility that the cysteines we introduced crosslinked with endogenous cysteines. The function of the resulting α1β2γ2 Cys-less receptor [α1(C234A,C293A)β2(C288A)γ2(C244A,C303A)] was identical to the wild-type with respect to sensitivity to GABA and maximal GABA-induced currents (Suppl. Figure 2A and B). Its modulation by zinc, flunitrazepam and ethanol was essentially the same as WT (Suppl. Figure 2C–E). To be certain that each GABAAR contained one γ2 subunit, we also took the precaution of injecting γ2 cRNAs in excess (10:1:1 with respect to α1 and β2). Both the small inhibition by zinc that is caused by γ subunits (Trudell et al. 2008) and the potentiation by flunitrazepam (that requires a γ subunit) suggest that the receptors that were expressed in this study included a γ2 subunit, which is the most common composition of native GABAARs (Sigel & Steinmann 2012). The inclusion of γ2 subunits not only produces the more physiological composition of GABAARs, but also delivers bigger currents. All mutants were made in this Cys-less background.
The receptors containing the β2(Y284C) mutation did not yield measurable currents. We characterized the responses of all the other double mutants through GABA concentration-response curves (Suppl. Table 1 and Suppl. Figs. 3 and 4). When a double mutant proved to be of interest, we measured the GABA concentration-response curves in the corresponding single mutants. The single mutants α1(Q229C)β2γ2, α1(L232C)β2γ2, α1β2(T225C)γ2 and α1β2(M286C)γ2 GABAARs were less sensitive to GABA compared to α1β2γ2 Cys-less. Most of the double mutants showed a decreased sensitivity to GABA in different degrees, except for α1β2(N265C,L285C)γ2.
3.1. Redox treatment and GABA responses
We measured EC50 GABA-induced currents after DTT (2 mM, reducing agent) and after Cu++:phenanthroline (100/200 μM, oxidizing agent). We observed significant changes in the GABA-induced currents in several receptors combining pairs of cysteines, suggesting crosslinks (disulfide bridges) were formed between two cysteine residues. The Cα-Cα distances between the cysteine pairs studied are listed in Suppl. Table 2.
3.2. Cysteine pairs containing α1(L232C) (inter-subunit)
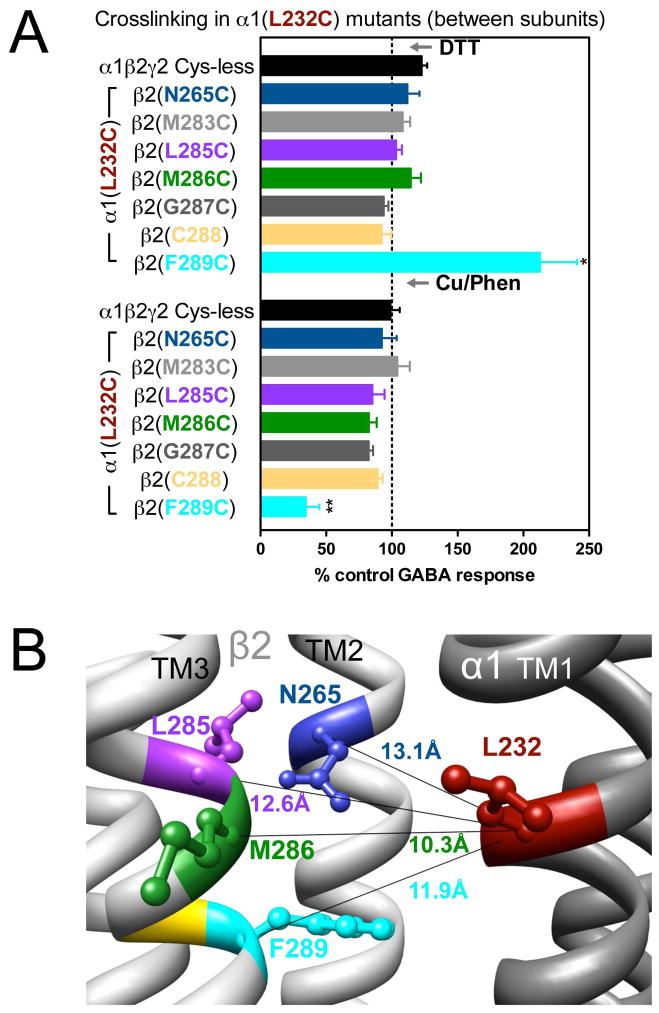
In α1(L232C)-containing GABAARs, only the combination α1(L232C)β2(F289C)γ2 showed significant changes in GABA-induced responses: an increase after DTT and a decrease after Cu++:phenanthroline (Fig. 3A). In Fig. 3B, the four shortest estimated Cα-Cα distances between α1(L232C) and amino acids in β2 TM3 are shown. Note that β2(M286C), the cysteine that, according to the model, is the closest to α1(L232C), did not show any evidence of crosslinking (Fig. 3A). We tried modifying the redox protocol, by applying Cu++:phenanthroline in the presence of GABA (3 mM GABA), and also using maximal GABA concentration instead of EC10 GABA, but we did not observe any changes in the GABA-induced currents under any conditions (data not shown).
Figure 3.
Inter-subunit crosslinking:
 (TM1) and cysteines in TM2 and TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application [EC50 GABA were used, except for
(TM1) and cysteines in TM2 and TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application [EC50 GABA were used, except for
 , in which spontaneous crosslinking prevented measuring EC50, therefore 100 μM GABA was used). *p< 0.05 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–7). B. The four closest amino acids to
, in which spontaneous crosslinking prevented measuring EC50, therefore 100 μM GABA was used). *p< 0.05 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–7). B. The four closest amino acids to
 are shown, along with the respective Cα-Cα distances, as seen from the membrane, between β2 and α1 subunits.
are shown, along with the respective Cα-Cα distances, as seen from the membrane, between β2 and α1 subunits.
3.3. Cysteine pairs containing α1(Q229C) (inter-subunit)
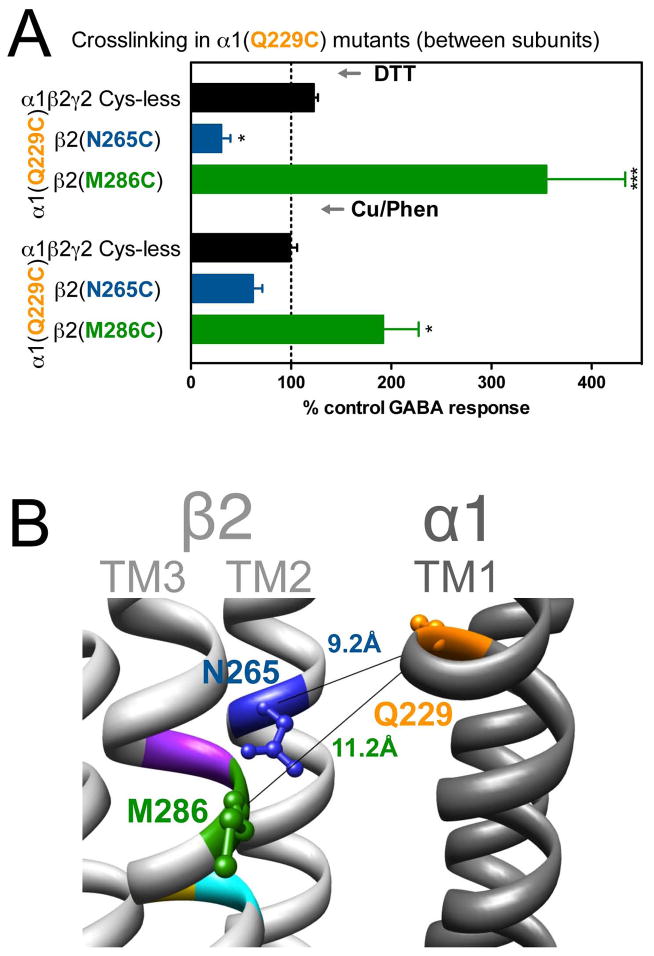
In α1(Q229C)-containing GABAARs, the two combinations tested showed significant changes in GABA-induced responses: in α1(Q229C)β2(N265C)γ2, GABA-induced responses were decreased after DTT, and in α1(Q229C)β2(M286C)γ2 GABA-induced responses were increased after DTT, with no reversal after treatment with Cu++:phenanthroline (Fig. 4A). The corresponding Cα-Cα distances are given in Fig. 4B.
Figure 4.
Inter-subunit crosslinking:
 (TM1) and cysteines in TM2 and TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used]. *p< 0.05, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 6). B. The three amino acids are shown in the model, along with the respective Cα-Cα distances, as seen from the membrane, between β2 and α1 subunits.
(TM1) and cysteines in TM2 and TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used]. *p< 0.05, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 6). B. The three amino acids are shown in the model, along with the respective Cα-Cα distances, as seen from the membrane, between β2 and α1 subunits.
3.4. Cysteine pairs containing β2(N265C) (intra-subunit)
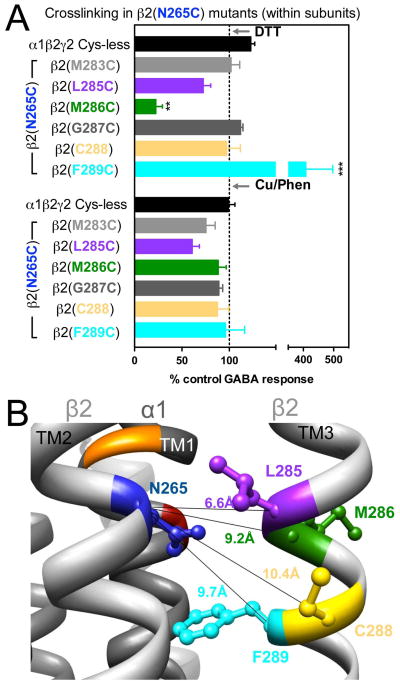
In β2(N265C)-containing GABAARs, two combinations showed significant changes in GABA-induced responses: in α1β2(N265C,M286C)γ2, GABA-induced responses were decreased after DTT, and in α1β2(N265C,F289C)γ2, GABA-induced responses were increased after DTT. In both cases, after Cu++:phenanthroline the GABA-induced responses tended to return to control levels (Fig. 5A), suggesting spontaneous crosslinking during expression. In Fig. 5B, the four shortest Cα-Cα distances between β2(N265C) and amino acids in β2 TM3 are shown.
Figure 5.
Intra-subunit crosslinking:
 (TM2) and cysteines in TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used). **p< 0.01, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–8). B. The four closest amino acids to
(TM2) and cysteines in TM3 of β2 subunit. A. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used). **p< 0.01, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–8). B. The four closest amino acids to
 are shown, along with the respective Cα-Cα distances, as seen from the center of the subunit.
are shown, along with the respective Cα-Cα distances, as seen from the center of the subunit.
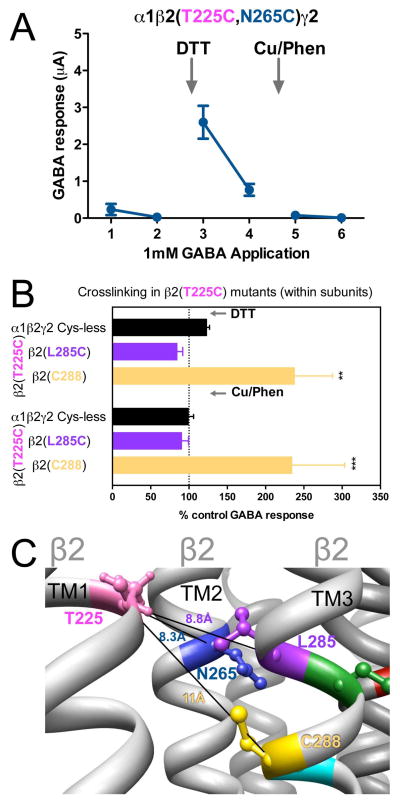
3.5. Cysteine pairs containing β2(T225C) (intra-subunit)
In β2(T225C)-containing GABAARs, two combinations showed significant changes in GABA-induced responses. In α1β2(T225C,N265C)γ2, GABA-induced responses were very small, increased after DTT treatment, spontaneously decreased with air oxidation, to finally become no longer measureable after Cu++:phenanthroline treatment (Fig. 6A). In α1β2(T225C,C288)γ2, GABA-induced responses were increased after DTT, and did not return to control levels after Cu++:phenanthroline treatment (Fig. 6B), suggesting spontaneous crosslinking during expression, probably during a step that brings the cysteines closer than in the final conformation, as the crosslink could not be regenerated in the presence of Cu++:phenanthroline. In Fig. 6C, the three Cα-Cα distances between β2(T225C) and the amino acids tested in β2 TM1 and TM3 are shown.
Figure 6.
Intra-subunit crosslinking:
 (TM1) and cysteines in TM2 and TM3 of β 2 subunit. A. GABA responses after DTT and Cu++:Phen application (1 mM GABA were used). B. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used). **p< 0.01, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–8). C. The four amino acids are shown, along with the respective Cα-Cα distances, as seen from TM4.
(TM1) and cysteines in TM2 and TM3 of β 2 subunit. A. GABA responses after DTT and Cu++:Phen application (1 mM GABA were used). B. GABA responses after DTT and Cu++:Phen application (EC50 GABA were used). **p< 0.01, ***p< 0.001 versus Cys-less receptor; two-way ANOVA, and Bonferroni post-tests (n= 5–8). C. The four amino acids are shown, along with the respective Cα-Cα distances, as seen from TM4.
3.6. Redox treatment in single cysteine mutants
To control for cysteines reacting with amino acids other than the introduced cysteines, we measured GABA-induced responses after DTT and Cu++:phenanthroline in the single mutants corresponding to the double mutants that showed changes after this protocol; no significant changes were observed (Suppl. Fig. 5).
3.7. Redox treatment and drug modulation of GABA responses
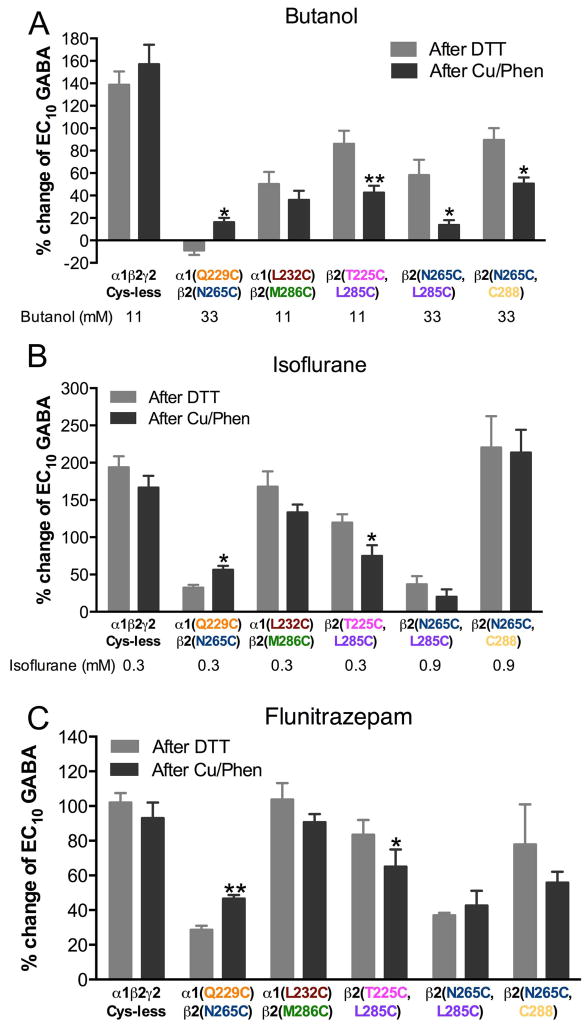
There were other cysteine combinations that, based on the GABAAR model, could be expected to crosslink, but their GABA-induced responses did not change after redox treatment. These double mutants were α1(Q229C)β2(N265C)γ2, α 1(L232C)β2(M286C)γ2, α1β2(T225C,L285C)γ2, α1β2(N265C,L285C)γ2 and α 1β2(N265C,C288)γ2. One possibility is that the cysteines are so close that, when they crosslink, the disulfide bridge does not have an impact on the GABAAR function; we could consider this a “silent” crosslink. However, if those cysteines are part of a drug-binding site, and they form a disulfide bridge across this site, this would prevent drug binding. To test this hypothesis, we studied butanol and isoflurane, two drugs assumed to bind in the vicinity of these amino acids. We also studied flunitrazepam, as a negative control, since the flunitrazepam binding site is located over 20 Å away at the extracellular interface between α1 and γ2 subunits (Trudell et al. 2012, Trudell 2002). We tested the effects of these compounds on EC10 GABA-induced currents, after DTT and Cu++:phenanthroline treatments. We used butanol instead of ethanol because several mutations decreased the ethanol effect to the point of eliminating it.
We observed no changes in drug effects on α1(L232C)β2(M286C)γ2 after DTT and Cu++:phenanthroline applications (Fig. 7). However, there was a significant reduction in the butanol potentiation of GABA-induced currents after oxidation (induction of disulfide bridges) in α1β2(T225C,L285C)γ2, α1β2(N265C,L285C)γ2 and α1β2(N265C,C288)γ2 (Fig. 7A). After oxidation, there was a decrease in isoflurane potentiation only in α1β2(T225C,L285C)γ2 (Fig. 7B). In the case of α1(Q229C)β2(N265C)γ2, all the drug effects were increased after oxidation (Fig. 7); because the latter changes also induced flunitrazepam potentiation, they are not considered specific. There were no changes in flunitrazepam potentiation for the other combinations (Fig. 7C), except for α1β2(T225C,L285C)γ2, which was relatively small (−22%) compared with the change in the butanol potentiation (−51%).
Figure 7.
Crosslinking and drug effects. The effects of three drugs on EC10 GABA responses were tested after DTT and after Cu++:Phen application. A. Butanol (11 mM and 33 mM). B. Isoflurane (0.3 and 0.9 mM). C. Flunitrazepam (0.1 μM). (n= 4–6). *p< 0.05, **p< 0.01 versus after DTT; Student’s t-test.
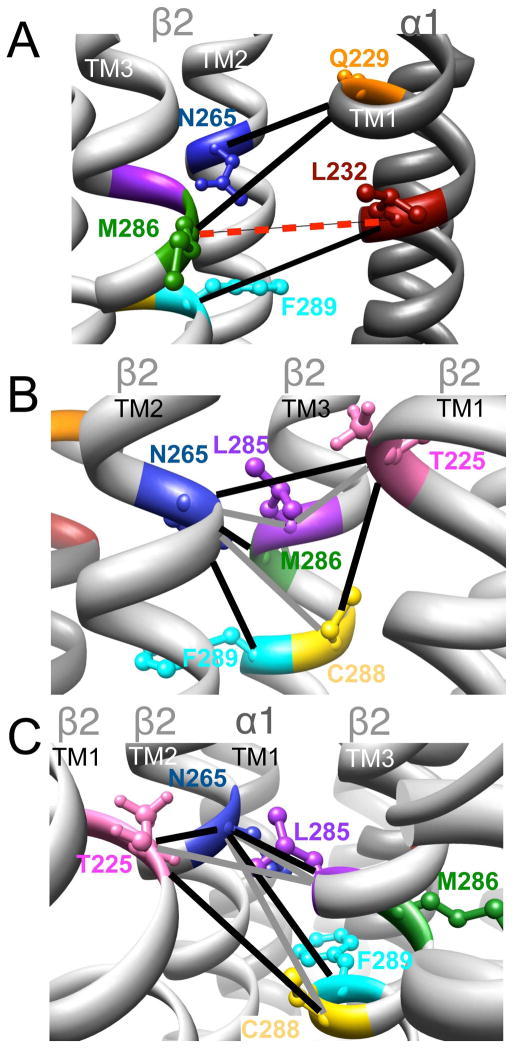
A summary of the changes observed in GABA responses and butanol effect after crosslinking is given in Table 1, and the corresponding amino acids are highlighted in the model in Fig. 8.
Table 1.
Changes observed in GABA-induced responses and butanol modulation of GABA-induced responses in selected combinations of GABAAR cysteine mutants after reducing (DTT) and oxidizing (Cu++/Phen) treatment. Change is compared with the control (Cys-less receptor) in equivalent conditions. The color indicates the color of the amino acid in the model representations.
| GABAA receptor | GABA responsesa | Butanol effectb | |
|---|---|---|---|
|
| |||
| After DTT | After Cu++/Phen | After crosslink | |
| α1β2γ2 Cys-less (control) | No change | No change | No change |
|
Inter-subunit
| |||
|
|
Decrease | No change | Increasec |
|
|
Increase | Increase | Spontaneous crosslink |
|
|
No change | No change | No change |
|
|
Increase | Decrease | Spontaneous crosslink |
|
Intra-subunit
| |||
|
|
Increase | Decrease | Spontaneous crosslink |
|
|
No change | No change | Decrease |
|
|
Increase | Increase | Spontaneous crosslink |
|
|
No change | No change | Decrease |
|
|
Decrease | No change | Spontaneous crosslink |
|
|
No change | No change | Decrease |
|
|
Increase | No change | Increasec |
EC50 GABA, except α1(L232C)β2(F289C)γ2 (100 μM) and α1β2(T225C,N265C)γ2 (1 mM)
Butanol modulation of EC10 GABA
Probably global effect, as it also showed changes in the flunitrazepam effect
Figure 8.
Homology model of the α1β2γ2 GABAAR, with relevant amino acids and their interaction between (A) and within (B and C) subunits. A. Three cysteine pairs [
 ,
,
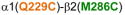 , and
, and
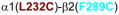 ] showed changes in GABA-induced responses (black lines) after redox treatment. The closest cysteine pair between the α1 and β2 subunits [
] showed changes in GABA-induced responses (black lines) after redox treatment. The closest cysteine pair between the α1 and β2 subunits [
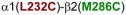 ] did not show any evidence of crosslinking (dotted line, in red). B and C (same TMs from two different viewpoints). Four cysteine pairs within the β2 subunit [
] did not show any evidence of crosslinking (dotted line, in red). B and C (same TMs from two different viewpoints). Four cysteine pairs within the β2 subunit [
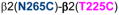 ,
,
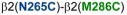 ,
,
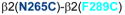 , and
, and
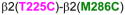 ] showed changes in GABA-induced responses (black lines) after redox treatment, indicative of crosslinking. Three additional pairs [
] showed changes in GABA-induced responses (black lines) after redox treatment, indicative of crosslinking. Three additional pairs [
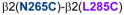 ,
,
 , and
, and
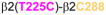 ] showed changes in the butanol potentiation (grey lines) after redox treatment, indicative of “silent crosslinking”: GABA responses were not affected, but the effect of the drug binding at the pocket where the disulfide bridge occurs is modified.
] showed changes in the butanol potentiation (grey lines) after redox treatment, indicative of “silent crosslinking”: GABA responses were not affected, but the effect of the drug binding at the pocket where the disulfide bridge occurs is modified.
3.8. Drug effects on single cysteine mutants
Butanol potentiation of GABA responses was decreased in α1(Q229C)-, α 1(L232C)-, β2(N265C)-, β2(L285C)-, β2(M286C)- and β2(F289C)-containing GABAARs compared with the Cys-less receptor (Suppl. Fig. 6A). Isoflurane and flunitrazepam potentiation of GABA responses was decreased only in β2(F289C)-containing GABAARs (Suppl. Figs. 6B and C). The mutation of β2F289 to cysteine seems to impair greatly the ability of the receptor to respond to any modulator.
3.9. BMTS labeling and GABA responses in single cysteine mutants
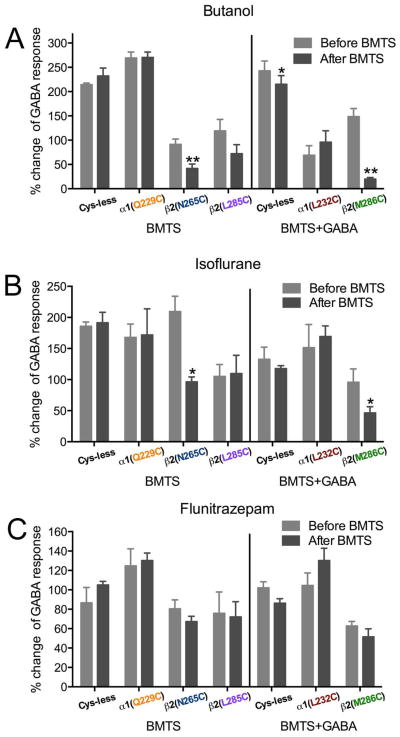
We tested eight single cysteine mutants by determining the EC10 GABA, applying BMTS, and then retesting the GABA responses (Fig. 9A). In two of the mutants, α 1(Q229C)- and β2(F289C)-containing GABAARs, GABA responses were decreased after BMTS treatment. In two others, β2(N265C)- and β2(L285C)- containing GABAARs, GABA responses were increased after BMTS treatment. In the other four mutants [α1(L232C)-, β2(T225C)-, β2(M286C)- and β2C288-containing GABAARs], there was no net change in the GABA responses, although the considerable dispersion of the data for β 2(T225C)- and β2(M286C)- containing GABAARs suggested that the labeling was producing an inconsistent effect.
Figure 9.
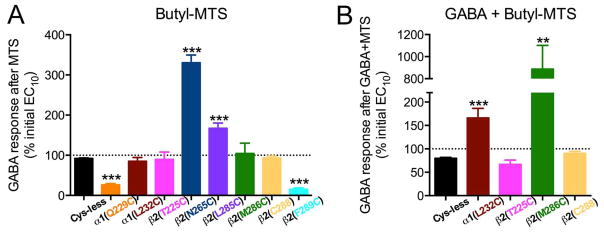
Alkyl labeling of single cysteine mutants and GABA responses. The effects of butyl methanethiosulfonate (BMTS) labeling on EC10 GABA responses were tested on single cysteine mutants. Changes observed after labeling in the absence (A) and presence (B) of maximal GABA. **p< 0.01, ***p< 0.001 versus Cys-less receptor; Student’s t- test, with post-hoc Bonferroni correction.
We tested labeling conditions in the presence of GABA for the four mutants that showed no net change in the GABA responses after BMTS treatment. After co-applying BMTS with a maximally effective GABA concentration, two of the mutants showed increased GABA responses: α1(L232C)- and β2(M286C)-containing GABAARs (Fig. 9B).
3.10. BMTS labeling and drug modulation of GABA responses in single cysteine mutants
We determined the butanol, isoflurane, and flunitrazepam effects on GABA responses in single cysteine mutants before and after labeling the cysteine with BMTS, under the conditions previously shown to induce changes in the GABA response, i.e., labeling (Fig. 10). After BMTS labeling, butanol potentiation was decreased in β2(N265C)- and β2(M286C)-containing GABAARs; it was not significantly changed in the control Cys-less GABAAR (Fig. 10A). Isoflurane potentiation was also decreased after BMTS labeling in those same mutants (Fig. 10B), while the flunitrazepam potentiation was not modified significantly by the treatment (Fig. 10C). The only case in which all drug effects were changed by the BMTS treatment was α1β2(F289C)γ2, in which all drug effects (including flunitrazepam) switched from very small, and even inhibitory effects, to potentiation (data not shown), and we therefore considered it to be a global effect on activation or gating.
Figure 10.
Alkyl labeling of single cysteine mutants and drug effects. The effects of three drugs on EC10 GABA responses were tested before and after butyl methanethiosulfonate (BMTS) labeling, in the absence or presence of GABA, in single cysteine mutants. A. Butanol (33 mM). B. Isoflurane (0.3 mM). C. Flunitrazepam (0.1 μM). (n= 3–5). *p< 0.05, **p< 0.01 versus Cys-less receptor; Student’s t-test.
A summary of the changes observed in GABA-induced responses and butanol effect after labeling with BMTS is given in Table 2.
Table 2.
Changes observed in GABA-induced responses and butanol modulation of GABA-induced responses in selected single cysteine GABAAR mutants after butyl methanethiosulfonate (BMTS) labeling. The color indicates the color of the amino acid in the model representations.
| GABAA receptor | GABA responses1 | Butanol effect2 | |
|---|---|---|---|
|
| |||
| BMTS | BMTS + GABA | ||
| α1β2γ2 Cys-less (control) | No change | No change | No change/ Small decrease |
|
|
Decrease | NT3 | No change |
|
|
No change | Increase | No change |
|
|
No net change | No net change | NT |
|
|
Increase | NT | Decrease |
|
|
Increase | NT | No change |
|
|
No net change | Increase | Decrease |
|
|
No change | No change | NT |
|
|
Decrease | NT | Increase4 |
EC10GABA
Butanol modulation of EC10 GABA
Not tested
Also changes in the flunitrazepam effect
4. Discussion
In order to assess the relative location of several amino acids in the extracellular half of the TM domain in the GABAAR and their relevance to alcohol binding, we introduced Cys in the relevant positions of α1 and β2 subunits. Several pairs of cysteines introduced in TM segments 1, 2, and 3 showed crosslinking. The two amino acids that showed the most influence on alcohol action were β2N265 and β2M286, previously identified in TM2 and TM3 of the β2 subunit as critical for alcohol modulation (Mihic et al. 1997, McCracken et al. 2010). Not only were they close enough to crosslink when replaced by Cys, but the GABA response was increased several fold after labeling the single Cys mutants with BMTS. In addition, this labeling reduced a subsequent potentiation by butanol and isoflurane, suggesting that these residues are lining a cavity where alcohols and inhaled anesthetics bind to produce potentiation of the GABA responses.
The cysteine in β2N265 also showed intra-subunit crosslinking with β2(F289C) in TM3 and β2(T225C) in TM1, as evidenced by changes in GABA-induced responses. Another pair that displayed intra-subunit crosslinking was β2(T225C) and β2C288, in TM1 and TM3 respectively. Three other Cys pairs showed changes in butanol potentiation after redox treatment, also indicative of crosslinking: β2(N265C) in TM2 with (1) β2(L285C) and (2) β2C288 in TM3, and (3) β2(T225C) in TM1 with β2(L285C) in TM3. In three out of four intra-subunit crosslinks that reduced butanol potentiation, one of the Cys involved was in the β2N265 position, highlighting the crucial role of this amino acid. Even though β2(N265C) and (L285C) are in close proximity in the model, we did not observe changes in the GABA responses after redox treatment. We hypothesize that these two Cys were in such close proximity that the presence or absence of a crosslink had no impact on the receptor function; therefore the GABA responses were not modified by the redox treatment. In two of the crosslinking pairs that were affected by butanol, β2L285 was present. The single β2(L285C)-containing mutant also showed a reduced sensitivity to butanol potentiation, but even though its labeling with BMTS produced a moderate increase in GABA responses, it did not significantly affect the potentiation of a subsequent application of butanol. This discrepancy between the results with BMTS labeling (alcohol binding mimic) and crosslinking (showing proximity between cysteines) may be due to the different consequences that each one produces: MTS labeling will occupy a water-filled pocket, and when said pocket is an alcohol binding site, it may have functional consequences, while crosslinking could hinder relative motion between TMs, and occlude or dramatically change the shape of the alcohol binding site. The lack of changes in alcohol action in the β2(L285C)-containing mutant after BMTS suggests that this position is not crucial for alcohol action, even though the changes observed in alcohol modulation after crosslinking suggest it is close to the alcohol binding pocket.
The closest Cys pair between the α1 and β2 subunits [α1(L232C) and β 2(M286C)] did not show any evidence of crosslinking, also shown by Bali et al. (2009). Three other pairs [α1(Q229C)-β2(N265C), α1(Q229C)-β2(M286C), and α1(L232C)-β2(F289C)] showed changes in GABA-induced responses after redox treatment. The last two of these three crosslinks had already been identified through changes in GABA responses and Western blots (Bali et al. 2009).
It is important to keep in mind that the models represent only one of the possible conformations that the receptor can adopt. In the case of the GABAAR in particular, crosslinking studies have shown that the extracellular half of the TM2 is highly dynamic (Goren et al. 2004, Horenstein et al. 2001, Bera & Akabas 2005). Although the mobility of TM3 is not certain, this segment is certainly not fixed in a static position (Jung et al. 2005, Otero-Cruz et al. 2007)
It is likely that the relative positions of the cysteines change as the receptor adopts different conformations. For instance, an etomidate binding site in GABAARs has been characterized using photolabeling techniques with etomidate analogs (Chiara et al. 2012, Chiara et al. 2013, Li et al. 2006). In all cases, βM286 is a clear participant in the etomidate binding site, along with αTM1 amino acids, indicating that the etomidate binding site is inter-subunit. Additionally, results from the Akabas’ (Bali et al. 2009) and our group have shown that β2(M286C) crosslinks with cysteines introduced into α1TM1. However, we showed here that β2(M286C) also crosslinked with β2(N265C) in TM2 of the same subunit, which means that TM3 can rotate enough for β2(M286C) to crosslink within the subunit, exhibiting a high degree of mobility.
Inter-subunit crosslinks occurred between α1(Q229C) and both β2(N265C) and β2(M286C), which have critical roles on alcohol modulation. However, the introduction of Cys in the α1Q229 position did not affect butanol potentiation in the single mutant, even though it greatly decreased GABA sensitivity. The MTS labeling of the single mutant yielded similar results: BMTS labeling decreased GABA-induced responses, but did not affect a subsequent butanol potentiation. This result suggests that α1Q229 is relevant for the gating process, but does not line an alcohol binding site, even though it is close enough to crosslink with β2(N265C) and β2(M286C). When we tested drug modulation in α1(Q229C)β2(N265C)γ2 receptors before and after redox treatment, both butanol and isoflurane potentiation were increased after the formation of a crosslink. We encountered a similar situation with α1β2(F289C)γ2, both before and after labeling with BMTS. Both α1Q229 and β2F289 face across the inter-subunit interface in at least in one conformation, and this would suggest the presence of an inhibitory inter-subunit alcohol binding pocket (when the crosslink is formed, butanol and isoflurane cannot bind and inhibit the GABA response, so the net drug effect through the other available binding pockets results in potentiation). However, the flunitrazepam effect was also markedly increased in both cases. As flunitrazepam binds at the α-γ interface of the extracellular domain, far from where the crosslink/labeling is taking place, we conclude that F289 is important in the transmission of agonist binding energy to the gating process.
Recent photoaffinity labeling studies support multiple inter-subunit binding sites for etomidate, propofol and pentobarbital (Chiara et al. 2013, Li et al. 2006). In addition, the X-ray crystal structure of an ethanol-sensitive GLIC supports an inter-subunit binding site for alcohol (Sauguet et al. 2013). Interestingly, neither octanol nor ethanol could block an etomidate analog photolabeling of bovine GABAAR, while isoflurane could (Li et al. 2010). However, octanol in the presence of GABA blocked photolabeling of recombinant α1β3γ2 GABAARs (Chiara et al. 2013). It is tempting to conclude that positive modulators of the GABAAR bind at inter-subunit cavities to exert their potentiation. Nevertheless, not all evidence points to inter-subunit sites: co-crystalization studies in GLIC crystal show desflurane and propofol bound in an intra-subunit cavity (Nury et al. 2011). Published data from photoaffinity labeling and X-ray crystallography are summarized in Suppl. Table 3.
The general correlation between the crosslinking data and the GluCl-based model we used is very good, corroborating the excellence of this model, which applies very well to intravenous anesthetic actions (Bertaccini et al. 2013). However, there were some inconsistencies: first, the closest cysteines studied at the interface, α1(L232C) and β2(M286C), were not able to crosslink under any experimental condition tested. Second, the crosslinks formed, both inter-subunit (αTM1-βTM3) and intra-subunit (within the βTMs), are clearly slanted (Figure 8), with TM3 seemingly displaced towards the intracellular side with respect to the other TMs. These observations suggest that the vertical alignment of TM3 with respect to the other TMs in the model is not precise, and that TM3 should be vertically displaced towards the extracellular side to adequately adjust to the experimental data. The GluCl crystal structure was obtained in the presence of ivermectin, which stabilizes an open-pore conformation (Hibbs & Gouaux 2011), so the model based on this structure could be more accurately reflecting an open (or desensitized) conformation of the channel. However, we tried crosslinking the pair of cysteines that was most in disagreement with the model [α1(L232C)-β2(M286C)] in the presence of GABA under different conditions, and the crosslinking never occurred, while two other pairs [α1(Q229C)-β2(M286C) and α1(L232C)-β2(F289C)] were formed in a regular pattern (the bridge-forming cysteines in each helix were separated by the same interval). Thus, the experimental data obtained from GABAARs presents a general agreement with the GluCl-based model, but not with respect to the vertical alignment of TM3 versus the other TMs.
We report in this study new intra- and inter-subunit crosslinking data in the GABAAR TM regions, focusing on amino acids critical for alcohol and inhaled anesthetic effects. Through crosslinking and labeling techniques, we conclude there are β2 intra-subunit alcohol/inhaled anesthetic binding pockets. The results provided no evidence for similar inter-subunit binding pockets for butanol and isoflurane. The homology model based on the GluCl structure has a general agreement with the crosslinking data within the β2 subunit, but overall, the relative position of the cysteines that crosslink suggest that the position of β2TM3 relative to the neighboring TMs (β2TM1, β2TM2, and α1TM1) is not represented accurately in the model.
We conclude that both GLIC and the superfamily of Cys-loop receptors possess alcohol/inhaled anesthetic binding sites in the extracellular half of the TM domains, but the precise binding location depends on the drug and the receptor. There appears to be no absolute rule about where (intra- or inter-subunit) anesthetics bind, and no relation between the location of the binding site and the kind of modulation obtained (see Suppl. Table 3). For example, in the mutated alcohol-sensitive GLIC, inter-subunit binding of ethanol potentiates the channel function (Howard et al. 2011), while intra-subunit binding of propofol and desflurane to wild-type GLIC inhibits (Weng et al. 2010); in GABAARs, binding of anesthetics to either inter- or intra-subunit binding sites appears to potentiate receptor function. In this study, we define β2 intra-subunit alcohol/inhaled anesthetic binding pockets critical for modulation of α1β2γ2 GABAARs.
Supplementary Material
Acknowledgments
This study was supported by a grant from the National Institutes of Health (AA06399). All authors declare no conflict of interest. C.M.B., J.A.H., D.J.L. and R.A.H. thank Mendy Black and Aanika Das for excellent technical assistance, and Jody Mayfield for outstanding editorial assistance. No conflicts of interest, financial or otherwise, are declared by the authors.
Abbreviations
- GABAAR
γ-aminobutyric acid type A receptor
- LGIC
ligand-gated ion channel
- TMs
transmembrane regions
- GluCl
C. elegans glutamate-gated chloride channel
- GLIC
Gloeobacter violaceus ligand-gated ion channel
- DTT
dithiothreitol
- MTS
methanethiosulfonate
- BMTS
butyl methanethiosulfonate
- LGIC
ligand-gated ion channel
Contributor Information
Cecilia M. Borghese, Email: cborghese@austin.utexas.edu.
Jessica A. Hicks, Email: jess_ica_08@hotmail.com.
Daniel J. Lapid, Email: dlapid@utexas.edu.
James R. Trudell, Email: trudell@stanford.edu.
References
- Bali M, Jansen M, Akabas MH. GABA-induced intersubunit conformational movement in the GABAA receptor α1M1-β2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3083–3092. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass RB, Butler SL, Chervitz SA, Gloor SL, Falke JJ. Use of site-directed cysteine and disulfide chemistry to probe protein structure and dynamics: applications to soluble and transmembrane receptors of bacterial chemotaxis. Methods in enzymology. 2007;423:25–51. doi: 10.1016/S0076-6879(07)23002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera AK, Akabas MH. Spontaneous thermal motion of the GABAA receptor M2 channel-lining segments. The Journal of biological chemistry. 2005;280:35506–35512. doi: 10.1074/jbc.M504645200. [DOI] [PubMed] [Google Scholar]
- Bertaccini EJ, Wallner B, Trudell JR, Lindahl E. Modeling anesthetic binding sites within the glycine alpha one receptor based on prokaryotic ion channel templates: the problem with TM4. Journal of chemical information and modeling. 2010;50:2248–2255. doi: 10.1021/ci100266c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini EJ, Yoluk O, Lindahl ER, Trudell JR. Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor. Anesthesiology. 2013 doi: 10.1097/ALN.0b013e31829e47e3. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. Sites of excitatory and inhibitory actions of alcohols on neuronal α2β4 nicotinic acetylcholine receptors. The Journal of pharmacology and experimental therapeutics. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [3H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–847. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 gamma-aminobutyric acid type A (GABAA) receptor. The Journal of biological chemistry. 2013;288:19343–19357. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren EN, Reeves DC, Akabas MH. Loose protein packing around the extracellular half of the GABAA receptor β1 subunit M2 channel-lining segment. The Journal of biological chemistry. 2004;279:11198–11205. doi: 10.1074/jbc.M314050200. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nature neuroscience. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Murail S, Ondricek KE, Corringer PJ, Lindahl E, Trudell JR, Harris RA. Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12149–12154. doi: 10.1073/pnas.1104480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Andreasen A, Trudell JR, Harrison NL. Tryptophan scanning mutagenesis in TM4 of the GABAA receptor α1 subunit: implications for modulation by inhaled anesthetics and ion channel structure. Neuropharmacology. 2002;43:669–678. doi: 10.1016/s0028-3908(02)00175-2. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, et al. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Akabas MH, Harris RA. Functional and structural analysis of the GABAA receptor α1 subunit during channel gating and alcohol modulation. The Journal of biological chemistry. 2005;280:308–316. doi: 10.1074/jbc.M409871200. [DOI] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to γ-aminobutyric acid type A (GABAA) receptors. The Journal of biological chemistry. 2010;285:8615–8620. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABAA receptors and alcohol. Pharmacology, biochemistry, and behavior. 2008;90:90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA, Trudell JR. Cross-linking of sites involved with alcohol action between transmembrane segments 1 and 3 of the glycine receptor following activation. Journal of neurochemistry. 2008;104:1649–1662. doi: 10.1111/j.1471-4159.2007.05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken ML, Borghese CM, Trudell JR, Harris RA. A transmembrane amino acid in the GABAA receptor β2 subunit critical for the actions of alcohols and anesthetics. The Journal of pharmacology and experimental therapeutics. 2010;335:600–606. doi: 10.1124/jpet.110.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Murail S, Howard RJ, Broemstrup T, Bertaccini EJ, Harris RA, Trudell JR, Lindahl E. Molecular mechanism for the dual alcohol modulation of Cys-loop receptors. PLoS computational biology. 2012;8:e1002710. doi: 10.1371/journal.pcbi.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- Otero-Cruz JD, Baez-Pagan CA, Caraballo-Gonzalez IM, Lasalde-Dominicci JA. Tryptophan-scanning mutagenesis in the αM3 transmembrane domain of the muscle-type acetylcholine receptor. A spring model revealed. The Journal of biological chemistry. 2007;282:9162–9171. doi: 10.1074/jbc.M607492200. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera — a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Adron Harris R, Delarue M. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nature communications. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. The Journal of biological chemistry. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell J. Unique assignment of inter-subunit association in GABAA α1β3γ2 receptors determined by molecular modeling. Biochimica et biophysica acta. 2002;1565:91–96. doi: 10.1016/s0005-2736(02)00512-6. [DOI] [PubMed] [Google Scholar]
- Trudell JR, Bertaccini E, Maciver MB. Teaching an old GABA receptor new tricks. Anesthesia and analgesia. 2012;115:270–273. doi: 10.1213/ANE.0b013e31824a0b3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell JR, Yue ME, Bertaccini EJ, Jenkins A, Harrison NL. Molecular modeling and mutagenesis reveals a tetradentate binding site for Zn2+ in GABAA αβ receptors and provides a structural basis for the modulating effect of the γ subunit. Journal of chemical information and modeling. 2008;48:344–349. doi: 10.1021/ci700324a. [DOI] [PubMed] [Google Scholar]
- Weng Y, Yang L, Corringer PJ, Sonner JM. Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesthesia and analgesia. 2010;110:59–63. doi: 10.1213/ANE.0b013e3181c4bc69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annual review of pharmacology and toxicology. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.