Abstract
Idiopathic generalized epilepsy represents about 30–35% of all epilepsies in humans. The bromodomain BRD2 gene has been repeatedly associated with the subsyndrome of juvenile myoclonic epilepsy. Our previous work determined that mice haploinsufficient in Brd2 (Brd2+/−) have increased susceptibility to provoked seizures, develop spontaneous seizures and have significantly decreased GABA markers in the direct basal ganglia pathway as well as in the neocortex and superior colliculus. Here we tested male and female Brd2+/− and wild type littermate mice in a battery of behavioral tests (open field, tube dominance test, elevated plus maze, Morris water maze and Barnes maze) to identify whether Brd2 haploinsufficiency is associated with the human behavioral patterns, so-called juvenile myoclonic epilepsy personality. Brd2+/− females but not males consistently displayed decreased anxiety. Further, we found a highly significant dominance trait (aggression) in the Brd2+/− mice compared to the wild type, more pronounced in females. Brd2+/− mice of either sex did not differ from wild type mice in spatial learning and memory tests. Compared to wild type littermates, we found decreased numbers GABA neurons in the basolateral amygdala, which is consistent with the increase in aggressive behavior. Our results indicate that Brd2+/− haploinsufficient mice show no cognitive impairment but have behavioral traits similar to those found in patients with juvenile myoclonic epilepsy (recklessness, aggression). This suggests that either the BRD2 gene is directly responsible for influencing many traits of juvenile myoclonic epilepsy or it controls upstream regulators of individual phenotypes.
Keywords: aggression, anxiety, cognition, idiopathic generalized epilepsy, personality, Brd2 haploinsufficiency, Morris Water Maze, Barnes maze, open field
Introduction
Idiopathic generalized epilepsy (IGE) represents about 30–35% of all epilepsies (Annegers, 1994). Previous and current research indicates that the patients with juvenile myoclonic epilepsy (JME; a subform of IGE) have personality disorders that include impulsive personality traits and higher novelty seeking behavior (Moschetta et al., 2011, Wandschneider et al., 2013) that are not associated with other forms of epilepsy. Patients with JME also show less self-control than matched healthy subjects (Plattner et al., 2007). Magnetic resonance spectroscopy and imaging studies suggest the involvement of prefrontal cortex in these epilepsy patients, and impairments in the prefrontal cortex may also be involved in associated behavioral traits (De Araujo Filho et al., 2009a, De Araujo Filho et al., 2009b, Koepp et al., 2013, Piazzini et al., 2008). On the other hand, JME patients usually do not have serious cognitive deficits (Moschetta & Valente, 2013) although, JME has been reported to be associated with verbal and visual memory deficits (Sonmez et al., 2004) related to the frontal lobe (Koepp, 2005, Piazzini et al., 2008). Although IGE is mostly genetic in origin (Greenberg et al., 1992), few IGE genes have been identified. Among those genes, BRD2 has been repeatedly both linked and associated with JME (Cavalleri et al., 2007, Durner et al., 1991, Greenberg et al., 1988a, Greenberg et al., 1988b, Greenberg et al., 2000, Pal et al., 2003, Sander et al., 1995, Weissbecker et al., 1991), specifically in Caucasian populations (Greenberg et al., 2000, Sander et al., 1997).
We generated a mouse model with the null mutation of the murine Brd2 gene (Shang et al., 2009). The homozygous mutation (Brd2−/−) is incompatible with life but Brd2+/− heterozygotes are viable. The heterozygotes have increased susceptibility to provoked seizures, develop unprovoked spontaneous seizures, and have significantly decreased GABA markers (such as GAD67 and/or parvalbumin) along the direct basal ganglia pathway, including in the neocortex and superior colliculus, indicating a GABA deficiency within the endogenous seizure-controlling pathway (Deransart & Depaulis, 2002). However, the hippocampus shows no such decrease in GABA markers (Velíšek et al., 2011).
In this study we investigated behavioral differences between Brd2+/− mice and wild type (wt) littermates with an emphasis on anxiety, cognition and aggression. We also determined the numbers of GAD67 and parvalbumin immunopositive cells in the basolateral amygdala, a structure strongly related to the control of aggression and fear (Mchugh et al., 2004, Schumann et al., 2011, Wang et al., 2011). In our mouse studies, we looked at the sexes separately because JME patients are predominantly female (Camfield et al., 2013, Kleveland & Engelsen, 1998, Pedersen & Petersen, 1998), an observation that is echoed in the mouse seizure susceptibility experiments (Velíšek et al., 2011).
Material and Methods
All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The respective protocols were approved by the IACUC of the Columbia University (New York, NY), New York Medical College (Valhalla, NY), and Nationwide Children’s Hospital (Columbus, OH). Brd2+/− mice and Brd2+/+ littermates (controls; referred here to as wt) were generated most frequently by mating wt Brd2+/+ females to heterozygous Brd2+/− males. All mice used in this study were 6–9 months old, both males and females, and at least the 7th generation of backcrossing onto a C57BL/6J background strain, so that their genetic background would be considered on average 99% C57BL/6J (Mouse Nomenclature, jaxmice.jax.org). The genotypes of the mice were determined by PCR using primers that spanned the gene-trap vector junction inserted into the Brd2 gene (Shang et al., 2009). The mice were kept in groups of three in a cage (same sex) on regular light:dark cycle (lights on at 07:00) with free access to food and water. All experiments were performed during the light phase of the cycle preferable between 10:00–14:00. Two separate cohorts of animals were used for behavioral testing (open field, elevated plus maze, Morris Water maze OR Barnes maze) with at least three day between individual tests. Tube dominance was tested in an additional cohort of mice. Immunohistochemistry was performed on brains of animals not subjected to any behavioral testing.
Behavioral testing
Initially, we investigated the mice in the open field to determine if there were any differences in normal behavioral patterns, in the velocity of walking, trajectory traveled, grooming, etc. (Goldstein et al., 2012, Velíšek, 2006). During this test, the mouse is placed in the center of an arena (42 × 42 cm) with translucent walls and 3D infrared diode arrays. The mouse is left to explore the environment freely for 5 minutes; all movements are recorded. Average speed, distance traveled, central zone entries and time spent in the central zone (centrally positioned 20 × 20 cm square) were calculated by means of software (Activities; Med-Associates, St. Albans, VT). In addition to motor performance, the open field test also provides information about anxiety level: Mice prefer to spend more time in the safety of the walls (thigmotaxis) than in the center of the arena. Relative increases in time spent close to the walls of the arena are consistent with increased anxiety, while more time spent in the center is consistent with decreased anxiety (Choleris et al., 2001).
As a corollary test for anxiety, we used the elevated “plus” maze (Pellow & File, 1986, Velíšek, 2006). During this test, the mouse is placed at the crossing of the two open and two closed arms of the device, facing the open arm. Number of entries into either arm and the time spent in the arms is recorded (videotracking using Stoelting’s ANY-Maze software; Wood Dale, IL) for total 3 minutes. Mice typically prefer the subjectively safer closed arms, although they tend to explore new areas.
Observations during routine handling of the mice had suggested that +/− mice were more aggressive than wt. Therefore, we used the dominance tube test to test and quantify aggressive behaviors, i.e., whether the heterozygotes are more aggressive compared to wt. The enclosed space of the tube represents a desired environment for the mice and the tube test is a non-fighting social dominance test. The tube diameter is chosen to allow the mouse to move either forward or backward without any obstacles, but not to be able to turn around (Kovacsics & Gould, 2010). Social dominance is determined by allowing a heterozygote and a wt of the same sex to enter the clear acrylic tube (diameter 2.5 cm; length 30 cm) from opposite ends (Korade et al., 2013). Both mice usually proceed in walking the tube until they meet in the middle followed by a non-aggressive exchange of olfactory information. Then, the more dominant mouse starts pushing back the less dominant mouse. This is considered to reflect the aggressive tendency controlled by the prefrontal cortex (Molina & O'donnell, 2008, Wang et al., 2011). We have tested each mouse three times (three sessions) allowing one hour between the sessions, always with a different sex-matched partner mouse of the opposite genotype and always from a different cage. We recorded which mouse pushed the less dominant one out of the tube (Harrison et al., 2009). The trial concluded when one mouse had its forepaws out of the tube (Semple et al., 2012). The tube test is a reliable method for hierarchical rating of mice as well as an indicator of significant prefrontal cortex involvement (Wang et al., 2011). All testing was done blind to genotype.
Additionally, we investigated whether the Brd2+/− genotype affects hippocampal learning and spatial memory using the Morris Water Maze (MWM) (Schenk & Morris, 1985). In this test, the mouse is released from the side of a circular water tank and will seek and swim to the rescue platform (hidden about ½ cm under the water level in fixed position in the tank). The mouse has constant visual cues to orientation in the tank because the platform itself is invisible. We did four training trials per day (with a one hour inter-trial interval) for four days; the starting quadrants were pseudo-randomly varied. At 24 hours after the last training trial, a retrieval test was performed: We placed the mouse in the tank but without the platform, and we recorded the time the mouse spent in the quadrant where the platform had been located during the training trials. A similar test was performed 7 days after the training phase. The swimming trajectory was always followed by videotracking (ANY-maze).
To confirm the spatial learning and memory results obtained in the MWM (Patil et al., 2009), we tested different groups of mice in the Barnes maze (Barnes, 1979, Goldstein et al., 2012), which does not involve the significant stressor of swimming. In the Barnes maze, the mouse is released from the starting box in the center of the circular platform around which 20 holes are spaced evenly in the periphery. One of the holes contains the escape box, the other 19 are blanks. The mouse needs to find the escape box. A fluorescent (not heat producing) light source provided 800 lx of illumination directed on the platform as an aversive stimulus. There were 4 days of training with four training trials per day with a one hour inter-trial interval (Goldstein et al., 2012). Similar to the MWM protocol, there was a 24 hour and a 7 day retrieval test in which the escape box is replaced with a blank for the test. During the retrieval test, we recorded the length of time the mouse sniffs around the former location of the escape box (±1 box).
Immunohistochemistry
We evaluated the expression of GABA markers (GAD67 and parvalbumin) in the amygdala and in the prefrontal cortex of mice not subjected to behavioral tests. The amygdala is involved in the control of anxiety and aggressive behaviors (Hale et al., 2010) and GABA has a key role in the control of anxiety (Smith & Rudolph, 2012). For free-floating immunohistochemical staining, 40 µm thick coronal hemi-sections were cut using a cryostat. To identify markers of GABAergic neurons, alternating sections were collected for separate parvalbumin (PVA) and GAD67 immunostaining. GAD67 is an isozyme of the GABA synthesizing enzyme glutamic acid decarboxylase that tags almost the entire population of GABAergic neurons. We used anti-parvalbumin (1:5000, Sigma, St. Louis, MO) and anti-GAD67 (1:4000; Millipore, Temecula, CA) antibodies. The immunostaining was visualized using the avidin-biotin horseradish peroxidase method (Vectastain AB kit, Vector Laboratories, Burlingame, CA) (Velíšek et al., 2011, Velíšková & Velíšek, 2007).
Counting of immunopositive cells
Section images were digitally captured. Counts centered on the basolateral nucleus of amygdala (defined as the cross-section of the nucleus) or the prefrontal cortex (defined as a rectangle in the area of prelimbic cortex and area 1 of the cingulate cortex). For counting, a minimum of three position-matched sections for each structure were selected from the brain of each of Brd2+/− and Brd2+/+ mice. There were four mice in each subgroup (genotype/sex) for the amygdala counts and three mice per each subgroup for the prefrontal cortex counts. All immunopositive neurons in the area of interest were counted (Ravizza et al., 2003, Rieux et al., 2002). Counts from three sections were averaged for each subject (mouse) and the average used for statistical evaluation. Our goal was to compare the relative number of immunopositive cells in the Brd2+/− mice to the number in wt mice (Velíšek et al., 2011).
Statistical Analysis
Initially, data were evaluated for distribution and variance. Data following a Gaussian distribution and with similar variance were analyzed using parametric tests (Student’s t-test for two groups, ANOVA for multiple groups). Proportions in two groups were evaluated using Fisher’s Exact Test. Data collected over time in spatial learning tests were analyzed using two-way ANOVA with repeated measures as one factor. Level of significance was preset to p<0.05.
Results
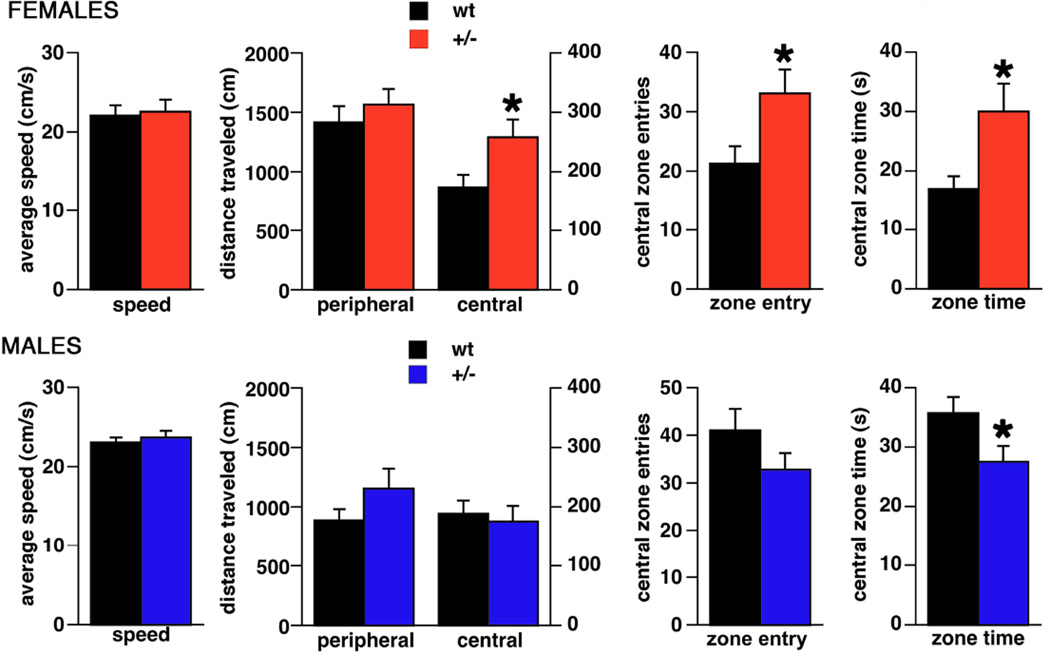
Open Field (Figure 1)
Figure 1. Performance of Brd2+/− mice and wt littermates in the open field.
Top – females (n+/−=14; nwt=12), bottom – males (n+/−=17; nwt=12). We determined speed, distance traveled in the central versus peripheral zone of the open field, number of entries in the central zone and the time spent in the central zone. Female Brd2+/− mice traveled longer distance in the central field, made more entries to the central field and spent more time there compare to wt female littermates (*p<0.05). None of these outcomes was recorded in male Brd2+/− mice. In contrast, Brd2+/− male mice spent less time in the central area of the open field compared to the wt male littermates.
In female mice, the average traveling speed was independent of the genotype. Both Brd2+/− (n=14) and wt (n=12) mice had average speeds around 22 cm/s (Student’s t-test; t(24)=0.251; p=0.804). This indicates that movement of the Brd2+/− mice is not impaired. There was no difference between the two groups of mice in total distance traveled (Student’s t-test; t(24)=1.090; p=0.287; not shown). However, when we compared the distance traveled in the periphery and in the central zone of the open field, we found that Brd2+/− females traveled significantly longer distance in the central area compared to wt females (Student’s t-test; t(24)=2.498; p=0.048). This finding could be associated either with significantly more entries of Brd2+/− females into the central zone and/or more time spent in the central zone. Statistics revealed that female Brd2+/− mice entered the central zone more frequently (Student’s t-test; t(24)=2.314; p=0.030) and stayed there longer (Student’s t-test; t(24)=2.403; p=0.024) compared to wt female mice. These observations of Brd2+/− females indicate significantly decreased preference for the peripheral parts of the open field (decreased thigmotaxis) compared to wt, a conclusion supported by 1) the longer time spent in the central area of the open field, 2) the longer distance traveled there, and 3) the increased number of entries. Decreased thigmotaxis is a sign of decreased anxiety (Simon et al., 1994). In male mice we did not find differences in open field behaviors between Brd2+/− (n=17) and wt (n=11) except for the time spent in the central zone. Brd2+/− males spent less time in the central zone than wt (Student’s t-test; t(26)=2.229; p=0.044), which might be suggestive of increased anxiety.
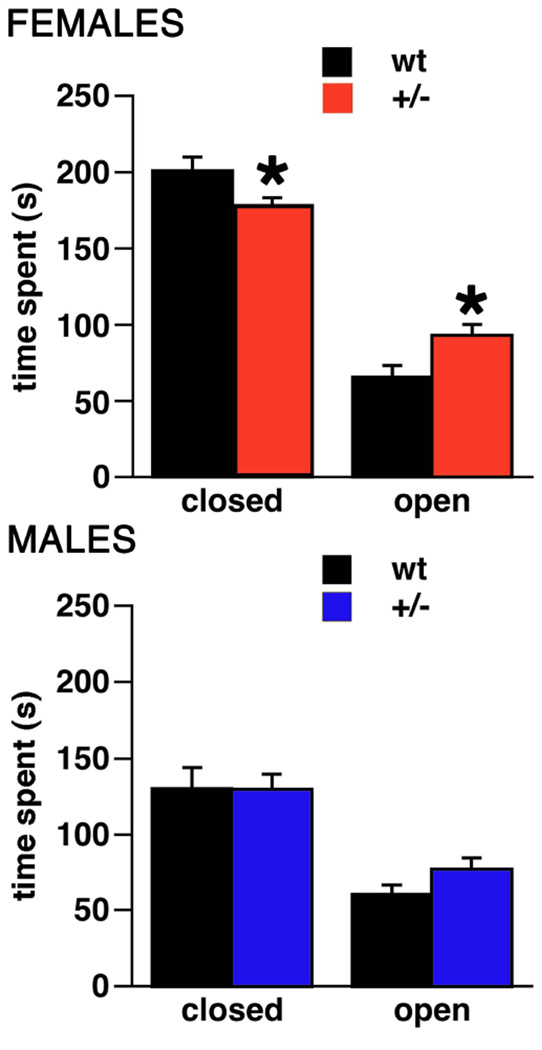
Elevated Plus Maze (Figure 2)
Figure 2. Activity of Brd2+/− mice and wt littermates in the elevated plus maze.
Top – females (n+/−=15, nwt=14), bottom – males (n+/−=17, nwt=12). In this test Brd2+/− females spent more time in the open arms and proportionally less time in the closed arms of the open field compared to their wt female littermates (*p<0.05). This was not the case in male Brd2+/− mice.
The findings in the elevated plus maze, confirmed the findings of decreased anxiety in Brd2+/− female mice compared to wt females. Overall, female Brd2+/−mice (n=15) spent significantly less time in the closed arms of the maze (Student’s t-test; t(27)=2.374; p=0.025) but spent significantly more time on the open arms compared to female wt mice (n=14; Student’s t-test; t(27)=2.112; DF=27; p=0.044). The remaining time (up to the 3 min session limit) was spent on the central square. However, we did not find any differences for elevated plus-maze behaviors between male Brd2+/− vs. wt mice. Thus, this test does not confirm the indication of increased anxiety in males that was seen in the previous test.
Tube dominance test (Figure 3)
Figure 3. Increased dominance in Brd2+/− mice compared to wt littermates tested in the tube.
a. Overview of three sequential trials (1 hour apart), during which pairs (+/− male – wt male or +/− female – wt female; n=10 per each subgroup) were placed in the opposite ends of clear acrylic tube. A “winner” was assigned to the mouse who after the encounter in the mid tube overpowered the other mouse having it back up out of the tube. Columns represent number of wins for individual subgroups separated by sex. For each trial, the pairs were built differently, so there was no repeated interaction with a former tube mate. Columns clearly indicate prevailing dominance of Brd2+/− females over wt females from the beginning, while +/− males developed their dominance over continuing trials 1–3.
b. Combined data from trials 1–3 indicating proportion of wins per in Brd2+/− and wt mice split by sex. Brd2+/− mice were clearly dominant in this test (*p=0.0001).
We had noted enhanced aggression in Brd2+/− mice in normal handling. We used tube dominance test to confirm and quantify this observation. We tested the mice in three trials. For each trial a different male-male or female-female pair was chosen. A total of 10 Brd2+/−/wt male pairs and 10 female pairs were tested (40 mice total). Figure 3A shows number of “winners” (dominant mice) in each category, i.e. male Brd2+/−, female Brd2+/−, male wt and female wt. The results unambiguously show that Brd2+/− females were consistently dominant over wt in this test across the trials. On the other hand, Brd2+/− males required repeated encounters with wt opponents to develop clear dominance. Figure 3B summarizes data from trials 1–3. Out of 60 encounters, Brd2+/− mice prevailed in 41, while wt prevailed in 19 (Fisher’s Exact test; *p=0.0001).
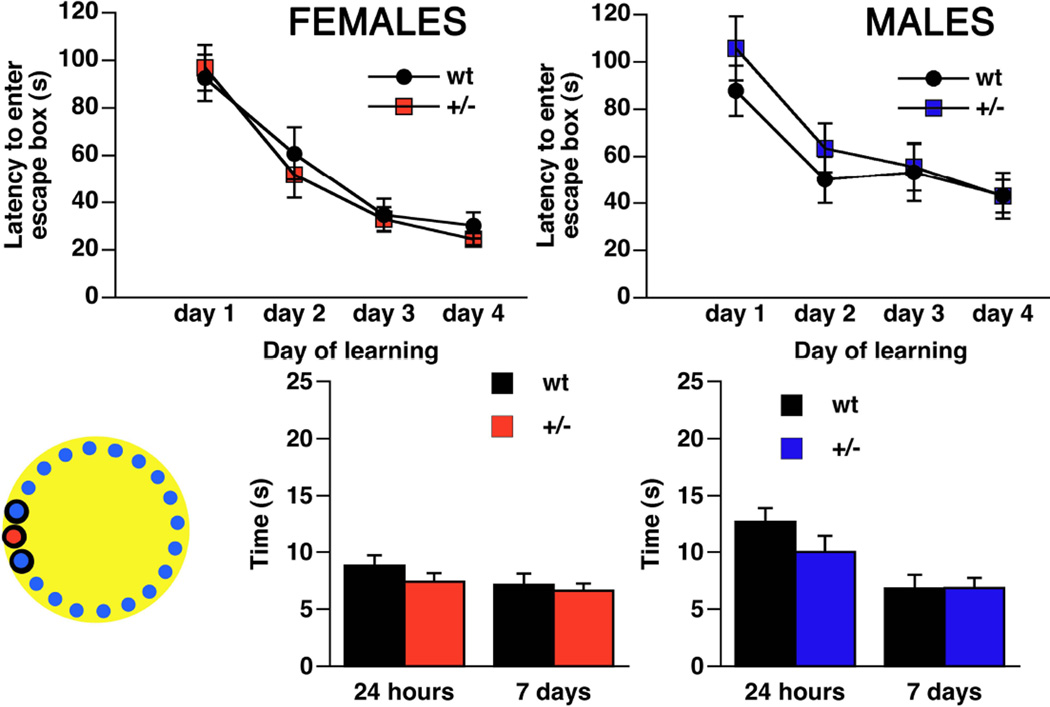
Morris Water Maze (MWM; Figure 4)
Figure 4. Learning and memory retrieval of Brd2+/− mice and wt littermates in the Morris water maze.
Left inset – a scheme of Morris Water maze with submerged platform location in the NW quadrant (red circle). Red – females (n+/−=16 ; nwt=12); blue – males (n+/−=12; nwt=11). Motivation to find a hidden platform is provided by water environment. While both males and females were capable of learning location of the platform over four days of training, there was no difference between +/− and wt littermates. Similarly, there was no difference between +/− and wt littermates in memory retrieval at either 24 hours or 7 days after learning has been completed (measured as time spent in the quadrant of former location of the platform).
We asked whether the GABAergic neuron marker deficits in Brd2+/− mice that are seen along a) the direct basal ganglia pathway, b) in the neocortex, and c) in the superior colliculus, would impair spatial learning and memory. In the water maze (MWM), we found no difference in learning the platform location between Brd2+/− female mice (n=16) and wt females [n=12; repeated measures ANOVA, between factor F(1,26)=0.167; p=0.687]. Indeed, learning (i.e., the gradual decrease in time needed to find the platform over the days of training; i.e., an eventual non-zero slope of the learning curve) was present and similar in both groups [repeated measures ANOVA within factor F(3,78)=21.384; p<0.0001; no significant interaction between the factors]. Similarly, there was no difference between Brd2+/− and wt female mice in the memory retrieval test at 24 hours or 7 days after completion of training. The results for males were similar to females. We did not find any differences in spatial learning in the MWM between Brd2+/− males (n=12) and wt males (n=11; repeated measures ANOVA between factor F(1,21)=0.001; p=0.980) while, again, male mice were capable of significant learning (a gradual decrease in the time to entry into the rescue box over the days of training; repeated measures ANOVA within factor F(3,63)=16.004; p<0.001). There were no differences between Brd2+/− and wt males in memory retrieval tests at 24 hours or 7 days after training.
Barnes Maze (Figure 5)
Figure 5. Learning and memory retrieval of Brd2+/− mice and wt littermates in the Barnes maze.
Left inset – a scheme of Barnes maze with 19 blank boxes (blue), one full escape box (red) and area of the adjacent boxes (encircled black) to the escape box evaluated as positive outcome during memory retrieval test. Red – females (n+/−=16 ; nwt=14); blue – males (n+/−=17; nwt=12). In the Barnes maze, bright light (800 lx) serves as motivation to escape from the open platform and seek a hiding place in the full escape box. While both males and females were capable of learning of the position of escape box over four days of training, there was no difference between +/− and wt littermates. Similarly, there was no difference between +/− and wt littermates in memory retrieval at either 24 hours or 7 days after learning has been completed.
Swimming may represent a confounder in the MWM because mice are not fond of swimming. Thus, stress effects of swimming might have overridden any possible (subtle) differences between +/− and wt mice of both sexes. Therefore, using a different sets of animals than in the MWM test, we validated our MWM data in the Barnes Maze, the dry version of the spatial learning and memory test. For female mice, there was again no difference in spatial learning between Brd2+/− (n=16) and wt (n=14; repeated measures ANOVA between factor F(1,28)=0.206; p=0.653); both groups displayed significant acquisition of the task (repeated measures ANOVA within factor F(3,84)=33.912; p<0.0001). There was no difference between Brd2+/− female mice and wt controls in memory retrieval at 24 hours and 7 days after learning. In male mice, we also did not see any difference between the groups of Brd2+/− (n=17) and wt mice (n=12; repeated measures ANOVA between factor F(1,27)=0.439; p=0.573). Similar to the female results, and similar to results collected in MWM, there was significant learning in both groups over 4 days of training (repeated measures ANOVA within factor F(3,81)=21.079; p<0.0001). There was no difference in memory retrieval after 24 hours or 7 days between male Brd2+/− and wt mice.
These results indicate that the spatial learning and memory are affected neither as a result of Brd2 haploinsuffiiency nor as a result of the earlier-reported GABA marker decrease in the basal ganglia and neocortex. It is noteworthy that there were no differences in GABAergic neuron markers in the hippocampus (Velíšek et al., 2011). Thus, the finding that genotype did not affect spatial learning and memory supports the observation that Brd2 haploinsufficiency does not affect hippocampal function.
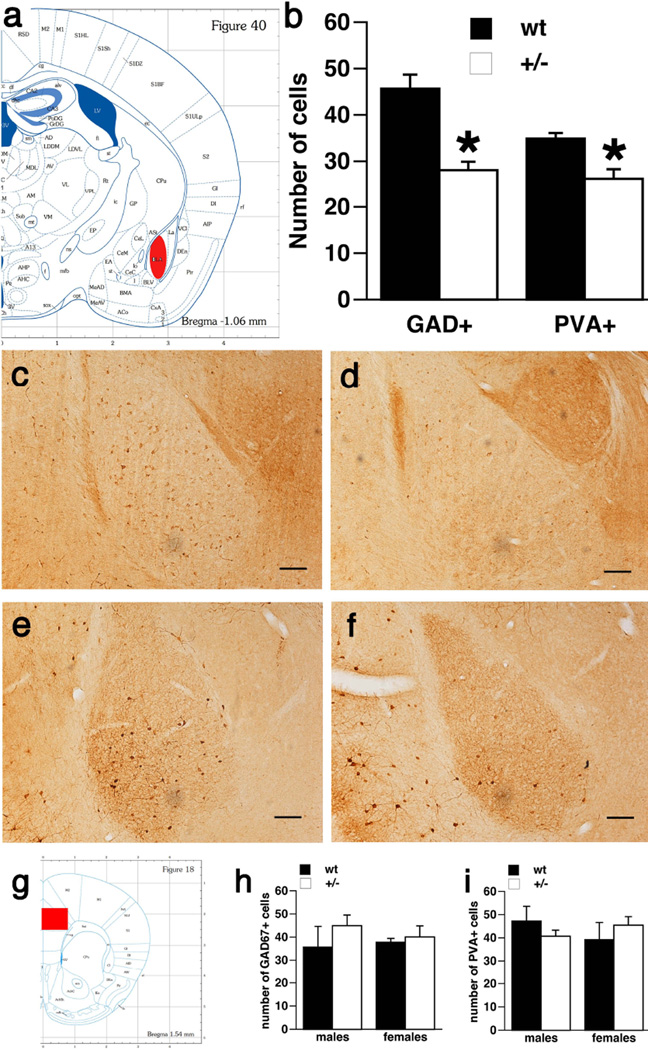
Expression of markers for GABAergic neurons in the basolateral amygdala and prefrontal cortex (Figure 6)
Figure 6. Decreased number of GAD67 and parvalbumin immunopositive neurons in the basolateral amygdala of Brd2+/− mice compared to wt littermates.
a. Plate 40 from (Paxinos & Franklin, 2004) showing the area of basolateral amygdala where the counts were performed (red fill).
b. Numbers of both GAD67-immunopositive cells and parvalbumin immunopositive cells were decreased in +/− mice irrespective of sex compared to wt littermates (two way ANOVA; *p<0.05).
c. Microphotograph of basolateral amygdala in a wt female mouse with immunostaining of GAD67 cells. Scale bars always 100 µm.
d. Microphotograph of basolateral amygdala in a Brd2+/− female mouse with immunostaining of GAD67 cells. Please note that number of dark stained cells is very low, in contrast to wt female.
e. Microphotograph of basolateral amygdala in a wt female mouse with immunostaining of parvalbumin cells.
f. Microphotograph of basolateral amygdala in a Brd2+/− female mouse with immunostaining of parvalbumin cells. Please note that number of dark stained cells is very low, in contrast to wt female.
g. Plate 18 from (Paxinos & Franklin, 2004) showing the area of prefrontal cortex (frame dimension) where the counts were performed (red fill).
h. There was no difference in the expression of GAD67 immunopositive cells in the prefrontal cortex area both between Brd2+/− and wt mice and between males and females.
i. There was no difference in the expression of PVA immunopositive cells in the prefrontal cortex area both between Brd2+/− and wt mice and between males and females.
We determined expression of GAD67 and PVA (GABA markers) in amygdala since amygdala function is associated with fear and with aggressive behaviors (Mchugh et al., 2004, Schumann et al., 2011, Treit et al., 1993). We found that both markers we examined, GAD67 and PVA, were significantly decreased in Brd2+/− mice, irrespective of sex. In GAD67 expression, there was a significant effect of genotype (ANOVA F(1,12)=24.735; p=0.0003), however there was no effect of sex (ANOVA F(1,12)=0.685; p=0.424) or interaction between the factors (ANOVA F(1,12)=1.330; p=0.271). Similar to GAD67, in PVA expression, there was a significant effect of genotype (ANOVA F(1,12)=10.946; p=0.0062), but there was no main effect of sex (ANOVA F(1,12)=0.030; p=0.866) or interaction between the effects (ANOVA F(1,12)=0.009; p=0.930).
We also investigated expression of GABA markers in prefrontal cortex because of the role prefrontal cortex plays in dominance and aggression (Wang et al., 2011). We determined the number of GAD67 and PVA immunopositive neurons in the perilimbic and cingulate portions of the prefrontal cortex. First, we found that the numbers of GAD67 and PVA immunopositive cells are virtually the same in this area (paired t-test; t(11)=0.834; p=0.422), indicating that most, if not all, GABAergic cells express PVA in this particular part of the prefrontal cortex. Second, in counts of GAD67 immunopositive cells, we found no main effect of genotype (ANOVA F(1,9)=1.170; p=0.310) or sex (ANOVA F(1,9)=0.065; p=0.805). There was no main effect of genotype in counts of PVA-immunopositive cells (ANOVA F(1,10)=0.0034; p=0.990) or main effect of sex (ANOVA F(1,10)=0.079; p=0.784). No interactions between the main effects were found in either cell type.
Discussion
Our data show that Brd2+/− mice have sex-specific alterations in anxiety as compared to wt mice. We found decreased anxiety traits in Brd2+/− female mice but these traits were not observed in Brd2+/− males. Further, both Brd2+/− female and male mice displayed increased aggression compared to wt, aggression that was more pronounced in females. In either sex, Brd2 haploinsuficiency was not associated with any problems in spatial learning and memory. Finally, we also found a significant decrease in GABA neuronal markers (GAD67 and PVA) in basolateral amygdala in Brd2+/− mice compared to wt, yet no difference was found in the prefrontal cortex. These findings correspond to our previously published study in Brd2+/− mice that showed sex-specific increases in susceptibility to provoked seizures, the occurrence of spontaneous seizures in females, and decreased numbers of GAD67 immunopositive neurons along the basal ganglia pathways compared to wt mice (Velíšek et al., 2011).
Our behavioral findings show decreases in anxiety in female Brd2+/− mice and increases in aggression in all Brd2+/− mice compared to wt. There are studies indicating that patients with JME (repeatedly linked to, and associated with, the BRD2 gene (Greenberg et al., 2000, Sander et al., 1997)) also show certain behavioral problems. These behavioral problems include increased risk-taking behaviors (Wandschneider et al., 2013) and impulsive personality traits (Moschetta et al., 2011). Indeed, in our mouse experiments, the enhanced exploration of the central area (the risk zone) of the open field and the increase in time spent on the open arms of the elevated plus maze, are indicators of increased risk-taking behaviors in the mice (Olsen et al., 2013). Interestingly, a study investigating deficits of a gene controlling circadian rhythms (Clock) (Easton et al., 2003) also found more robust increases in risk-taking behavior specifically in females. The findings suggest that some genes such as Clock (or Brd2 in this study) may interact with gonadal steroid hormones or sex to produce behavioral changes.
We did not find any cognitive deficits (determined in the Barnes maze or Morris Water Maze) in Brd2+/− mice compared to controls. Similarly, clinical data do not show significant cognitive deficiencies in patients with JME (Moschetta & Valente, 2013). The cognitive tasks used in our study in the mice depend on intact hippocampal function (Stewart et al., 2011) and, in fact, we did not find any changes in GABA markers in the hippocampus of Brd2+/− mice compared to wt in our previous work (Velíšek et al., 2011). This is also in accordance with neuropsychological studies, which generally report normal IQ in JME patients; the functions of the temporal lobes specifically (verbal and nonverbal episodic memory) seem to be unaffected (Delgado-Escueta et al., 2013, Roebling et al., 2009, Wandschneider et al., 2010). These parallel findings in patients with JME and in Brd2+/− mice indicate that hippocampal structure and function are unaffected in both JME patients and in our mouse JME model.
On the other hand, in the Brd2+/− mice we did find increased social dominance in the tube test, which tests dominance as well as aggression (Johns et al., 2010). The main structures responsible for these behaviors involve circuitry among prefrontal and orbital cortices, striatum and amygdala (Cho et al., 2013, Molina & O'donnell, 2008, Nelson & Trainor, 2007, Wang et al., 2011). Previously, we found impairment in the striatal number of GAD67 and parvalbumin immunopositive neurons in Brd2+/− mice (suggesting a deficit in GABA neurons (Velíšek et al., 2011)). In the current study, we expanded those findings and we show, in addition, that there are decreased GABA markers in the amygdala of the Brd2+/− mice as well. In patients with JME, changes in fronto-striatal circuitry have been demonstrated, with abnormalities in the dorsolateral prefrontal cortex, premotor cortex, basal frontal cortex, thalamus, and putamen (Koepp et al., 2013, Woermann et al., 1999) associated with decreased cognitive flexibility (Mcdonald et al., 2006, Van Schouwenburg et al., 2014, Wandschneider et al., 2012). Although JME’s major pathology is a form of generalized epilepsy, EEG recordings and current imaging techniques provide evidence of the major involvement of the frontal lobes (Koepp et al., 2013, Wandschneider et al., 2013), all in accordance with the findings of behavioral and neuropsychological studies (De Araujo Filho et al., 2013, Schmitz et al., 2013). Another study of JME patients investigated risk-taking behaviors and determined that JME patients with ongoing seizures display increased risk-taking traits as well as activation of the prefrontal cortex compared to controls without JME or JME patients without ongoing seizures (Wandschneider et al., 2013). While we did not find any modifications of GABA markers directly in the prefrontal cortex of the Brd2+/− mice, we found that, in addition to increased aggression, these mice have decreases in anxiety traits, which can be related to risk-taking behaviors (Easton et al., 2003, Keers et al., 2012). These behaviors also involve prefrontal cortex, but we hypothesize that a neurotransmitter system other than the GABA system is involved (Vialou et al., 2014).
Previously, we found sex-specific phenotypic differences between Brd2+/− and wt mice in both seizure susceptibility and in the expression of GABA markers (Velíšek et al., 2011), and now we see such differences in behaviors as well. It is striking that many of these observations in the mouse are similar to observations in humans (Craiu, 2013), [although, to our knowledge, no one has investigated sex-specific differences in human JME patients]. Sex-specific occurrence of traits may indicate: (a) A possible role of sex chromosome differences and their interaction with the partial Brd2 deficit in heterozygotes. (b) Interactions of Brd2 function with the effects of gonadal sex steroids, as shown previously for the interaction of the Clock gene with sex in anxiety behaviors (Easton et al., 2003). (c) The role of circulating sex steroids (specifically progesterone) during the ovarian cycle on anxiety behaviors in females (Galeeva et al., 2003, Gangitano et al., 2009, Maguire et al., 2005). Our ongoing experiments in animals during phases of postnatal development will further elucidate the first two possibilities of genetically-based sex differences versus interaction with early developmental organizational effects of steroid hormones (sexually dimorphic effects) (Velíšková & Moshé, 2001). Testing both Brd2+/− and wt females at random time points throughout the ovarian cycle speaks against the last option.
The finding that the decreased anxiety in female Brd2+/− mice is associated with decreased expressions of GABA markers in the basolateral amygdala seems counterintuitive, as anxiolytic effects are usually dependent on strengthening GABA-mediated inhibition (Smith & Rudolph, 2012); for example, benzodiazepines, which strengthen such inhibition, have significant anxiolytic properties (Griebel & Holmes, 2013). However, mice with high anxiety traits have increased levels of GAD65 and GAD67 mRNAs and protein, as well as increased levels of GABA in the amygdala (Tasan et al., 2011). Lesions of basolateral amygdala, on the other hand, lead to decreased anxiety traits in rats (Treit et al., 1993). Finally, one study directly and positively links the number of active PVA-containing neurons in the amygdala to the level of anxiety (Hale et al., 2010). A very recent study in patients with JME, using Magnetic Resonance Spectroscopy with ultra short echo time, determined that there is a decrease in thalamic GABA neurotransmission with an increase in N-acetyl-aspartate. These observations together suggest damage to thalamic GABAergic neurons (Hattingen et al., 2014). Unfortunately, relatively low resolution of this technique does not make possible to investigate relatively small brain structures such as dorsolateral striatum, substantia nigra pars reticulata or amygdala.
Our data suggest that the Brd2 gene (in addition to governing seizure susceptibility) plays a role in the expression of sex-specific behavioral traits. Since the Brd2 gene product is likely a transcription factor controlling production of many different transcripts (Belkina et al., 2013) (ergo regulating many different functions), these transcripts may participate in brain development and control of seizure susceptibility, cell proliferation, and behavior, and also may be responsible for differential outcomes in males and females. Since these control functions, or interactions, of Brd2 with other transcripts may also occur at certain specific developmental stages, the data indicate that JME is a developmental disorder (De Nijs et al., 2013).
Acknowledgments
Supported by the NIH grants NS072966 (LV); NS056093 (JV); GM081767 (DJW); NS027941 (DAG); NS061829 (DAG); NS070323 (DAG).
References
- Annegers JF. Epidemiology and genetics of epilepsy. Neurol Clin. 1994;12:15–29. [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Belkina AC, Nikolajczyk BS, Denis GV. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol. 2013;190:3670–3678. doi: 10.4049/jimmunol.1202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 28(Suppl 1):S15–S17. doi: 10.1016/j.yebeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Walley NM, Soranzo N, Mulley J, Doherty CP, Kapoor A, Depondt C, Lynch JM, Scheffer IE, Heils A, Gehrmann A, Kinirons P, Gandhi S, Satishchandra P, Wood NW, Anand A, Sander T, Berkovic SF, Delanty N, Goldstein DB, Sisodiya SM. A multicenter study of BRD2 as a risk factor for juvenile myoclonic epilepsy. Epilepsia. 2007;48:706–712. doi: 10.1111/j.1528-1167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci. 2013;33:14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Craiu D. What is special about the adolescent (JME) brain? Epilepsy Behav. 2013;28(Suppl 1):S45–S51. doi: 10.1016/j.yebeh.2012.12.008. [DOI] [PubMed] [Google Scholar]
- de Araujo Filho GM, de Araujo TB, Sato JR, Silva I, Lin K, Junior HC, Yacubian EM, Jackowski AP. Personality traits in juvenile myoclonic epilepsy: evidence of cortical abnormalities from a surface morphometry study. Epilepsy Behav. 2013;27:385–392. doi: 10.1016/j.yebeh.2013.02.004. [DOI] [PubMed] [Google Scholar]
- de Araujo Filho GM, Jackowski AP, Lin K, Guaranha MS, Guilhoto LM, da Silva HH, Caboclo LO, Junior HC, Bressan RA, Yacubian EM. Personality traits related to juvenile myoclonic epilepsy: MRI reveals prefrontal abnormalities through a voxel-based morphometry study. Epilepsy Behav. 2009a;15:202–207. doi: 10.1016/j.yebeh.2009.03.011. [DOI] [PubMed] [Google Scholar]
- de Araujo Filho GM, Lin K, Lin J, Peruchi MM, Caboclo LO, Guaranha MS, Guilhoto LM, Carrete H, Jr, Yacubian EM. Are personality traits of juvenile myoclonic epilepsy related to frontal lobe dysfunctions? A proton MRS study. Epilepsia. 2009b;50:1201–1209. doi: 10.1111/j.1528-1167.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- de Nijs L, Wolkoff N, Grisar T, Lakaye B. Juvenile myoclonic epilepsy as a possible neurodevelopmental disease: role of EFHC1 or Myoclonin1. Epilepsy Behav. 2013;28(Suppl 1):S58–S60. doi: 10.1016/j.yebeh.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Koeleman BP, Bailey JN, Medina MT, Duron RM. The quest for juvenile myoclonic epilepsy genes. Epilepsy Behav. 2013;28(Suppl 1):S52–S57. doi: 10.1016/j.yebeh.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Deransart C, Depaulis A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002;4(Suppl 3):S61–S72. [PubMed] [Google Scholar]
- Durner M, Sander T, Greenberg DA, Johnson K, Beck-Mannagetta G, Janz D. Localization of idiopathic generalized epilepsy on chromosome 6p in families of juvenile myoclonic epilepsy patients. Neurology. 1991;41:1651–1655. doi: 10.1212/wnl.41.10.1651. [DOI] [PubMed] [Google Scholar]
- Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Galeeva AY, Tuohimaa P, Shalyapina VG. The role of sex steroids in forming anxiety states in female mice. Neurosci Behav Physiol. 2003;33:415–420. doi: 10.1023/a:1022864011385. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8:398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velíšek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Delgado-Escueta AV, Maldonado HM, Widelitz H. Segregation analysis of juvenile myoclonic epilepsy. Genet Epidemiol. 1988a;5:81–94. doi: 10.1002/gepi.1370050204. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Delgado-Escueta AV, Widelitz H, Sparkes RS, Treiman L, Maldonado HM, Park MS, Terasaki PI. Juvenile myoclonic epilepsy (JME) may be linked to the BF and HLA loci on human chromosome 6. Am J Med Genet. 1988b;31:185–192. doi: 10.1002/ajmg.1320310125. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Delgado-Escueta AV. Evidence for multiple gene loci in the expression of the common generalized epilepsies. Neurology. 1992;42:56–62. [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Keddache M, Shinnar S, Resor SR, Moshe SL, Rosenbaum D, Cohen J, Harden C, Kang H, Wallace S, Luciano D, Ballaban-Gil K, Tomasini L, Zhou G, Klotz I, Dicker E. Reproducibility and complications in gene searches: linkage on chromosome 6, heterogeneity, association, and maternal inheritance in juvenile myoclonic epilepsy. Am J Hum Genet. 2000;66:508–516. doi: 10.1086/302763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, Lowry CA. Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1285–1293. doi: 10.1016/j.pnpbp.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingen E, Luckerath C, Pellikan S, Vronski D, Roth C, Knake S, Kieslich M, Pilatus U. Frontal and thalamic changes of GABA concentration indicate dysfunction of thalamofrontal networks in juvenile myoclonic epilepsy. Epilepsia. 2014 doi: 10.1111/epi.12656. [DOI] [PubMed] [Google Scholar]
- Johns JM, McMurray MS, Joyner PW, Jarrett TM, Williams SK, Cox ET, Black MA, Middleton CL, Walker CH. Effects of chronic and intermittent cocaine treatment on dominance, aggression, and oxytocin levels in post-lactational rats. Psychopharmacology (Berl) 2010;211:175–185. doi: 10.1007/s00213-010-1877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S, Nothen MM, Rietschel M, Schalkwyk LC, Fernandes C. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS One. 2012;7:e38263. doi: 10.1371/journal.pone.0038263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleveland G, Engelsen BA. Juvenile myoclonic epilepsy: clinical characteristics, treatment and prognosis in a Norwegian population of patients. Seizure. 1998;7:31–38. doi: 10.1016/s1059-1311(98)90005-x. [DOI] [PubMed] [Google Scholar]
- Koepp MJ. Juvenile myoclonic epilepsy--a generalized epilepsy syndrome? Acta Neurologica Scandinavica. Supplementum. 2005;181:57–62. doi: 10.1111/j.1600-0404.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Woermann F, Savic I, Wandschneider B. Juvenile myoclonic epilepsy--neuroimaging findings. Epilepsy Behav. 2013;28(Suppl 1):S40–S44. doi: 10.1016/j.yebeh.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Korade Z, Folkes OM, Harrison FE. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol Biochem Behav. 2013;106:101–108. doi: 10.1016/j.pbb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacol Biochem Behav. 2010;94:380–386. doi: 10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Swartz BE, Halgren E, Patell A, Daimes R, Mandelkern M. The relationship of regional frontal hypometabolism to executive function: a resting fluorodeoxyglucose PET study of patients with epilepsy and healthy controls. Epilepsy Behav. 2006;9:58–67. doi: 10.1016/j.yebeh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Molina Y, O'Donnell S. Age, sex, dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev Neurobiol. 2008;68:950–959. doi: 10.1002/dneu.20633. [DOI] [PubMed] [Google Scholar]
- Moschetta S, Fiore LA, Fuentes D, Gois J, Valente KD. Personality traits in patients with juvenile myoclonic epilepsy. Epilepsy Behav. 2011;21:473–477. doi: 10.1016/j.yebeh.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Moschetta S, Valente KD. Impulsivity and seizure frequency, but not cognitive deficits, impact social adjustment in patients with juvenile myoclonic epilepsy. Epilepsia. 2013;54:866–870. doi: 10.1111/epi.12116. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Olsen D, Kaas M, Schwartz O, Nykjaer A, Glerup S. Loss of BDNF or its receptors in three mouse models has unpredictable consequences for anxiety and fear acquisition. Learn Mem. 2013;20:499–504. doi: 10.1101/lm.032045.113. [DOI] [PubMed] [Google Scholar]
- Pal DK, Evgrafov OV, Tabares P, Zhang F, Durner M, Greenberg DA. BRD2 (RING3) is a probable major susceptibility gene for common juvenile myoclonic epilepsy. Am J Hum Genet. 2003;73:261–270. doi: 10.1086/377006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SS, Sunyer B, Hoger H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009;198:58–68. doi: 10.1016/j.bbr.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Amsterdam ; Boston: Elsevier Academic Press; 2004. [Google Scholar]
- Pedersen SB, Petersen KA. Juvenile myoclonic epilepsy: clinical and EEG features. Acta Neurol Scand. 1998;97:160–163. doi: 10.1111/j.1600-0404.1998.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia. 2008;49:657–662. doi: 10.1111/j.1528-1167.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- Plattner B, Pahs G, Kindler J, Williams RP, Hall RE, Mayer H, Steiner H, Feucht M. Juvenile myoclonic epilepsy: a benign disorder? Personality traits and psychiatric symptoms. Epilepsy Behav. 2007;10:560–564. doi: 10.1016/j.yebeh.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Friedman LK, Moshé SL, Velíšková J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Rieux C, Carney R, Lupi D, Dkhissi-Benyahya O, Jansen K, Chounlamountri N, Foster RG, Cooper HM. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: comparison of different methods of quantification. J Biol Rhythms. 2002;17:121–136. doi: 10.1177/074873002129002410. [DOI] [PubMed] [Google Scholar]
- Roebling R, Scheerer N, Uttner I, Gruber O, Kraft E, Lerche H. Evaluation of cognition, structural, and functional MRI in juvenile myoclonic epilepsy. Epilepsia. 2009;50:2456–2465. doi: 10.1111/j.1528-1167.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Sander T, Bockenkamp B, Hildmann T, Blasczyk R, Kretz R, Wienker TF, Volz A, Schmitz B, Beck-Mannagetta G, Riess O, Epplen JT, Janz D, Ziegler A. Refined mapping of the epilepsy susceptibility locus EJM1 on chromosome 6. Neurology. 1997;49:842–847. doi: 10.1212/wnl.49.3.842. [DOI] [PubMed] [Google Scholar]
- Sander T, Hildmann T, Janz D, Wienker TF, Neitzel H, Bianchi A, Bauer G, Sailer U, Berek K, Schmitz B, et al. The phenotypic spectrum related to the human epilepsy susceptibility gene "EJM1". Ann Neurol. 1995;38:210–217. doi: 10.1002/ana.410380213. [DOI] [PubMed] [Google Scholar]
- Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Yacubian EM, Feucht M, Hermann B, Trimble M. Neuropsychology and behavior in juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S72–S73. doi: 10.1016/j.yebeh.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Canchola SA, Noble-Haeusslein LJ. Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J Neurotrauma. 2012;29:2672–2683. doi: 10.1089/neu.2012.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang E, Wang X, Wen D, Greenberg DA, Wolgemuth DJ. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev Dyn. 2009;238:908–917. doi: 10.1002/dvdy.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonmez F, Atakli D, Sari H, Atay T, Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–336. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Stewart S, Cacucci F, Lever C. Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer's mouse model. Journal of Alzheimer's disease : JAD. 2011;26:105–126. doi: 10.3233/JAD-2011-101827. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Bukovac A, Peterschmitt YN, Sartori SB, Landgraf R, Singewald N, Sperk G. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience. 2011;183:71–80. doi: 10.1016/j.neuroscience.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Pesold C, Rotzinger S. Noninteractive effects of diazepam and amygdaloid lesions in two animal models of anxiety. Behav Neurosci. 1993;107:1099–1105. doi: 10.1037//0735-7044.107.6.1099. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, Onnink AM, ter Huurne N, Kan CC, Zwiers MP, Hoogman M, Franke B, Buitelaar JK, Cools R. Cognitive flexibility depends on white matter microstructure of the basal ganglia. Neuropsychologia. 2014;53:171–177. doi: 10.1016/j.neuropsychologia.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Velíšek L. Prenatal exposure to betamethasone decreases anxiety in developing rats: Hippocampal neuropeptide Y as a target molecule. Neuropsychopharmacology. 2006;31:2140–2149. doi: 10.1038/sj.npp.1301016. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Shang E, Velíšková J, Chachua T, Macchiarulo S, Maglakelidze G, Wolgemuth DJ, Greenberg DA. GABAergic neuron deficit as an idiopathic generalized epilepsy mechanism: the role of BRD2 haploinsufficiency in juvenile myoclonic epilepsy. PLoS One. 2011;6:e23656. doi: 10.1371/journal.pone.0023656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšková J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Velíšek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandschneider B, Centeno M, Vollmar C, Stretton J, O'Muircheartaigh J, Thompson PJ, Kumari V, Symms M, Barker GJ, Duncan JS, Richardson MP, Koepp MJ. Risk-taking behavior in juvenile myoclonic epilepsy. Epilepsia. 2013 doi: 10.1111/epi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandschneider B, Kopp UA, Kliegel M, Stephani U, Kurlemann G, Janz D, Schmitz B. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology. 2010;75:2161–2167. doi: 10.1212/WNL.0b013e318202010a. [DOI] [PubMed] [Google Scholar]
- Wandschneider B, Thompson PJ, Vollmar C, Koepp MJ. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia. 2012;53:2091–2098. doi: 10.1111/epi.12003. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Weissbecker KA, Durner M, Janz D, Scaramelli A, Sparkes RS, Spence MA. Confirmation of linkage between juvenile myoclonic epilepsy locus and the HLA region of chromosome 6. Am J Med Genet. 1991;38:32–36. doi: 10.1002/ajmg.1320380109. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122:2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]