Abstract
Aspects of immune system dysregulation associated with long-duration spaceflight have yet to be fully characterized and may represent a clinical risk to crewmembers during deep space missions. Plasma cytokine concentration may serve as an indicator of in vivo physiological changes or immune system mobilization. The plasma concentrations of 22 cytokines were monitored in 28 astronauts during long-duration spaceflight onboard the International Space Station. Blood samples were collected 3 times before flight, 3–5 times during flight (depending on mission duration), at landing, and 30 days after landing. Analysis was performed by bead array immunoassay. With few exceptions, minimal detectable mean plasma concentrations were observed at baseline (launch minus 180) for innate inflammatory cytokines or adaptive regulatory cytokines; however, interleukin (IL)-1ra and several chemokines and growth factors were constitutively present. An increase in the plasma concentration, tumor necrosis factor-α (TNFα), IL-8, IL-1ra, thrombopoietin (Tpo), vascular endothelial growth factor (VEGF), C-C motif chemokine ligand 2 (CCL2), chemokine ligand 4/macrophage inhibitory protein 1b (CCL4), and C-X-C motif chemokine 5/epithelial neutrophil-activating protein 78 (CXCL5) was observed associated with spaceflight. No significant alterations were observed during or following spaceflight for the inflammatory or adaptive/T-regulatory cytokines: IL-1α, IL-1β, IL-2, interferon-gamma (IFN-γ), IL-17, IL-4, IL-5, IL-10, G-CSF, GM-CSF, FGF basic, CCL3, or CCL5. This pattern of cytokine dysregulation suggests multiple physiological adaptations persist during flight, including inflammation, leukocyte recruitment, angiogenesis, and thrombocyte regulation.
Introduction
Immune system dysregulation in humans has long been a recognized phenomenon immediately following short- and long-duration spaceflight, consisting of altered leukocyte distribution, diminished function of specific immunocyte populations, and altered cytokine production profiles following mitogenic stimuli (Gueguinou and others 2009). Decades of terrestrial space-analog studies, both modeled microgravity cell culture and using animal subjects, have provided additional insight regarding the specific microgravity-induced defects among immunocyte populations (Borchers and others 2002; Sonnenfeld 1994). For example, modeled microgravity effects on immune cells include reduced interleukin (IL)-2 production (Cooper and Pellis 1998; Licato and Grimm 1999), increased TNFα production (Batkai and others 1999), reduced motility (Meloni and others 2006), altered cytoskeletal structure (Janmey 1998), and reduced proliferative responses (Cooper and Pellis 1998). During 2 Space Shuttle flights, the alterations observed during terrestrial analog cell culture conditions were found to correlate with those observed during cell culture during spaceflight (Hashemi and others 1999). Altered cytokine production has also been observed in other terrestrial spaceflight analogs, such as prolonged bed rest (Shearer and others 2009) and rodent hindlimb unloading (Aviles and others 2005). The utility for such models lies in the mechanistic understanding of microgravity-associated immune changes or in the evaluation of potential countermeasures. Boonyaratanakornkit and others (2005) used modeled microgravity cell culture to identify gravisensitive genes, which may contribute to spaceflight-associated immune dysregulation. Using the rodent hindlimb unloading model, Aviles and others (2003, 2004) demonstrated that treatment with an active hexose correlated compound restored some of the microgravity-associated immune dysfunction. More recent data have confirmed that immune dysregulation also occurs during short-duration spaceflight in humans (Crucian and others 2012), confirming immune dysregulation is an in-flight phenomenon, not merely an artifact of microgravity cell culture or postflight landing effect. The reactivation of latent herpes viruses, likely as a result of diminished immune control, has also been documented to occur in astronauts during spaceflight (Mehta and others 2000; Pierson and others 2005). Indeed, postflight alterations in cytokine profiles following short-duration spaceflight were recently found to positively correlate with astronaut reactivation of latent herpesviruses (Mehta and others 2012).
Plasma cytokine concentration may serve as an indicator of in vivo physiological changes or immune system mobilization (Kroemer and Martinez 1991; Sonnenfeld 1994). Increases in plasma concentrations may reflect large magnitude localized reactions, or the manifestation of systemic reactions. Being very diverse group of immune regulatory mediators, cytokines may be subdivided into several categories, allowing monitoring of different types of biological responses. These include innate immunity [(eg, TNFα, IL-1β, IL-6, IL-12, IFN-α, transforming growth factor-β (TGF-β)], adaptive immunity (eg, IFN-γ, IL-4, IL-10, IL-17, IL-21), growth factors that regulate hematopoiesis (eg, G-, M-, GM-CSF, Tpo, erythropoietin/EPO, FGFb), and chemokines that orchestrate chemotactic trafficking of immunocompetent cells (eg, IL-8, CCL2, CCL3, CCL4, etc.). Furthermore, many of the very same cytokines can be subdivided into 2 major groups based on their physiological functions: pro- versus anti-inflammatory (eg, TNFα, IL-1b, IL-6, IL-12, or IL-10, TGF-b, IL-1ra, respectively). Therefore, the assessment of many cytokines, and the determination of specific pattern shifts, can indicate the presence of specific disease types. For example, it is well established that rheumatoid arthritis and multiple sclerosis are “Th1” diseases, whereas systemic autoimmune diseases and allergies are “Th2” diseases (Kasakura 1998).
Aside from autocrine and paracrine effects, many cytokines can act in endocrine manner following exogenous (eg, pathogens) or endogenous (eg, other cytokines, autoantigens) stimulation and produce systemic outcomes, such as cytokine storm (rev in Harrison 2010; Tisoncik and others 2012). Most cytokines possess a short half-life and are locally acting; therefore, the plasma level for most cytokines is generally low. Exceptions include that some chemokines, which due to their role in leukocyte trafficking and recruitment, must leave a localized site of inflammation to recruit specific cell types from the general circulation.
Plasma cytokine assessment has been demonstrated to have clinical utility as a biomarker for various specific immunologic diseases or other disruptions in physiological homeostasis. Elevated levels of IL-6, IL-7, IL-10, and IFN-γ have been detected in the plasma of HIV-infected patients and correlated well with prognosis (Chuenchitra and others 2012). Plasma levels of cytokines have also been found to correlate with disease presence or prognosis in rheumatoid arthritis (Khan and others 2009), myelofibrosis (Tefferi and others 2011), Sjogren's syndrome (Szodoray and others 2004), COPD (Bon and others 2010), and pelvic inflammatory disease (Chen and others 2008).
It has been suggested that persistent immune dysregulation may increase specific clinical risks for astronaut crewmembers participating in exploration-class deep space missions (Crucian and Sams 2009). A broad human survey of human immunity during long-duration spaceflight has not yet been performed. To determine in vivo immune homeostasis during long-duration orbital spaceflight, we investigated astronaut plasma cytokine levels as a biomarker of immune status during missions to the International Space Station (ISS).
Materials and Methods
Subjects and missions
Twenty-eight ISS astronaut crewmembers participated in this study. Mission durations were approximately 6 months, which is considered long-duration spaceflight. Of the 28 subjects, 21 were male and 7 were female, and their mean age was 49±4 years. Approval was obtained from the Institutional Review Board at the NASA Johnson Space Center, Houston, TX. Informed consent was obtained from all subjects before participation.
Plasma samples
Whole blood samples (5.0 mL) were collected at the following time points: 180, 45, or 10 days before flight (L-180, L-45, L-10, respectively), at 5 time points during spaceflight [flight days (FD) 15, 30, 60, 120, and 180], within 24 h after landing (R+0), and 30 days after landing (R+30). Samples were collected as the first morning activity for the crew, after a minimum 8-h period of fasting and no exercise. Sample collections generally did not coincide with either extravehicular activities (spacewalks) or the docking/undocking of other visiting vehicles. These provisions were enacted to sample within periods believed to be less stressful for the crew. “Morning” is defined by the crew sleep/wake schedule as immediately following waking, despite the crew living according to a different time zone while on orbit. There were no other constraints placed on the sample collection to have schedule flexibility within the myriad of other on-orbit operations. All sample collection time points are approximations and could vary somewhat based on operational constraints. Samples were collected in EDTA tubes containing plasma-separation gel. Following collection, samples were centrifuged for 30 min to allow plasma separation. All samples (pre-, in-, and postflight) were frozen until batch analysis after the in-flight samples were returned to the Earth onboard either the U.S. Space Shuttle or the SpaceX Dragon capsule.
Plasma cytokine concentration
The concentrations for 22 plasma cytokines representing 5 broad categories of function (Table 1) were determined simultaneously in duplicate using a commercially available multiplex bead immunoassay (R&D Systems). Samples were processed according to the manufacturer's instructions. Briefly, 50 μL of plasma were incubated with beads bound to a cytokine capture antibody. The 22 bead populations vary by fluorescence intensity so that they may be resolved for individual analysis. Bead cytokine concentrations were then washed and incubated with a fluorescent secondary antibody, specific for each cytokine, but fluorescing along a single channel distinct from the bead populations. The assay was performed in a 96-well plate, and the analysis was performed using a Luminex 100 instrument (Luminex, Inc.).
Table 1.
Twenty Two Cytokines Assessed on International Space Station Astronauts, by Category
| Inflammatory | Anti-inflammatory | Adaptive/regulatory | Growth factors | Chemokines |
|---|---|---|---|---|
| IL-1α | IL-1ra | IFN-γ | G-CSF | CCL2/MCP-1 |
| IL-1β | IL-2 | GM-CSF | CCL3/MIP-1α | |
| TNFα | IL-17 | FGF basic | CCL4/MIP-1β | |
| IL-6 | IL-4 | Tpo | CCL5/RANTES | |
| IL-8 | IL-5 | VEGF | CXCL5/ENA-78 | |
| IL-10 |
Statistical analysis
Mean concentration was calculated for all sample values over the indicated assay threshold (Table 1). A repeated-measures one-way analysis of variance was performed to determine the effect of spaceflight on plasma cytokine levels. If there was a significant main effect observed for spaceflight, then an additional post hoc Bonferroni t-test was performed comparing each time point to the L-180 time point. For these analyses, in the event that the baseline value was below the assay threshold, the threshold value itself was used for statistical analysis. In the event that all mean time point data were below the assay threshold of sensitivity, no statistical analysis was performed. Statistical analyses were performed using Sigma Stat 3.11 (Systat Software).
Results
Inflammatory/anti-inflammatory
Mean L-180 baseline levels of IL-1α, IL-1β, TNFα, IL-6, and IL-8 were all below the limit of sensitivity for each cytokine (Table 2). There were no statistically significant in-flight alterations for IL-1α, IL-1β, or IL-6. The concentration of TNFα was increased during spaceflight (main effect P<0.01); however, the more conservative post hoc analysis did not indicate significant increases for any specific in-flight time points. A main-effect increase in IL-8 was observed during spaceflight (P<0.001), with several in-flight time points achieving individual statistical significance when compared to L-180 (Table 2). Although no in-flight increase was observed for plasma IL-6, there appeared to be a trend toward elevated concentration immediately postflight. Among all premission samples (3 time points per crewmember, 28 crewmembers), only 2 samples had detectable levels of IL-6 above the limit of sensitivity, whereas immediately following the spaceflight, 10 crewmembers possessed IL-6 concentrations above the limit (data not shown). There was a fairly stable preflight baseline concentration of IL-1ra between L-180 and L-45 (378 and 363 pg/mL, respectively). By L-10, IL-1ra concentration was trending upward and was significantly higher than baseline (L-180) for all in-flight time points (R+0), resulting in a significant main-effect difference (P<0.001; Table 2). Individual analysis of the IL-1ra increase achieved significance at FD120 and R+0 (P<0.001).
Table 2.
Mean Plasma Cytokine Concentration (pg/ml) for International Space Station Astronauts Before, During, and After Spaceflight
| Spaceflight | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Sensitivity | L-180 | L-45 | L-10 | FD15 | FD30 | FD60 | FD120 | FD180 | R+0 | R+30 | |
| n | P value | (pg/mL) | 26 | 28 | 24 | 28 | 26 | 28 | 24 | 21 | 28 | 27 |
| IL-1a | 0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | <0.36 | |
| IL-1b | 0.57 | <0.57 | <0.57 | <0.57 | <0.57 | <0.57 | 0.6±0.3 | 0.9±0.7 | 0.8±0.6 | <0.57 | <0.57 | |
| TNFα | <0.05 | 1.5 | <1.5 | <1.5 | <1.5 | 2.1±0.6 | 1.6±0.3 | 1.7±0.3 | 1.9±0.5 | 1.7±0.4 | <1.5 | <1.5 |
| IL-6 | 1.11 | <1.11 (n=23) | <1.11 (n=25) | <1.11 (n=21) | <1.11 (n=25) | <1.11 (n=23) | <1.11 (n=25) | <1.11 (n=21) | <1.11 (n=20) | <1.11 (n=25) | <1.11 (n=24) | |
| IL-8 | <0.001 | 1.97 | <1.97 | <1.97 | 2.7±0.4 | 7.6*±2.2 | 6.2±1.4 | 7.0*±1.7 | 6.7±2.0 | 6.7*±2.3 | <1.97 | 2.0±0.4 |
| IL-1ra | <0.01 | 10.91 | 379±37 (n=23) | 363±35 (n=25) | 505±61 (n=21) | 556±61 (n=25) | 558±77 (n=23) | 635±99 (n=25) | 722*±128 (n=21) | 631±82 (n=20) | 687*±117 (n=25) | 576±144 (n=24) |
| INF-g | 1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | <1.27 | |
| IL-2 | 2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | <2.23 | |
| IL-17 | 1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | <1.1 | |
| IL-4 | 4.46 | <4.46 (n=23) | <4.46 (n=25) | <4.46 (n=21) | <4.46 (n=25) | <4.46 (n=23) | <4.46 (n=25) | <4.46 (n=21) | <4.46 (n=20) | <4.46 (n=25) | <4.46 (n=24) | |
| IL-5 | 0.71 | <0.71 (n=23) | <0.71 (n=25) | <0.71 (n=21) | <0.71 (n=25) | <0.71 (n=23) | <0.71 (n=25) | <0.71 (n=21) | <0.71 (n=20) | <0.71 (n=25) | <0.71 (n=24) | |
| IL-10 | 0.3 | <0.3 (n=23) | <0.3 (n=25) | <0.3 (n=21) | 0.3±0.2 (n=25) | <0.3 (n=23) | <0.3 (n=25) | 0.3±0.2 (n=21) | <0.3 (n=20) | <0.3 (n=25) | <0.3 (n=24) | |
| G-CSF | 1.48 | 5.4±1.6 | 4.7±1.2 | 6.2±1.6 | 5.0±1.3 | 3.1±0.6 | 5.3±1.5 | 10.7±5.9 | 6.6±2.3 | 7.2±1.6 | 4.3±1.0 | |
| GM-CSF | 1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | <1.98 | |
| FGFb | 4.91 | 7.7±3.4 | 6.8±2.8 | 8.9±3.3 | 6.2±1.9 | 11.4±3.5 | 11.6±3.3 | 7.7±2.7 | 6.8±2.2 | 5.6±2.2 | 6.4±2.4 | |
| Tpo | <0.001 | 9.94 | 141±16 | 139±17 | 165±22 | 183±18* | 186*±28 | 184*±21 | 194*±27 | 215*±22 | 138±16 (n=27) | 129±15 |
| VEGF | <0.001 | 1.84 | 3.7±0.8 (n=23) | 5.1±1.4 (n=25) | 6.7±2.2 (n=21) | 10.2±1.9 (n=25) | 14.3*±4.5 (n=23) | 10.5±1.7 (n=25) | 11.5±2.9 (n=21) | 10.6±1.8 (n=20) | 3.5±0.8 (n=25) | 3.7±0.9 (n=23) |
| CCL2/MCP-1 | <0.001 | 0.47 | 72.4±6.4 | 77.7±7.6 | 69.4±7.6 | 70.7±5.4 | 66.6±5.5 | 77.6±6.6 | 85.4±7.1 | 86.6±7.5 | 122.5*±17.4 | 90.9±7 |
| CCL3/MIP-1α | 1.45 | 9.4±3 (n=23) | 7.6±3.1 (n=25) | 8.6±3.2 (n=21) | 10.2±4 (n=25) | 5.2±2.2 (n=23) | 7.3±3 (n=25) | 9.8±3.9 (n=21) | 7.9±4.1 (n=20) | 7.1±2.9 (n=25) | 6.6±2.8 (n=24) | |
| CCL4/MIP-1β | <0.05 | 0.72 | 16.2±2.1 | 16.5±2.7 | 17.2±3.0 | 22.1±2.8 | 20.1±2.4 | 21.8±2.8 | 24.3*±5.0 | 21.1±3.2 | 17.0±2.2 | 18.7±3.8 |
| CCL5/RANTES | 1.91 | 3427±306 (n=23) | 3247±242 (n=25) | 3986±180 (n=21) | 3609±190 (n=25) | 3741±197 (n=23) | 3546±182 (n=25) | 3720±232 (n=21) | 4003±199 (n=20) | 3366±262 (n=25) | 3562±216 (n=24) | |
| CXCL5/ENA-78 | <0.001 | 4.14 | 239±60 | 348±203 | 898*±251 | 2020*±363 | 1882*±319 | 1933*±330 | 1741*±309 | 1841*±385 | 189±48 | 204±53 |
Data are expressed as mean concentration (pg/mL)±SEM. Main effects were determined by performing a repeated-measures one-way ANOVA analysis compared to the baseline L-180 sample, resulting in the indicated “P” values. In the event that the baseline value was below the assay threshold, the threshold value itself was used for statistical analysis. In the event that all mean time point data were below the assay threshold of sensitivity, no statistical analysis was performed. Individual time point significance was determined by a post hoc Bonferroni t-test, with significant differences indicated by bold+*. Overall “n” was 28 subjects, the specific “n” for each time point/cytokine indicated.
Adaptive immunity cytokines
Mean L-180 baseline levels of IFN-γ, IL-2, IL-17, IL-4, IL-5, and IL-10 were all below the limit of sensitivity (Table 2). There were no statistically significant differences in any adaptive immunity cytokines at any measured in-flight or postflight time points.
Growth factors
The mean baseline concentration for the cytokines identified as “growth factors” varied from <1.0 pg/mL (GM-CSF) to 141 pg/mL for thrombopoietin (Tpo) (Table 2). During spaceflight, there were no significant alterations in the plasma concentration of G-CSF, GM-CSF, or FGF basic. Main-effect in-flight increases were detected in the plasma concentration of both Tpo and vascular endothelial growth factor (VEGF) (P<0.001), with Tpo significantly elevated at all 5 individual in-flight time points. In-flight alterations for all growth factors returned to baseline immediately after landing.
Chemokines
Generally, the baseline concentration for chemokines was much higher than for all other measured cytokines. The mean baseline concentration for chemokines ranged from 9.4 pg/mL (CCL3) to 3427 pg/mL (CCL5) (Table 2). During spaceflight, a main-effect increase was observed for CCL2, CCL4, and CXCL5 (P<0.001), with single point significance detected for CXCL5 at all 5 in-flight points, and on FD120 for CCL4. No single-time point significant in-flight increases were observed for CCL2, but a postflight significant increase was detected at R+0 (Table 2). No deviations from baseline were observed for CCL3 or CCL5.
Discussion
Alterations in plasma cytokine levels are an established biomarker for many diseases. As the dysregulation of the immune system is an established postflight phenomenon (Gueguinou and others 2009), and persistent dysregulation may be a clinical risk to crewmembers (Crucian and Sams 2009), it is appropriate to survey plasma cytokine levels in astronauts during long-duration spaceflight. For this study, 22 plasma cytokines were assessed at 5 time points during 6-month flight onboard the ISS. Time points were spaced to include the early adaptation phase (FD15, 30) and subsequent long-adaptation points through the entire 6-month mission (FD60, 120, 180). This approach allowed the kinetics of an entire mission to be assessed, while freezing all samples for batch analysis eliminated any interassay variability concerns. All in-flight data were compared with the baseline sample collected ∼180 days before launch. A second prelaunch sample (L-45) allows another correlative prelaunch sampling and a general measure of intrasubject variability, and a third preflight sample opportunity was available at 10 days before launch.
This survey activity was a component of a larger flight study, and no control group was included in the original design to parallel the astronaut subjects. However, we recognize that such a control would have value for assessing variability within individual subjects over time. The reader may observe that the concentrations of some cytokines, which are elevated during spaceflight, are actually trending toward an increase by L-10. This is most likely a result of premission stress influences on the crewmembers. In fact, Stowe and others (1999, 2000) have characterized immunological and stress alterations in Space Shuttle crewmembers at both 10 and 3 days before launch. The L-180 sampling is considered early enough to avoid any prelaunch or training stresses, thus establishing a legitimate baseline value for each crewmember. We also included the additional baseline data points for reader consideration. Unfortunately, it cannot be said with certainty if the alterations observed during spaceflight result purely from stress or include synergic influences of microgravity, radiation, and so on. It may be noted that the effect of “spaceflight” on human physiology does indeed represent the summary effect of all these influences, which are unlikely to change during future deep-space exploration-class missions.
Although the in-flight samples are most relevant for an assessment of long-duration spaceflight, samples were also collected immediately following landing (R+0). Sample collection after landing assesses postflight physiological changes that are associated with a high-G re-entry following prolonged microgravity-associated deconditioning. Such data are relevant for the immediate postlanding phase of interplanetary exploration. The “recovery” sample, collected 30 days after landing, was included to ensure that any flight or landing-associated alterations would be trending toward baseline values. This is relevant to establishing how quickly crews recover from the adverse effects of spaceflight.
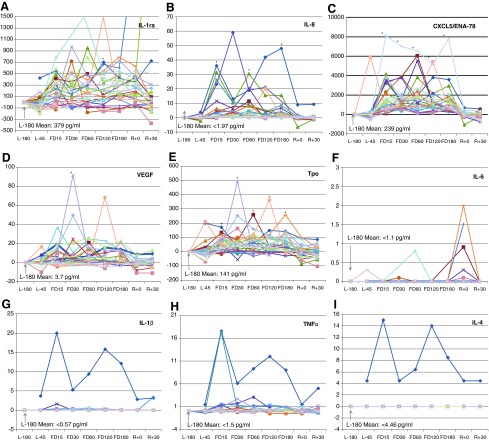
Individual subject data analysis indicated that where statistically significant alterations occurred, crewmember data were remarkably consistent. Setting the L-180 baseline concentration to zero to observe deviations from baseline, single-subject analysis of IL-1ra, IL-8, CXCL5, VEGF, and Tpo shows that most crewmembers generally manifested a positive alteration during flight, with few obvious negative outliers (Fig. 1A–E). Plotting the individual data also may resolve in-flight effects from postflight trends such as an apparent increase in plasma IL-6 present for most crewmembers after landing, which unfortunately remained below the threshold of sensitivity for the assay for most in-flight data points (Fig. 1F). When tracking individual crew data, occasional outlier crewmember data may be observed that show, usually at a few specific time points only, relatively high concentrations of certain cytokines. Examples are shown in Fig. 1G–I, where clear single-subject outliers are visible for IL-1β, TNFα, and IL-4. Although these alterations were not frequent enough to influence statistical significance, on a case-by-case basis, these shifts may be clinically relevant. Crewmembers do occasionally experience adverse medical events during spaceflight, including infectious disease or hypersensitivity responses. For this study, we cannot know if these particular outliers correlated with adverse medical events; however, such a correlation would appear possible even if the underlying mechanistic cause was subclinical. Unfortunately, individual crew health data were not captured as part of the current study. However, it may be feasible that monitoring crewmember plasma cytokine concentration could have routine use for either clinical monitoring or augmenting primary diagnostic/prognostic laboratory measures.
FIG. 1.
Representative individual crewmember plasma cytokine concentrations during long-duration spaceflight. The graphs depict (A) IL-1ra, (B) IL-8, (C) CXCL5/ENA-78, (D) VEGF, (E) Tpo, (F) IL-6, (G) IL-1β, (H) TNFα, (I) IL-4. For all crewmembers, baseline concentrations (L-180) were normalized to zero, and deviations from baseline were plotted for all other time points. For all measured values below the assay sensitivity limit, the limit value itself was plotted. Statistically significant alterations in raw mean values are indicated (*) where P≤0.05; n=28.
Inflammatory cytokines recruit leukocytes to a localized immune reaction, promote fever, vascular changes, cellular activation, and acute phase responses. With rare exceptions, minimal detectable mean plasma levels were observed at baseline (L-180) for innate inflammatory cytokines (IL-1α, IL-1β, TNFα, IL-6) and adaptive regulatory cytokines (IL-2, IFN-γ, IL-17, IL-4, IL-5, IL-10); however, IL-1ra as well as all measured chemokines and growth factors were constitutively present. This pattern is consistent with generally healthy astronauts free of infectious disease or inflammatory processes during premission training.
During spaceflight, there was a significant increase in plasma IL-8 at multiple in-flight time points, whereas TNFα demonstrated a significant main-effect increase associated with spaceflight (Table 1). Although the levels of other inflammatory cytokines (IL-1a, IL-1b, IL-6) were not elevated during spaceflight, the increase in IL-8 and TNFα is indicative of mild inflammation and would be consistent with sensitized innate immunocyte function associated with persistent low-level inflammation during spaceflight. Persistent inflammation during flight could derive from flight-associated alterations in the gut microbiome or the consistent exposure to increased environmental radiation. It is interesting that plasma levels of IL-1ra, an inhibitor of the proinflammatory effects of IL-1, were also consistently elevated during spaceflight. IL-1ra demonstrated both a main-effect increase associated with flight and a specific increase at the FD120 time point (Table 2). Assuming that spaceflight factors induce persistent low-grade systemic or localized inflammation, elevations in circulating IL-1ra may represent an adaptive physiological response to the inflammatory stress (Suzuki and others 2002). In fact, similar stimuli induce cells to secrete both IL-1 and IL-1ra, but plasma concentrations of IL-1ra are consistently about 100-fold higher than those of IL-1 (Burger and Dayer 2000), which would support the current observation of levels of IL-1ra in astronauts during spaceflight being 300- to 600-fold higher than those of IL-1a or IL-1b (Table 2). It is also noteworthy that systemic plasma IL-1a or IL-1b did not increase during spaceflight. It is therefore possible that the in-flight cytokine profile does reflect some localized inflammation (ie, gut), whereas the major systemic observation was the significant increase in IL-1ra. In fact, it has been previously demonstrated that localized inflammation does result in a primarily anti-inflammatory systemic response (Rivera-Chavez and others 2003) and that increased systemic levels of IL-1ra may serve as a good prognosis indicator for a localized inflammatory disease (John and others 2008).
Further supporting an inflammatory state during flight are the observed elevations in CCL2, CCL4, and CXCL5 during spaceflight (Table 2). Many cytokines have pleotropic redundant or overlapping functions. Although classified separately in this article as “chemokines,” these molecules are also directly involved in the process of inflammation. CXCL5 (ENA-78), a potent neutrophil chemoattractant, is produced by cells subsequent to stimulation with inflammatory cytokines, such as IL-1 or TNFα (Walz and others 1993). CCL4 (MIP-1b) is a proinflammatory molecule produced by macrophages and an attractant for monocytes, NK cells, and other immunocytes (Maurer and von Stebut 2004). CCL2, also known as monocyte chemotactic protein-1, recruits monocytes, memory T cells, and dendritic cells to a site of localized inflammation. CCL4 (and other MIP-1 family members) upregulates the process of inflammation via the recruitment of inflammatory cells, inducing the release of proinflammatory mediators, and modulating T-helper cell differentiation (Maurer and von Stebut 2004). Increased plasma levels of CCL4 have been found to correlate with clinical diseases, such as multiple myeloma (Terpos and others 2005) and hepatitis C (Zeremski and others 2007). Increased levels of CXCL5 have been found to correlate with inflammatory diseases, such as rheumatoid arthritis (Walz and others 1997). Although it is unclear why only these 3 chemokines displayed consistent in-flight increases, their elevation also supports some degree of a chronic proinflammatory state during spaceflight. Levels of CXCL5 increased by nearly 10-fold during flight, remained elevated for an entire 6-month spaceflight, and return to baseline almost immediately upon landing (Table 2).
Normal human levels of adaptive immunity cytokines are very low in plasma, as adaptive immune processes should end to protect the host from the damaging effects of a continuous uncontrolled immune activation. In this study, no increases were observed for any Th1, Th17, or Th2 cytokines during spaceflight (Table 2). Astronauts onboard the ISS are essentially in a well-maintained isolation chamber and unlikely to be exposed to various transmissible pathogens and therefore may mount fewer adaptive immune responses. The plasma cytokine data may suggest that the astronauts were simply free from infectious disease; however, there may be an alternative explanation. Numerous microgravity or microgravity-analog cell culture experiments have demonstrated that T cells do not activate normally during reduced gravity conditions (Hashemi and others 1999; Boonyaratanakornkit and others 2005). There have also been previous reports of diminished T-cell function in astronauts associated with spaceflight. In-flight and postflight mitogen-stimulated cell cultures from astronauts produce greatly reduced levels of cytokines than preflight baseline cultures (Crucian and others 2008). This has been interpreted as a generalized reduction in T-cell function during spaceflight. Consequently, astronauts display persistent in-flight reactivation of various latent herpesviruses (Mehta and others 2000; Pierson and others 2005), known to be associated with reduced cytotoxic T-cell function. Likely causes for adaptive immunosuppression during flight include physiological stress, circadian misalignment, isolation and confinement, or a microgravity-associated defect in T-cell intracellular signal transduction (Boonyaratanakornkit and others 2005). Therefore, the absence of increased levels of adaptive cytokine during spaceflight could also result from, to some degree, crewmember inability to mount adaptive responses during flight. Further complicating the astronaut risk scenario, the recently described alterations in microbial virulence may yet alter susceptibility for infectious diseases during spaceflight (Wilson and others 2007).
The observed increases in growth factors and chemokines may indicate other types of adaptation, such as enhanced innate immune parameters, or attempts to overcome diminished immunocyte function. Increased Tpo and VEGF were previously reported in a single subject participating in a 21-day short-duration spaceflight. Tpo was found to be elevated throughout the flight, but VEGF elevated only during the early stages of flight and then returned to baseline (Gunga and others 1999). The increase in VEGF was suggested to be related to intravascular fluid shifts. However, Gunsilius and others (1999) have reported a striking correlation between platelet levels and VEGF, with VEGF substantially lower in thrombocytopenic patients. They suggested that platelets are a predominant source of VEGF in serum, therefore when Tpo levels are elevated (and platelet levels would be correspondingly low), VEGF levels would be expected to be low. Gunsilius suggested that the rapid decrease in VEGF in the single subject may correlate with thrombocyte depletion. Thrombocytopenia is known to occur during spaceflight (Kalandarova 1991; Davis and others 1996) and would be supported by elevated plasma Tpo levels observed in the same single subject. Our data, however, indicate that both Tpo and VEGF remain elevated throughout a 6-month orbital spaceflight. Tpo was significantly elevated at all 5 in-flight time points, whereas the levels of VEGF tended to be elevated at all in-flight points (main-effect), with a significant point-specific increase on FD30 (Table 2). These data suggest that the rapid in-flight return to baseline in VEGF previously reported (Gunga and others 1999) may not be reflective of astronauts in general. Tpo stimulates platelet production but is regulated via a negative feedback loop. Tpo is bound onto the surface of platelets via the CD110 surface receptor, therefore removing it from circulation. When platelet levels decrease, Tpo therefore increases and stimulates new platelet production. It would seem logical therefore that since thrombopenia is associated with spaceflight, Tpo levels would be elevated. It is unclear if the rise in VEGF may therefore be associated with a platelet decrease (destruction and release into plasma) or if a VEGF rise may be associated with other physiological processes (fluid shifts and angiogenesis). It is noteworthy that the chemokine CXCL5, also consistently elevated during spaceflight, also displays angiogenic properties and supports tumor formation (Li and others 2011). Although activated platelets are a rich source of several chemokines (Gear and Camerini 2003; Gleissner and others 2008), in this study, other platelet-associated chemokines (RANTES, MIP-1a) were not elevated during spaceflight. It is therefore questionable if the elevations in CXCL5 result from platelet activation, even though the levels of Tpo increased during spaceflight.
Unfortunately, due to existing study constraints, only plasma samples were collected for this study, so other beneficial parameters such as a white blood cell count, differential, and functional assays could not be performed. It is noteworthy that in this specific study, plasma samples were generally collected during relatively lower-stress mission phases away from vehicle dockings, extravehicular activities (spacewalks), or docked vehicle operations. Docked vehicle operations, such as the arrival of a visiting cargo vehicle, are periods of intensive work usually accompanied by a circadian shift. This was the preferred sampling option since samples could be frozen on-orbit for storage, and later returned for analysis. Other studies onboard the ISS, which sample crewmembers and return ambient blood for analysis, will define the immunological changes associated with periods of elevated stress. To determine clinical risks for exploration-class deep-space missions, a thorough understanding of immune system dysregulation is necessary for all mission phases. This includes the postlaunch space adaptation phase, the “space normal” transit phase equilibration, and the stressful postlanding phase following prolonged deconditioning.
It is noteworthy that the in-flight concentration of 9 plasma cytokines has been previously reported for short-duration Space Shuttle missions (Crucian and others 2012). Significant in-flight increases, compared to preflight baseline, were reported for IL-1β, TNFα, IFNα, IFNg, IL-17, IL-4, IL-10, and IL-12. For IL-6, concentration was not increased during flight, but a significant postflight increase was observed. Only 7 of these cytokines were also measured during the current ISS study. For ISS astronauts, an in-flight increase was observed only for TNFα (Table 2), and a trend toward increasing concentration of IL-6, although not significant, was observed postflight (Fig. 1). We may speculate why increases in certain plasma cytokines would be observed in Space Shuttle crewmembers and not in ISS crewmembers. Certainly, Shuttle missions are dramatically different from ISS missions in several respects. Shuttle missions have been described as a “sprint”, consisting of exhaustive in-flight work schedules and frequently dealing with operational anomalies. In contrast, ISS missions have been described as a “marathon”, consisting of prolonged durations with a (relatively) more reasonable work schedule. In fact, Shuttle missions may represent a more “acute” stress, whereas the ISS mission may represent a more “chronic” stress model. There are some research data to support this notion, indicating Shuttle crewmembers manifest some increased immune cellular function postflight (Crucian and others 2008).
From the data described here, it is clear that a pattern of persistent physiological adaptations occur during spaceflight that include shifts in immune/hormonal regulation. It has yet to be determined if these adaptations increase crew risk for adverse medical events during spaceflight. The pattern of cytokine elevations observed during spaceflight is somewhat surprising, in that within categories there are differential increases: some inflammatory cytokines/chemokines are elevated, whereas others are not. This may be explained by different plasma half-life among the cytokines, different kinetics/magnitude of expression versus resorption by target cells, or the location/nature of the proinflammatory stimuli that may affect crewmembers during spaceflight. Further investigations will be required to precisely define the mechanistic causes of in-flight immune dysregulation. It is clear, however, that immunity is dysregulated during spaceflight, including the previously described alterations in cellular distribution and function (Crucian and others 2012), and now evidence of in vivo immunoregulatory alterations. Even if this phenomenon is subclinical during orbital flight, clinical risk to crewmembers could be elevated for deep-space missions. As future studies continue to characterize in-flight immune alterations, the development of countermeasures to enable exploration missions to be conducted safely may be warranted.
Acknowledgments
The authors thank the ISS astronauts for participating in this study. The authors are particularly grateful to the JSC experiment support staff for both the NASA Nutritional (SMO-018) and Immunology (SMO-016) flight studies onboard the ISS.
Author Disclosure Statement
The authors have no commercial associations that would result in a conflict of interest regarding this publication.
References
- Aviles H, Belay T, Fountain K, Vance M, Sun B, Sonnenfeld G. 2003. Active hexose correlated compound enhances resistance to Klebsiella pneumoniae infection in mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol 95:491–496 [DOI] [PubMed] [Google Scholar]
- Aviles H, Belay T, Vance M, Sonnenfeld G. 2005. Effects of space flight conditions on the function of the immune system and catecholamine production simulated in a rodent model of hindlimb unloading. Neuroimmunomodulation 12:173–181 [DOI] [PubMed] [Google Scholar]
- Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G. 2004. Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol 97:1437–1444 [DOI] [PubMed] [Google Scholar]
- Batkai L, Varkonyi A, Minarovits J. 1999. The effect of simulated microgravity conditions on the TNF-alpha production by human PBMCS. J Gravit Physiol 6:P109–P110 [PubMed] [Google Scholar]
- Bon JM, Zhang Y, Duncan SR, Pilewski JM, Zaldonis D, Zeevi A, McCurry KR, Greenspan SL, Sciurba FC. 2010. Plasma inflammatory mediators associated with bone metabolism in COPD. Copd 7:186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit JB, Cogoli A, Li CF, Schopper T, Pippia P, Galleri G, Meloni MA, Hughes-Fulford M. 2005. Key gravity-sensitive signaling pathways drive T cell activation. Faseb J 19:2020–2022 [DOI] [PubMed] [Google Scholar]
- Borchers AT, Keen CL, Gershwin ME. 2002. Microgravity and immune responsiveness: implications for space travel. Nutrition 18:889–898 [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer J-M. 2000. IL-1 RA. In: Oppenheim J, and Feldmann M, eds. Cytokine Reference, Vol. September2000. Miamisburg (OH): Elsevier Science [Google Scholar]
- Chen KS, Wang PH, Yang SF, Lin DB, Lin YJ, Kuo DY, Lin LY, Wu MT, Lin CW, Lee S, Chou MC, Tsai HT, Hsieh YS. 2008. Significant elevation of a Th2 cytokine, interleukin-10, in pelvic inflammatory disease. Clin Chem Lab Med 46:1609–1616 [DOI] [PubMed] [Google Scholar]
- Chuenchitra T, Chaitaveep P, Sukwit S, Dettrairat S, Tabprasit S, Srisurapanon S, Kohreanudom S, Kuvanont D, Sutthent R, Sirisopana N, Nitayaphan S. 2012. Cytokine profiles in HIV-1 subtype CRF01_AE infected individuals with different rates of diseases progression: a multiplex immunoassay. J Med Assoc Thai 95Suppl5:S116–S123 [PubMed] [Google Scholar]
- Cooper D, Pellis NR. 1998. Suppressed PHA activation of T lymphocytes in simulated microgravity is restored by direct activation of protein kinase C. J Leukoc Biol 63:550–562 [DOI] [PubMed] [Google Scholar]
- Crucian B, Sams C. 2009. Immune system dysregulation during spaceflight: clinical risk for exploration-class missions. J Leukoc Biol 86:1017–1018 [DOI] [PubMed] [Google Scholar]
- Crucian B, Stowe R, Mehta S, Uchakin P, Quiriarte H, Pierson D, Sams C. 2013. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J Clin Immunol 33:456–465 [DOI] [PubMed] [Google Scholar]
- Crucian BE, Stowe RP, Pierson DL, Sams CF. 2008. Immune system dysregulation following short- vs long-duration spaceflight. Aviat Space Environ Med 79:835–843 [DOI] [PubMed] [Google Scholar]
- Davis TA, Wiesmann W, Kidwell W, Cannon T, Kerns L, Serke C, Delaplaine T, Pranger A, Lee KP. 1996. Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J Leukoc Biol 60:69–76 [DOI] [PubMed] [Google Scholar]
- Gear AR, Camerini D. 2003. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation 10:335–350 [DOI] [PubMed] [Google Scholar]
- Gleissner CA, von Hundelshausen P, Ley K. 2008. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol 28:1920–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguinou N, Huin-Schohn C, Bascove M, Bueb JL, Tschirhart E, Legrand-Frossi C, Frippiat JP. 2009. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth's orbit? J Leukoc Biol 86:1027–1038 [DOI] [PubMed] [Google Scholar]
- Gunga HC, Kirsch K, Roecker L, Jelkmann W. 1999. Haemopoietic, thrombopoietic, and vascular endothelial growth factor in space. Lancet 353:470. [DOI] [PubMed] [Google Scholar]
- Gunsilius E, Petzer AL, Gastl G. 1999. Space flight and growth factors. Lancet 353:1529. [DOI] [PubMed] [Google Scholar]
- Harrison C. 2010. Sepsis: calming the cytokine storm. Nat Rev Drug Discov 9:360–361 [DOI] [PubMed] [Google Scholar]
- Hashemi BB, Penkala JE, Vens C, Huls H, Cubbage M, Sams CF. 1999. T cell activation responses are differentially regulated during clinorotation and in spaceflight. Faseb J 13:2071. [DOI] [PubMed] [Google Scholar]
- Janmey PA. 1998. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev 78:763–781 [DOI] [PubMed] [Google Scholar]
- John CC, Park GS, Sam-Agudu N, Opoka RO, Boivin MJ. 2008. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine 41:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandarova MP. 1991. [Changes in hematologic indicators in personnel testing during 370-day anti-orthostatic hypokinesia]. Kosm Biol Aviakosm Med 25:15–18 [PubMed] [Google Scholar]
- Kasakura S. 1998. [A role for T-helper type 1 and type 2 cytokines in the pathogenesis of various human diseases]. Rinsho Byori 46:915. [PubMed] [Google Scholar]
- Khan IH, Krishnan VV, Ziman M, Janatpour K, Wun T, Luciw PA, Tuscano J. 2009. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom 76:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Martinez C. 1991. Cytokines and autoimmune disease. Clin Immunol Immunopathol 61:275. [DOI] [PubMed] [Google Scholar]
- Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, Li G, Lu X, Strieter RM, Burdick M, Go VL, Reber HA, Eibl G, Hines OJ. 2011. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol 178:1340–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licato LL, Grimm EA. 1999. Multiple interleukin-2 signaling pathways differentially regulated by microgravity. Immunopharmacology 44:273. [DOI] [PubMed] [Google Scholar]
- Maurer M, von Stebut E. 2004. Macrophage inflammatory protein-1. Int J Biochem Cell Biol 36:1882–1886 [DOI] [PubMed] [Google Scholar]
- Mehta SK, Crucian BE, Stowe RP, Simpson RJ, Ott CM, Sams CF, Pierson DL. 2012. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine 61:205–209 [DOI] [PubMed] [Google Scholar]
- Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. 2000. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 182:1761–1764 [DOI] [PubMed] [Google Scholar]
- Meloni MA, Galleri G, Pippia P, Cogoli-Greuter M. 2006. Cytoskeleton changes and impaired motility of monocytes at modelled low gravity. Protoplasma 229:243–249 [DOI] [PubMed] [Google Scholar]
- Pierson DL, Stowe RP, Phillips TM, Lugg DJ, Mehta SK. 2005. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun 19:235–242 [DOI] [PubMed] [Google Scholar]
- Rivera-Chavez FA, Wheeler H, Lindberg G, Munford RS, O'Keefe GE. 2003. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg 237:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer WT, Ochs HD, Lee BN, Cohen EN, Reuben JM, Cheng I, Thompson B, Butel JS, Blancher A, Abbal M, Aviles H, Sonnenfeld G. 2009. Immune responses in adult female volunteers during the bed-rest model of spaceflight: antibodies and cytokines. J Allergy Clin Immunol 123:900–905 [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G. 1994. Effect of space flight on cytokine production. Acta Astronaut 33:143–147 [DOI] [PubMed] [Google Scholar]
- Stowe RP, Pierson DL, Feeback DL, Barrett AD. 2000. Stress-induced reactivation of Epstein-Barr virus in astronauts. Neuroimmunomodulation 8:51–58 [DOI] [PubMed] [Google Scholar]
- Stowe RP, Sams CF, Mehta SK, Kaur I, Jones ML, Feeback DL, Pierson DL. 1999. Leukocyte subsets and neutrophil function after short-term spaceflight. J Leukoc Biol 65:179–186 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. 2002. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8:6–48 [PubMed] [Google Scholar]
- Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. 2004. Circulating cytokines in primary Sjogren's syndrome determined by a multiplex cytokine array system. Scand J Immunol 59:592–599 [DOI] [PubMed] [Google Scholar]
- Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. 2011. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 29:1356–1363 [DOI] [PubMed] [Google Scholar]
- Terpos E, Politou M, Viniou N, Rahemtulla A. 2005. Significance of macrophage inflammatory protein-1 alpha (MIP-1alpha) in multiple myeloma. Leuk Lymphoma 46:1699–1707 [DOI] [PubMed] [Google Scholar]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. 2012. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. 1997. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol 62:604–611 [DOI] [PubMed] [Google Scholar]
- Walz A, Strieter RM, Schnyder S. 1993. Neutrophil-activating peptide ENA-78. Adv Exp Med Biol 351:129. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Ott CM, Honer zu Bentrup K, Ramamurthy R, Quick L, Porwollik S, Cheng P, McClelland M, Tsaprailis G, Radabaugh T, Hunt A, Fernandez D, Richter E, Shah M, Kilcoyne M, Joshi L, Nelman-Gonzalez M, Hing S, Parra M, Dumars P, Norwood K, Bober R, Devich J, Ruggles A, Goulart C, Rupert M, Stodieck L, Stafford P, Catella L, Schurr MJ, Buchanan K, Morici L, McCracken J, Allen P, Baker-Coleman C, Hammond T, Vogel J, Nelson R, Pierson DL, Stefanyshyn-Piper HM, Nickerson CA. 2007. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci U S A 104:16299–16304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeremski M, Petrovic LM, Talal AH. 2007. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat 14:675–687 [DOI] [PubMed] [Google Scholar]