Abstract
Exposure to moderate levels of ethanol during brain development has a number of effects on social behavior but the molecular mechanisms that mediate this are not well understood. Gaining a better understanding of these factors may help to develop therapeutic interventions in the future. Zebrafish offer a potentially useful model in this regard. Here, we introduce a zebrafish model of moderate prenatal ethanol exposure. Embryos were exposed to 20 mM ethanol for seven days (48hpfs–9dpf) and tested as adults for individual social behavior and shoaling. We also tested their basal anxiety with the novel tank diving test. We found that the ethanol-exposed fish displayed reductions in social approach and shoaling, and an increase in anxiety in the novel tank test. These behavioral differences corresponded to differences in hrt1aa, slc6a4 and oxtr expression. Namely, acute ethanol caused a spike in oxtr and ht1aa mRNA expression, which was followed by down-regulation at 7dpf, and an up-regulation in slc6a4 at 72hpf. This study confirms the utility of zebrafish as a model system for studying the molecular basis of developmental ethanol exposure. Furthermore, it proposes a putative developmental mechanism characterized by ethanol-induced OT inhibition leading to suppression of 5-HT and up-regulation of 5-HT1A, which leads, in turn, to possible homeostatic up-regulation of 5-HTT at 72hpf and subsequent imbalance of the 5-HT system.
Keywords: Moderate prenatal ethanol, Oxytocin, Serotonin, Social, Vasopressin, Zebrafish
1. Introduction
Maternal alcohol consumption during pregnancy results in a range of effects on the developing fetus, collectively referred to as Fetal Alcohol Spectrum Disorders (FASD; Paintner et al., 2012). FASD are characterized by a range of teratogenic and psychological defects, and represent the leading non-hereditary cause of mental retardation, with the prevalence estimated at between 2 and 5% of the population of the USA and western Europe (May et al., 2009). At the extreme end of the spectrum, when mothers drink heavily during pregnancy, fetal alcohol syndrome (FAS) typically results in gross skeletal and craniofacial abnormalities, and severe CNS dysfunction (Hanson et al., 1976). Moderate alcohol consumption (e.g., equivalent to 1–2 drinks/day, average BAC ~0.01–0.04 g/dL Valenzuela et al., 2012) is associated with a range of more subtle cognitive and behavioral defects, including aggression and depression (Sood et al., 2001), vulnerability to stress (Hellemans et al., 2008), impulsivity and inattention (Streissguth et al., 1989; Suess et al., 1997), and poor scholastic performance (Olson et al., 1998).
Social behavior in offspring exposed to alcohol during gestation (PNE) has been extensively studied, with deficits ranging from problems forming social relationships to severe antisocial behavior (Keil et al., 2010; Kelly et al., 2000; McGee et al., 2008; Rasmussen et al., 2011; Roebuck et al., 1999; Thomas et al., 1998). The heterogeneous nature of social relationships in humans is such that social deficits observed in PNE children are likely to be the result of numerous additive or interactive factors, ranging from insecure attachment styles in both the offspring (O’Connor et al., 2002) and caregivers (Swanson et al., 2000), to deficits in perception of social cues or the ability to sustain functional relationships (Kelly et al., 2000).
Preclinical models have typically used animals to gain insight into neurobiological processes underlying the social deficits associated with PNE. For example, recent work from Hamilton et al. (2010) demonstrated that the adult offspring of rats exposed to moderate levels of ethanol (PNE rats) during pregnancy exhibited changes in some aspects of social behavior (social investigation and aggression), especially in males. These changes appeared to be related to alterations in experience-dependent structural plasticity in frontal cortical regions (agranular insular cortex [AID], the rat homologue of the primate orbital–prefrontal cortex). These data strongly suggest that structural and synaptic plasticity, particularly in brain regions associated with social behavior (e.g., areas of the neocortex), is affected by moderate PNE. The cellular and molecular factors that underpin and modulate this, however, are less well understood.
Zebrafish are a widely used model system in developmental neuroscience. This is due predominantly to their small size, prolific breeding and unparalleled genetic tractability (Guo, 2004). Zebrafish offer a potentially excellent model for studying the molecular processes resulting from PNE because, a) embryos develop ex utero meaning that very precise volumes of ethanol can be added to the embryo medium, and b) the embryos are completely transparent, facilitating real-time visual inspection of developing cells. Zebrafish are also a social (shoaling) species, and provide a potentially excellent model for studying the social aspects of PNE (Buske and Gerlai, 2011; Fernandes and Gerlai, 2009; Oliveira, 2013; Pham et al., 2012). Previous work by Buske and Gerlai (2011) and Fernandes and Gerlai (2009) showed that brief (1–2 h) exposure to high concentrations of ethanol (50 mM [0.25% v/v], 100 mM [0.5% v/v], 200 mM [1% v/v]) alters adult social behavior (operationalized by nearest neighbor and by proximity to a virtual fish) and reduces levels of 2-(5-hydroxy-1H-indol-3-yl)acetic acid (5HIAA; a 5-HT metabolite) in the adult brain. An assessment of more moderate levels of ethanol on these aspects of zebrafish behavior, however, has not been previously carried out. Therefore, here, we examined the effect of moderate developmental ethanol exposure on mRNA expression of genes that code for components of neurotransmitter systems implicated in the control of social behavior, namely serotonin receptor 1a (5-HT1A; htr1a, Bell and Hobson, 1993; Strobel et al., 2003), serotonin transporter (5-HTT; slc6a4 Wendland et al., 2006; Canli and Lesch, 2007) and receptors for the neuropeptides oxytocin and vasopressin (OT; oxtr and AVP; avpr Winslow et al., 1993; Heinrichs et al., 2009).
2. Materials and methods
2.1. Subjects
Adult Tubingen (mixed male/female) zebrafish were kept in a recirculating system, on a 14/10-hour light/dark cycle, at 28.5 °C within our zebrafish aquarium. Fish were fed with a mixture of flake food, fresh brine shrimp and bloodworm. Adults were bred in house and fry reared according to the above protocols. Larvae from each condition (20 mM ethanol and control) were sacrificed at 24hpf (i.e., before ethanol exposure), 50hpf (acute ethanol exposure: 2 h after ethanol added), 72hpf and finally at 7dpf. All animal work was carried following approval from the Queen Mary Research Ethics Committee, and under license from the Animals (Scientific Procedures) Act 1986. Care was taken to minimize the numbers of animals used in this experiment in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk/page.asp?id=1357). Specifically, we examined data from previous pilot studies and studies with other species to carry out a power calculation and assess the minimum number of animals necessary for the expected effect size with power of 0.8.
2.2. Developmental ethanol exposure
Tubingen zebrafish embryos were treated by transferring them into 20 mM (0.12% v/v, equating to ~0.04 g/dL BAC [see below]) ethanol in aquarium water at 48hpf, the long-pec stage (Kimmel et al., 1995). All embryos were carefully staged before treatment. Prior to adding the ethanol, it was our policy to dechorionate the larvae if they had not hatched (in practice, this was rarely necessary as most embryos had hatched by this point). This developmental stage was chosen as it represents a key stage in brain ontogeny, including the development of monoaminergic neurons (Guo et al., 1999). Previous research in adult zebrafish found brain concentrations of ethanol following chronic exposure typically reach ~80–90% bath concentration in adults (Dlugos and Rabin, 2003; Mathur et al., 2011) and ~30–40% in embryos and larvae (Reimers et al., 2004). The relationship between brain and blood alcohol content is not straightforward, and estimates for the ratio of brain:blood ethanol range from 0.6 to 1.5 (Moore et al., 1997). Therefore, we estimate that the larvae would have had a blood alcohol concentration (BAC) of ~0.02–0.07 g/dL putting the zebrafish model in the moderate prenatal ethanol classification (Valenzuela et al., 2012).
Fish water was changed on alternate days and the tanks were cleaned in order to reduce the buildup of yeast and control variation in the ethanol concentration due to evaporation. Embryos were kept in Petri dishes until they were five days old, after which they were transferred into tanks with dimensions 10 × 10 × 20 cm (depth × width × length cm) and a volume of 500 mL with airlines. A maximum of 40 fish were kept in each tank and numbers of ethanol and control fish were balanced; dividing equally the quantity of fish receiving ethanol or aquarium water for the controls between the tanks. Feeding of the embryos ZM000 and paramecium commenced at five days. After seven day swimming in fish water containing ethanol (i.e., aged nine days), the fry were transferred back to pure aquarium water. At this stage, we photographed a selection of larvae from each treatment (ethanol and control) and measured their size (analysis of pixel density) to ensure that there were no gross morphological differences. At age three weeks the volume of water in the tanks was increased to 1 L to provide more space for growth and the ZM000 was replaced with ZM100 and artemia. At age five weeks they were transferred into 7 L tanks and cleaning was reduced to once a week. For the qPCR analysis, embryos were removed at 24hpf, 50hpf, 72hpf, and 7dpf (see below for details).
2.3. Stress reactivity (tank diving)
The tank diving task was carried out in 1.5 L trapezoid tanks (15.2 height × 27.9 top × 22.5 bottom × 7.1 width cm) filled with aquarium treated water from the main aquarium supply. Prior to tank diving, all fish were pair housed for 2-weeks as previously described (Parker et al., 2012). For housing and transport, fish were placed into individual holding tanks, measuring height × width × length: 10 cm × 11 cm × 20 cm. All fish were transported from the aquarium to the test room the day before testing in order to acclimate them to the test-room conditions. Within the holding tanks were located tank inserts with perforated bases. This allowed the fish to be removed easily for testing, thus controlling for the potentially confounding factor of difficulties netting the fish for testing. The order in which the fish were tested was fully counterbalanced according to both pre- and post-natal exposure to ethanol. Testing was carried out during the light phase (i.e., between 9 am and 5 pm) over a four-day period. During the procedure, each fish was individually placed in the novel tank. They were filmed over a 5 minute period, during which time we recorded the duration of time spent in the bottom third of the tank, as well as the distance travelled (see Fig. 1). The filming and analysis were carried out using Noldus Ethovision XT software (TrackSys, Nottingham, UK). Following the tank dive, the fish was removed from the novel tank and placed back in its holding tank.
Fig. 1. Tank used for tank-diving assay.
The fish was placed in the tank for 5-minutes, during which it was filmed from the side to ascertain time spent in the lower third.
2.4. Individual social behavior
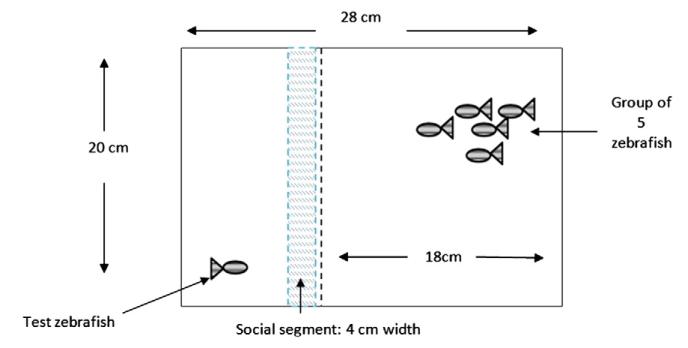
Fig. 2 displays the apparatus used for the individual fish social behavioral assay. Five adult zebrafish were placed into one side of the tank; in the other side was the test zebrafish. Perforated sheets divided the two segments such that the test zebrafish could both see and smell the group of conspecifics. The tank was filled with aquarium treated water. The five fish in the group were either PNE fish or controls, matching the test fish’s developmental treatment. Using Etho-Vision XT 5.0, the duration out of a 20-minute trial that the test zebrafish spent swimming in the segment closest to the divide was recorded for adult control and ethanol treated zebrafish. This assay was performed on four-month-old fish (~50% male/female) with n = 33 fish in the control and n = 30 fish in the PNE group on both occasions.
Fig. 2. Individual social behavioral assay.
The test fish was placed in the testing area, and the shoal was placed in the larger component. The time spent in the social segment by the individual fish was compared between PNE and control fish.
2.5. Shoaling
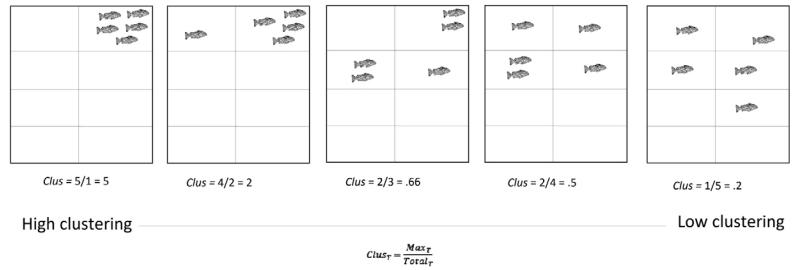
The shoaling procedure (Fig. 3) was based on an earlier study (Parker et al., 2013). In order to carry out the shoaling assay, fish from each treatment were split randomly into four groups of five (familiar individuals; mixed male/female). They were placed into an open arena (W × L × H: 42 × 49 × 15 cm) filled with 6 L aquarium treated water. The fish were left for 5 min to acclimate to the arena, and then filmed from above for 10-min. Behavioral sampling took place from the video recording. The arena was separated into eight equal sections (see Fig. 3). At each 30-second interval during the 10-min, the maximum number of fish in one section (Max) was divided by the total number of sections occupied by the fish (Total), thus providing a cluster score for each time point (t) (Collins et al., 2011).
Fig. 3. Cluster scoring protocol.
A group of 5 fish were placed in the arena and filmed for 10-min following 5-minute habituation. The number of fish occupying each section of the arena and the total number of sections occupied were recorded at 30-second intervals. Adapted from Parker et al. (2013).
2.6. Quantitative real-time PCR
Batches of zebrafish larvae (aged 24hpf, 50hpf, 72hpf, and 7dpf) were digested in 200 μL Lysis buffer with 2 μL Proteinase K for 30–45 min (55 °C) (n = 3 larvae/sample, n = 4 samples/age group). These embryos were checked for any differences in gross morphology and size (quantified by pixel density of photographs) to rule out any potential changes being due to developmental delay caused by the ethanol exposure. mRNA was isolated using 40 μL Dynabeads® Oligo(dT)25 according to the manufacturer’s instructions, and cDNA was synthesized and tested in a quantitative real-time polymerase chain reaction (qPCR). Reference genes (see Table 1 for primer sequences) were chosen according to previous research β-actin, ef1α and rpl13α (Tang et al., 2007). Target genes used were slc6a4, htr1a, oxtr, and avpr (see Table 1). Absolute quantification was obtained by making standards for each gene, prepared using the relevant primers to amplify fragments from cDNA. Samples were then PCR purified and diluted to 1011 fragments using the Avogadro constant. All qPCR reactions were carried out in triplicate. 2 μL of cDNA and 2 μL each of forward and reverse primers (see Table 1) was added to 10 μL SYBR® Green PCR Master mix (Applied Biosystems) on a LightCycler LC480 instrument (Roche Diagnostic). For detailed methods see Gemenetzidis et al. (2010) and Teh et al. (2012).
Table 1. Quantitative real-time PCR primer sequences.
| Gene name | Primers |
|---|---|
| β-actin-F | CGA GCT GTC TTC CCA TCC A |
| β-actin-R | TCA CCA ACG TAG CTG TCT TTC TG |
| eF1α-F | CTG GAG GCC AGC TCA AAC AT |
| eF1α-R | ATC AAG AAG AGT AGT ACC GCT AGC ATT AC |
| rpl13α-F | TCT GGA GGA CTG TAA GAG GTA TGC |
| rpl13α-R | AGA CGC ACA ATC TTG AGA GCA G |
| avpr-F | ACC TTC GTG ATC GTG CTC GC |
| avpr-R | CGG CCG TGT TCT TCG AGT C |
| htr1aa-F | GGA GCC CGC CAT GCG TCT T |
| htr1aa-R | CGT CGC GTT CCC GCT CCA A |
| slc6a4-F | GCC ACA GGC CCC GCT GTT A |
| slc6a4-R | ACC AGG GGC GAA GCC AAG CA |
| oxtr-F | ACA TCT TCA AGG ATC AAG ACT TTT GG |
| oxtr-R | ACC TCT TCG TTC CGC TTG AG |
2.7. Data preparation and statistical analysis
Tank diving data were fitted to a linear mixed effects model, with group (PNE vs handling control) and time (1–5 min), and their interaction, as fixed effects. Distance traveled was entered as a covariate to control for individual differences in freezing/darting in the novel tank (Parker et al., 2012). The dependent variable was time spent in the bottom third of the tank. Individual social behavioral data were analyzed using between-subjects t-tests. The independent variable was group (PNE vs handling control) and the dependent variable was the total time spent in social zone during the test (s). Cluster analysis data were fitted to a linear mixed effect model, with group (PNE vs handling control) as a fixed effect and time (20-levels) as a repeated effect with a structured identity covariance matrix specified. The dependent variable was cluster score (0.2–5). Relative mRNA expression ratios in the qPCR were calculated with respect to reference gene cycle-threshold (Ct) values, and then subjected to a two-way factorial (between-subjects) analysis of variance (ANOVA). Significant main effects and interactions were followed up with pairwise comparisons. The between-subject factors were age (4-levels: 24hpf, 50hpf, 72hpf, 7dpf) and ethanol treatment (2-levels: 20 mM ethanol vs handling control) and their interaction. Homogeneity and normality were ascertained by visual inspection of quantile–quantile (q–q) plots, and residual vs fitted values. All test statistics were evaluated with respect to a type-1 error rate of 0.05. All descriptive statistics are reported as estimated marginal means ± SE unless otherwise indicated. Statistical analyses were carried out in IBM SPSS for Macintosh (v. 19).
3. Results
3.1. Tank diving
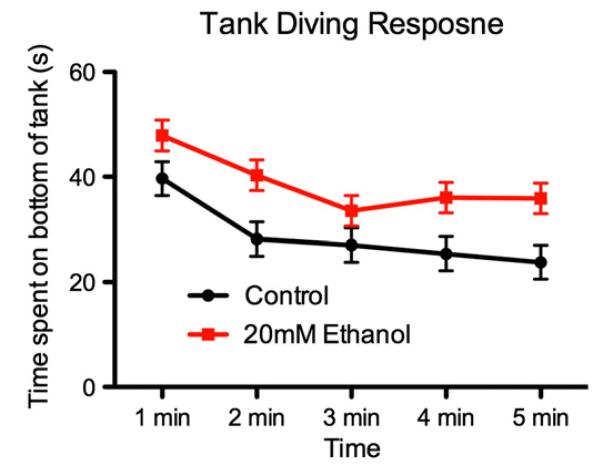
Fig. 4 displays the time spent in the bottom third during the 5 minute exposure to the novel tank. As is clear, although both groups displayed the typical tank diving response, gradually exploring the upper regions of the tank over the course of the exposure) the PNE fish appeared to spend longer in the bottom third of the tank. This was fitted to a linear mixed effects model, which confirmed a significant main effect for time, F4,179 = 7.24, p < 0.001 and for group, F1,179 = 25.87, p < 0.001. There was no time × group interaction (F < 1).
Fig. 4. Mean (±SE) time (s) spent in the bottom of the novel tank during the tank diving test for ethanol-treated and handling controls.
3.2. Individual social behavior
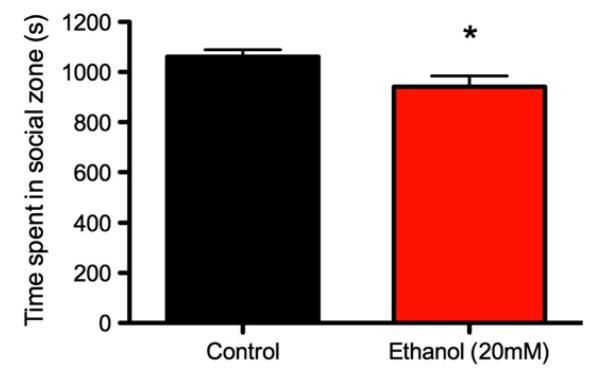
Fig. 5 displays the time spent in the social segment according to PNE treatment. The PNE 20 mM ethanol fish spent significantly less time in the social zone than the control animals, as confirmed with a between-subject t-test, t (61) = 2.35, p = 0.02.
Fig. 5. Mean (±SE) time spent in social sector over 20-minute period according to ethanol treatment.
* p = 0.02.
3.3. Shoaling behavior
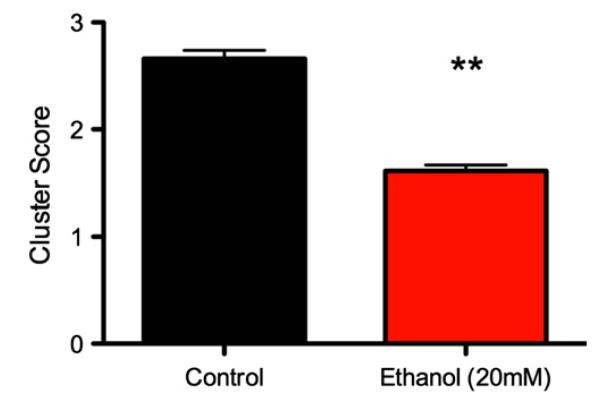
Fig. 6 displays the mean cluster scores for each shoal according to developmental ethanol exposure. The PNE shoals showed significantly less group cohesion during the course of the 10-minute observation period, and this difference was confirmed with a linear mixed effects model, F1,158 = 18.34, p < 0.001.
Fig. 6. Mean (±SE) cluster scores according to ethanol treatment.
A cluster score of 5 represents high clustering, and 0.2 represents low clustering (high dispersal). Scores were averaged for each shoal over a 10-minute observation period. ** p < 0.001.
3.4. Real-time quantitative PCR
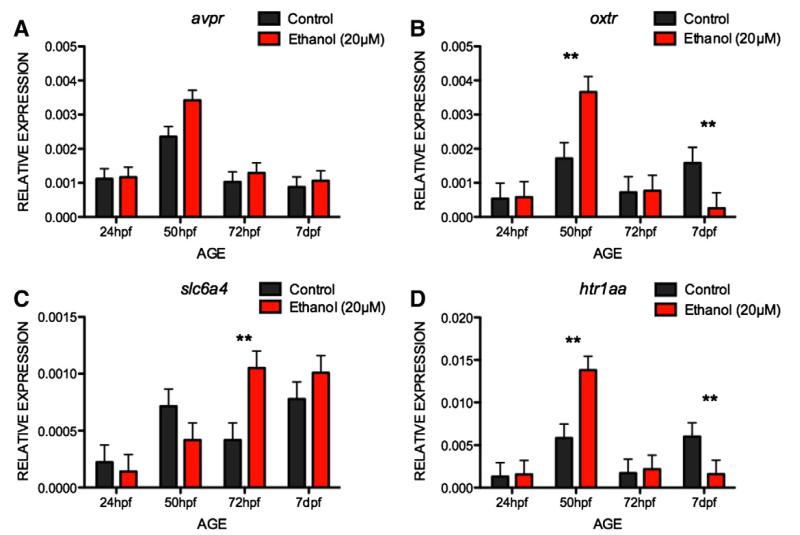
Fig. 7 displays the mRNA expression ratios for PNE and control embryos for genes relating to social behavior. Visual inspection of the data suggests that for all of the genes we tested, there was a spike in expression at 50hpf (i.e., 2 h after addition of the ethanol). In addition, for ht1aa and oxtr, it appears that the difference between ethanol and control embryos reverses by 7dpf. These differences were further characterized with two-way ANOVAs. For avpr (Fig. 7A), there was a significant effect of age, F3,24 = 18.49, p < 0.001, with mRNA expression higher at 50hpf than at any other age (ps < 0.001). There was no significant effect of treatment, although this approached significance, F1,24 = 3.47, p = 0.07, nor was there a significant age × treatment interaction, F3,24 = 1.2, p = 0.3. For oxtr (Fig. 7B), there was a significant effect of age, F3,24 = 9.37, p < 0.001, but not significant effect of treatment, F < 1. There was an age × treatment interaction, F3,24 = 4.38, p = 0.01. The interaction was characterized as ethanol treated embryos showing higher oxtr mRNA expression at 50hpf than controls, but this effect reversing by 7dpf. For slc6a4 (see Fig. 7C), there was a significant effect for age, F3,24 = 8.18, p = 0.001, but not for treatment, F1,24 = 1.28, p = 0.27. There was also a significant age × treatment interaction, F3,24 = 3.57, p = 0.03, characterized as a significant increase in slc6a4 mRNA expression at 72hpf in the ethanol treated group, but no differences at other stages. For ht1aa (Fig. 7D), there was a significant effect of age, F3,24 = 10.94, p < 0.001, but not for treatment, F < 1. There was also a significant age × treatment interaction, F3,24 = 4.82, p < 0.01, characterized as ethanol increasing ht1aa mRNA expression at 50hpf (p < 0.01), but this effect reversing at 7dpf (p < 0.01).
Fig. 7. Mean (±SE) mRNA expression ratios of social behavior-related genes to β-actin, ef1α and rpl13α of PNE and control zebrafish at different periods post-fertilization.
Note: ** p < 0.01.
4. Discussion
In both humans and in comparative animal models, exposure to moderate levels of alcohol during early brain development leads to social behavioral deficits. Here, we chronically exposed developing zebrafish embryos to a moderate level of ethanol (20 mM, equivalent to a BAC of ~0.04 g/dL), and observed both reduced social cohesion (shoaling) and reduced individual social behavior in this species. Analysis of developmental mRNA expression in genes relevant to social behavior revealed transient up-regulation of slc6a4 at 72hpf, and putative adaptive changes in oxtr and htr1aa expression following exposure to ethanol during early brain development, suggesting a developmental mechanism by which the observed effects on social behavior may manifest. This supports and extends previous work by Buske and Gerlai (2011) and Fernandes and Gerlai (2009), who showed that brief (1–2 hour) exposure to much higher concentrations of ethanol (0.25%, 0.5%, 1% v/v, equivalent to 0.08, 0.16 and 0.32 g/dL BAC) alters adult social behavior (operationalized by nearest neighbor and by proximity to a virtual fish) and reduced levels of 5HIAA (5-HT metabolite) in the adult brain. We also observed that PNE fish spent longer in the bottom third in the novel tank diving test, suggesting higher levels of trait anxiety in these fish (Stewart et al., 2012).
ht1aa mRNA expression initially increased following 2 hour exposure to ethanol, and subsequently decreased after 5 days suggesting adaptation of mRNA expression. ht1aa codes for the 5-HT1A receptor, and our findings support previous work demonstrating reduced binding of the 5-HT1A agonist 8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT) to 5-HT1A receptors in frontal cortical regions following PNE (Kim et al., 1997). This suggests that acute ethanol causes a spike in mRNA expression, and subsequent down-regulation at 7dfp. The role of the 5HT1A receptor in mammalian social behavior is well established. For example, the selective 5HT1A receptor agonist 8-OH-DPAT increased social interactions in gerbils (Cheeta et al., 2001), and the 5-HT1A antagonist pindobind decreased defensive and other social behavior in a resident-intruder test in mice (Bell and Hobson, 1993). Our data therefore support the hypothesis that alterations in social behavior observed here, and in many other PNE studies, may be the result of altered 5-HT activity.
We also found an increase in trait anxiety, as operationalized by time spent in the bottom third of a novel tank (Stewart et al., 2012), in the PNE fish. Previous work in mammals has identified differences in anxiety and stress reactivity following exposure to ethanol during early brain development (Osborn et al., 1998; Weinberg et al., 1996), and our findings confirm that similar mechanisms may be in place in fish. 5-HT1A knock-out mice show that increased anxiety (Heisler et al., 1998) and allelic variation at the 5-HT1A locus are related to pathological anxiety and depression in humans (Strobel et al., 2003). It could be hypothesized therefore that the increase in anxiety is the result of decreased htr1aa mRNA expression observed here in the 7pdf fish. An alternative interpretation is that the novel tank test is simply an extension of the reduced social interactions observed in the fish. We previously reported that individually housed zebrafish show markedly lower ‘anxiety’ responses on the novel tank test, while at the same time showed higher basal cortisol (CORT) than group-housed conspecifics (Parker et al., 2012). We suggested on the basis of this that the novel tank test might represent a social ‘searching’ assay, rather than an assay of anxiety per se, which would be consistent with the current data. PNE has a complex effect on stress reactivity and adaptation (Giberson and Weinberg, 1995; Weinberg, 1993; Weinberg et al., 1996). For example, PNE does not cause changes in baseline CORT or adrenocorticotrophic hormone (ACTH) in rats (Weinberg et al., 1995) but does alter hypothalamic pituitary–adrenal–cortical axis (HPA) adaptation to repeated stressors (Weinberg et al., 1996). In addition, Osborn et al. (1998) demonstrated sex differences in the physiological and behavioral responses to the elevated plus maze exposure following PNE. Hofmann et al. (2007) also showed that PNE female rats exhibit a decreased ACTH response to the 5-HT1A agonist, 8-OH-DPAT, but an increased ACTH response to the 5-HT2A agonist, DOI. Male rats showed no differences. This suggests that, in females at least, there is some disruption by PNE of the interaction between the serotonin system and HPA axis. This would imply a link between the social and stress-reactivity effects of PNE. An examination of the molecular structure of the HPA system (hypothalamic–pituitary–interrenal axis [HPI] in fish) and how this interacts with the 5-HT system during early brain development following developmental ethanol exposure may help to elucidate this. Although beyond the scope of this paper, our findings here demonstrate that zebrafish offer an excellent model system for examining this mechanism in vivo.
We also observed transient up-regulation of slc6a4 at 72hpf, with expression normalizing by 7dpf. slc6a4 codes for the serotonin transporter molecule (5-HTT), which is located pre-synaptically, and is responsible for 5-HT reuptake (Blakely et al., 1991). Interestingly, we observed that a change in slc6a4 was preceded by changes in ht1aa and oxtr, which codes for the oxytocin (OT) receptor. 5-HTT is known to be an important mediator of social behavior and social cognition (Canli and Lesch, 2007). For example, Wendland et al. (2006) demonstrated that allelic variation in the 5-HTT-linked polymorphic region (5-HTTLDR) explained variance in aggression and social cohesion in macaques. Similar variations have subsequently been cited as a potential mediator of impulse control (Paaver et al., 2007; Retz et al., 2004) and a variety of other psychopathologies in humans (Risch et al., 2009). PNE in rats causes permanent alterations in 5-HTT sites in the hypothalamus (Zafar et al., 2000) and further adds to the support for the involvement of the 5-HT system in social behavioral deficits observed in FASD.
In addition to the 5-HT system, the hypothalamic neuropeptides OT and AVP modulate and mediate aspects of social behavior (Heinrichs et al., 2009; Winslow et al., 1993). Here we observed a spike in oxtr mRNA expression as an acute response to addition of ethanol to the larvae at 50hpf. We then observed what appeared to be adaptation, with expression significantly down-regulated at 7dpf in a similar manner to ht1aa expression patterns. We did not observe a significant change in avpr expression at 50hpf. In mammals, as both 5-HT and OT regulate social behavior, the mechanisms by which the two interact have been of interest, and the co-localization of 5-HTT and OT expressing neurons in the hypothalamus is thought to constitute a mechanism by which 5-HTT regulates OT release (Emiliano et al., 2007). Acute ethanol directly inhibits OT release (Eisenhofer and Johnson, 1982; Fuchs and Wagner, 1963; Kalant, 1975). Our findings that there is a sharp spike in oxtr mRNA at 50hpf (2 h after ethanol exposure) are consistent with this, and suggest that ethanol initially increases, then ultimately decreases oxtr expression. Thus, as OT facilitates 5-HT release in the raphe nucleus in mammals (Yoshida et al., 2009), this suggests that acute ethanol will reduce 5-HT release in 5-HT neurons expressing OT receptors. This could result in initial homeostatic up-regulation of ht1aa expression as seen here. As the system re-balances, by 72hpf we saw an increase in slca6, possibly as a result of an increase in cells or as adaptation to remove any excess 5-HT. Collectively, this suggests a possible mechanism by which moderate ethanol exerts its effects on molecular factors associated with social behavior.
In conclusion, we have shown that zebrafish may represent a good model for translational work on the effects of ethanol exposure during brain development on behavior and gene expression. We found that moderate levels of ethanol exposure affect social behavior, and that this appears to be mediated by changes in 5-HT and OT mRNA expression levels. It also appeared that this reduction specifically in ht1aa and oxtr mRNA expression at 7dpf was an adaptation following an initial spike in expression on acute ethanol exposure. Finally, we found evidence for some role of ethanol during early brain development on anxiety. More research will be needed to elucidate the mechanisms by which these changes occurred, but this does suggest that zebrafish may be a suitable model for examining the molecular aspects of the stress–social interactions relating to PNE. Recognition and characterization of these processes will aid in the development of therapeutic interventions to help ameliorate negative symptoms of FASD and related psychiatric disorders.
Acknowledgments
MP was funded by project grant G1000053 from the National Centre for the Replacement, Reduction and Refinement of Animals in Research (NC3Rs; UK). CHB is a Royal Society (UK) Industrial Research Fellow. The funders played no part in study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Abbreviations
- 5-HT
serotonin
- 5-HTTLDR
5-HTT-linked polymorphic region
- 5HIAA
2-(5-hydroxy-1H-indol-3-yl)acetic acid
- 8-OH-DPAT
8-hydroxy-2-dipropylaminotetralin
- ACTH
adrenocorticotrophic hormone
- AID
agranular insular cortex
- AVP
vasopressin
- BAC
blood alcohol concentration
- CORT
cortisol
- FAS
Fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorder
- HPA
hypothalamic pituitary adrenocortical axis
- HPI
hypothalamic pituitary interrenal axis
- OT
oxytocin
- PNE
prenatal ethanol
- qPCR
quantitative real-time polymerase chain reaction
References
- Bell R, Hobson H. Effects of pindobind 5-hydroxytryptamine1A (5-HT1A), a novel and potent 5-HT1A antagonist, on social and agonistic behaviour in male albino mice. Pharmacol Biochem Behav. 1993;46:67–72. doi: 10.1016/0091-3057(93)90318-n. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Caron MG, Peek MM, Prince HK, et al. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol Teratol. 2011;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–9. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Tucci S, Sandhu J, Williams A, Rupniak N, File S. Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils. Brain Res. 2001;915:170–5. doi: 10.1016/s0006-8993(01)02846-3. [DOI] [PubMed] [Google Scholar]
- Collins LM, Asher L, Pfeiffer DU, Browne WJ, Nicol CJ. Clustering and synchrony in laying hens: the effect of environmental resources on social dynamics. Appl Anim Behav Sci. 2011;129:43–53. [Google Scholar]
- Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacol Biochem Behav. 2003;74:471–80. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Johnson RH. Effect of ethanol ingestion on plasma vasopressin and water balance in humans. Am J Physiol. 1982;242:R522–7. doi: 10.1152/ajpregu.1982.242.5.R522. [DOI] [PubMed] [Google Scholar]
- Emiliano AB, Cruz T, Pannoni V, Fudge JL. The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: a substrate for SSRIs therapeutic effects? Neuropsychopharmacology. 2007;32:977–88. doi: 10.1038/sj.npp.1301206. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–9. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AR, Wagner G. Effect of alcohol on release of oxytocin. Nature. 1963;198:92–4. doi: 10.1038/198092b0. [DOI] [PubMed] [Google Scholar]
- Gemenetzidis E, Elena-Costea D, Parkinson EK, Waseem A, Wan H, Teh M-T. Induction of human epithelial stem/progenitor expansion by FOXM1. Cancer Res. 2010;70:9515–26. doi: 10.1158/0008-5472.CAN-10-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giberson PK, Weinberg J. Effects of prenatal ethanol exposure and stress in adulthood on lymphocyte populations in rats. Alcohol Clin Exp Res. 1995;19:1286–94. doi: 10.1111/j.1530-0277.1995.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, et al. Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999;24:555–66. doi: 10.1016/s0896-6273(00)81112-5. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JW, Jones KL, Smith DW. Fetal alcohol syndrome. JAMA. 1976;235:1458. [PubMed] [Google Scholar]
- Heinrichs M, Von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu H-M, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci. 1998;95:15049–54. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann CE, Ellis L, Yu WK, Weinberg J. Hypothalamic–pituitary–adrenal responses to 5-HT1A and 5-HT2A/C agonists are differentially altered in female and male rats prenatally exposed to ethanol. Alcohol Clin Exp Res. 2007;31:345–55. doi: 10.1111/j.1530-0277.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- Kalant H. Direct effects of ethanol on the nervous system. Fed Proc. 1930–1941;34:1975. [PubMed] [Google Scholar]
- Keil V, Paley B, Frankel F, O’connor MJ. Impact of a social skills intervention on the hostile attributions of children with prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:231–41. doi: 10.1111/j.1530-0277.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gillespie RA, Druse MJ. Effects of maternal ethanol consumption and buspirone treatment on 5-HT1A and 5-HT2A receptors in offspring. Alcohol Clin Exp Res. 1997;21:1169–78. [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Mathur P, Berberoglu MA, Guo S. Preference for ethanol in zebrafish following a single exposure. Behav Brain Res. 2011;217:128–33. doi: 10.1016/j.bbr.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mcgee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. Am J Drug Alcohol Abuse. 2008;34:423–31. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kunsman GW, Levine BS, Herman MM, Cervenak J, Hyde TM. A comparison of ethanol concentrations in the occipital lobe and cerebellum. Forensic Sci Int. 1997;86:127–34. doi: 10.1016/s0379-0738(97)02129-4. [DOI] [PubMed] [Google Scholar]
- O’connor MJ, Kogan N, Findlay R. Prenatal alcohol exposure and attachment behavior in children. Alcohol Clin Exp Res. 2002;26:1592–602. doi: 10.1097/01.ALC.0000034665.79909.F0. [DOI] [PubMed] [Google Scholar]
- Oliveira RF. Mind the fish: zebrafish as a model in cognitive social neuroscience. Front Neural Circuits. 2013;7:131. doi: 10.3389/fncir.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998;22:685–96. [PubMed] [Google Scholar]
- Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology. 2007;194:545–54. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- Paintner A, Williams AD, Burd L. Fetal alcohol spectrum disorders—implications for child neurology, part 1: prenatal exposure and dosimetry. J Child Neurol. 2012;27:258–63. doi: 10.1177/0883073811428376. [DOI] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Millington ME, Brennan CH. Behavioral phenotyping of casper mutant and 1-pheny-2-thiourea treated adult zebrafish. Zebrafish. 2013;10:466–71. doi: 10.1089/zeb.2013.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Millington ME, Combe FJ, Brennan CH. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoS ONE. 2012;7:e34992. doi: 10.1371/journal.pone.0034992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, et al. Zebrafish protocols for neurobehavioral research. Springer; 2012. Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference, and mirror biting tests; pp. 231–46. [Google Scholar]
- Rasmussen C, Becker M, Mclennan J, Urichuk L, Andrew G. An evaluation of social skills in children with and without prenatal alcohol exposure. Child Care Health Dev. 2011;37:711–8. doi: 10.1111/j.1365-2214.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Reimers MJ, Flockton AR, Tanguay RL. Ethanol-and acetaldehyde-mediated developmental toxicity in zebrafish. Neurotoxicol Teratol. 2004;26:769–81. doi: 10.1016/j.ntt.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Retz W, Retz-Junginger P, Supprian T, Thome J, Rösler M. Association of serotonin transporter promoter gene polymorphism with violence: relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behav Sci Law. 2004;22:415–25. doi: 10.1002/bsl.589. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. JAMA. 2009;301:2462. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–6. [PubMed] [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose–response effect. Pediatrics. 2001;108:E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology. 2012;62:135–43. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests. Neurotoxicol Teratol. 1989;11:493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mössner R, Zeng Y, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety-and depression-related personality traits. J Neural Transm. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Suess PE, Newlin DB, Porges SW. Motivation, sustained attention, and autonomic regulation in school-age boys exposed in utero to opiates and alcohol. Exp Clin Psychopharmacol. 1997;5:375–87. doi: 10.1037//1064-1297.5.4.375. [DOI] [PubMed] [Google Scholar]
- Swanson K, Beckwith L, Howard J. Intrusive caregiving and quality of attachment in prenatally drug-exposed toddlers and their primary caregivers. Attach Hum Dev. 2000;2:130–48. doi: 10.1080/14616730050085527. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, Mcnabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin. 2007;39:384–90. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh MT, Hutchison IL, Costea DE, Neppelberg E, Liavaag PG, Purdie K, et al. Exploiting FOXM1-orchestrated molecular network for early squamous cell carcinoma diagnosis and prognosis. Int J Cancer. 2013;132:2095–2106. doi: 10.1002/ijc.27886. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain?. Insights from animal models. Trends Neurosci. 2012;35:284–92. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. Neuroendocrine effects of prenatal alcohol exposure. Ann N Y Acad Sci. 1993;697:86–96. doi: 10.1111/j.1749-6632.1993.tb49925.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Kim CK, Yu W. Early handling can attenuate adverse effects of fetal ethanol exposure. Alcohol. 1995;12:317–27. doi: 10.1016/0741-8329(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic–pituitary– adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20:122–31. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, et al. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 2006;36:163–72. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, Insel TR. Oxytocin and complex social behavior: species comparisons. Psychopharmacol Bull. 1993;29:409–14. [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–71. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H, Shelat SG, Redei E, Tejani-Butt S. Fetal alcohol exposure alters serotonin transporter sites in rat brain. Brain Res. 2000;856:184–92. doi: 10.1016/s0006-8993(99)02350-1. [DOI] [PubMed] [Google Scholar]