Summary
The bacterial pathogen Listeria monocytogenes uses actin-based motility to spread from infected human cells to surrounding healthy cells. Cell-cell spread involves the formation of thin extensions of the host plasma membrane (‘protrusions’) containing motile bacteria. In cultured enterocytes, the Listeria protein InlC promotes protrusion formation by binding and antagonizing the human scaffolding protein Tuba. Tuba is a known activator of the GTPase Cdc42. In this work, we demonstrate an important role for Cdc42 in controlling Listeria spread. Infection of the enterocyte cell line Caco-2 BBE1 induced a decrease in the level of Cdc42-GTP, indicating that Listeria downregulates this GTPase. Genetic data involving RNA interference indicated that bacterial impairment of Cdc42 may involve inhibition of Tuba. Experiments with dominant negative and constitutively activated alleles of Cdc42 demonstrated that the ability to inactivate Cdc42 is required for efficient protrusion formation by Listeria. Taken together, these findings indicate a novel mechanism of bacterial spread involving pathogen-induced downregulation of host Cdc42.
Introduction
Listeria monocytogenes is a gram-positive, food-borne pathogen capable of causing gastroenteritis, meningitis, or abortion (Posfay-Barbe and Ward, 2009; Vazquez-Boland et al., 2001). Critical for disease is the ability of Listeria to replicate within mammalian cells and spread between cells using a motility process dependent on the host actin cytoskeleton (Gouin et al., 2005; Haglund and Welch, 2011; Ireton, 2013). The initiation of spreading is promoted by the bacterial surface protein ActA, which stimulates the formation of F-actin ‘comet tails’ that propel bacteria through the cytosol. Motile bacteria contact the inner surface of the host cell plasma membrane, deforming the membrane into a thin extension called a ‘protrusion’. Finally, Listeria-containing protrusions are internalized by neighboring cells, resulting in cell-cell spread.
ActA is thought to be required for spreading in all cell types. In cells that do not form tight barriers, such as macrophages or fibroblasts, actin-dependent motility may be the sole factor governing bacterial protrusion formation and spread (Monack and Theriot, 2001). However, many cell types infected by Listeria are connected by cell-cell junctions that provide a barrier function. Such cells include enterocytes lining the intestinal lumen, hepatocytes, and the brain endothelium (Vazquez-Boland et al., 2001). In the polarized enterocyte cell line Caco-2 BBE1, ActA-mediated motility is not the sole process controlling Listeria spread (Rajabian et al., 2009) In these cells, the secreted bacterial protein InlC acts after F-actin comet tail formation to promote the formation of Listeria protrusions (Rajabian et al., 2009).
InlC enhances bacterial protrusion production by interfering with the function of a human cytoplasmic protein called Tuba (Rajabian et al., 2009). Tuba is a large scaffolding protein with several functional domains, including a carboxyl terminal SH3 domain that binds the human actin regulatory protein N-WASP (Salazar et al., 2003). Genetic, biochemical, and structural data indicate that one of the ways that InlC promotes Listeria spreading is by interacting with the Tuba SH3 domain (Rajabian et al., 2009; Polle et al., 2013). Binding of InlC to this domain displaces host N-WASP, thereby alleviating an inhibition in protrusion formation normally imposed by Tuba/N-WASP complexes. Importantly, the requirement for InlC/Tuba interaction in Listeria spread is observed not only in cultured cells, but also in a mouse model of infection (Leung et al., 2013). How InlC-mediated antagonism of Tuba and N-WASP enhances spreading is not fully understood. Based on the effects of Listeria infection on the structure of apical junctions, it was hypothesized that InlC might relieve cortical tension, thereby reducing the force needed by motile bacteria to remodel the plasma membrane into protrusions (Rajabian et al., 2009).
Apart from N-WASP, Tuba has additional cellular effectors (Bryant et al., 2010; Kodani et al., 2009; Kovacs et al., 2006; Kovacs et al., 2011; Otani et al., 2006; Qin et al., 2010; Salazar et al., 2003). One such effector is the human GTPase Cdc42, which is activated by a Dbl Homology (DH) domain in Tuba (Otani et al., 2006, Salazar et al., 2003). Cdc42 is a member of the evolutionarily conserved Rho family of small GTPases. These GTPases are active when bound to GTP, and inactive when complexed with GDP (Jaffe and Hall, 2005). The nucleotide-bound state of Cdc42 is regulated by several cellular proteins that activate or inactivate the GTPase (Jaffe and Hall, 2005; Schmidt and Hall, 2005, Tcherkezian and Lamarche-Vane, 2007). Tuba is one of many guanine nucleotide exchange factors (GEFs) capable of activating Cdc42 by catalyzing exchange of GTP for GDP (Kodani et al., 2009; Otani et al., 2006; Qin et al., 2010; Salazar et al., 2003; Schmidt and Hall, 2002; Sinha and Yang, 2008). Interestingly, the Tuba DH domain is specific for Cdc42 and does not efficiently activate other Rho family GTPases such as Rac1 (Otani et al., 2006; Salazar at al., 2003). The diverse biochemical functions of Cdc42 include regulation of actin polymerization, microtubule dynamics, and gene expression (Citi et al. 20013; Jaffe and Hall, 2005). Through these biochemical activities, Cdc42 controls multiple biological processes in mammalian cells, such as membrane trafficking, motility, cell cycle, cell-cell adhesion, and polarity (Citi et al., 2011; Harris and Tepass, 2010; Jaffe and Hall, 2005). Importantly, Cdc42 does not participate in F-actin tail formation by Listeria (Suzuki et al., 2000). However, whether this GTPase might act after actin-dependent motility to control bacterial protrusions during spread has not been investigated.
In this work, we demonstrate that Listeria antagonizes host Cdc42, and that this antagonism is needed for efficient bacterial spread in Caco-2 BBE1 cells. Infection of human cells with Listeria resulted in a ~ 65% decrease in Cdc42-GTP levels. RNA interference (RNAi)-based experiments supported the idea that bacterial antagonism of Cdc42 may occur, at least partly, through effects on Tuba. Importantly, experiments with dominant negative or constitutively activated alleles of Cdc42 indicated that inhibition of Cdc42 is needed for efficient formation of Listeria protrusions. Based on confocal microscopy analysis, bacterial inhibition of Cdc42 was required for perturbations in apical junctions associated with Listeria spreading. Collectively, these results identify a novel mechanism of control of microbial spread involving pathogen-induced downregulation of host Cdc42.
Results
Tuba activates Cdc42 in Caco-2 BBE1 cells
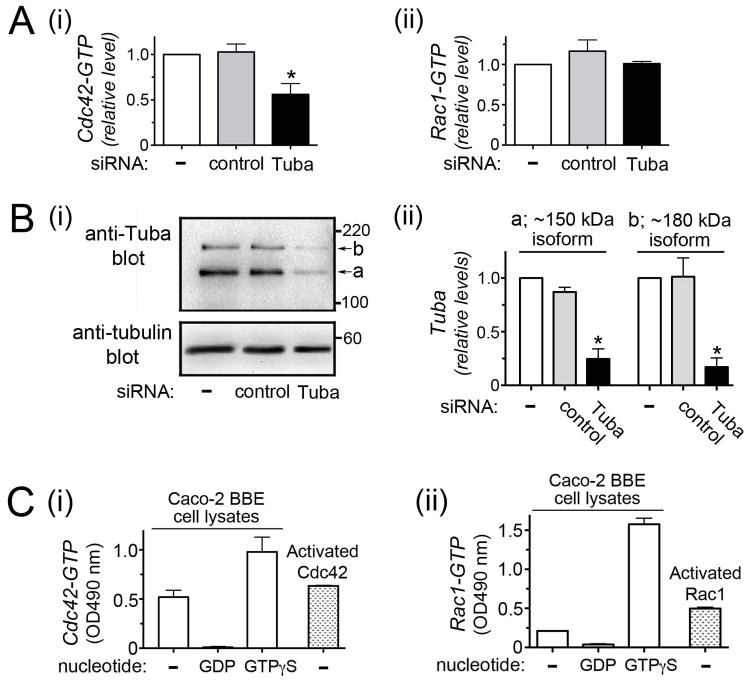
In vitro experiments demonstrate that Tuba has guanine nucleotide exchange factor (GEF) activity for the GTPase Cdc42 but not for the related GTPase Rac1 (Otani et al. 2003; Salazar et al., 2003). In order to determine if Tuba controls Cdc42 activity in Caco-2 BBE1 cells, we depleted Tuba through RNA interference and determined the resulting effect on Cdc42 or Rac1 activity using ELISA-based assays. siRNA-mediated knockdown of Tuba resulted in an approximately 35% reduction in Cdc42-GTP levels (Fig. 1). This effect on Cdc42 was observed with two different siRNAs targeting distinct regions in Tuba mRNA (Fig. 1Ai and data not shown). Activity of Rac1 was not affected by Tuba depletion (Fig. 1Aii). These results indicate an important role for Tuba in activation of Cdc42 in Caco-2 BBE1 cells.
Figure 1. Tuba controls Cdc42 activity in Caco-2 BBE1 cells.
Caco-2 BBE1 cells were either left untransfected or transfected with siRNAs targeting Tuba or a control non-targeting siRNA. Approximately 72 hours post-transfection, cells were solubilized in G-LISA lysis buffer for determination of GTPase activity or RipA buffer for confirmation of Tuba depletion. A. Effect of Tuba depletion on Cdc42 or Rac1 activity. Cdc42-GTP or Rac1-GTP levels in lysates were determined using G-LISA kits (Cytoskeleton). Relative Cdc42-GTP or Rac1-GTP values were obtained by normalizing absolute values to those from the no siRNA control. Mean relative Cdc42-GTP or Rac1-GTP values +/− SEM from three experiments are presented. Statistical analysis by ANOVA indicated P < 0.0001. *, P < 0.05 relative to control untransfected cells. B. Confirmation of siRNA-mediated Tuba depletion. i. A Western blot, representation of three experiments, is presented. As previously reported (Otani et al., 2006; Rajabian et al., 2009), two Tuba isoforms of ~ 150 kDa and ~180 kDa are expressed in Caco-2 cells. ii. Quantified Western blotting data (mean +/− SEM) from three experiments are shown. Statistical analysis by ANOVA indicated P = 0.0002 (150 kDa Tuba isoform) or P = 0.0027 (180 kDa Tuba isoform). *, P < 0.05 relative to control untransfected cells. C. Control experiments with the G-LISA kit indicating that the assays selectively detect activated Cdc42 or Rac1. Lysates from uninfected Caco-2 BBE1 cells were either left alone (−) or loaded with 1 mM GDP or 1 mM GTPγS to modulate GTPase activity. Purified constitutively activated Cdc42 or Rac1 proteins (Cytoskeleton) served as positive controls.
Genetic inhibition of Cdc42 restores normal spreading to Listeria lacking InlC
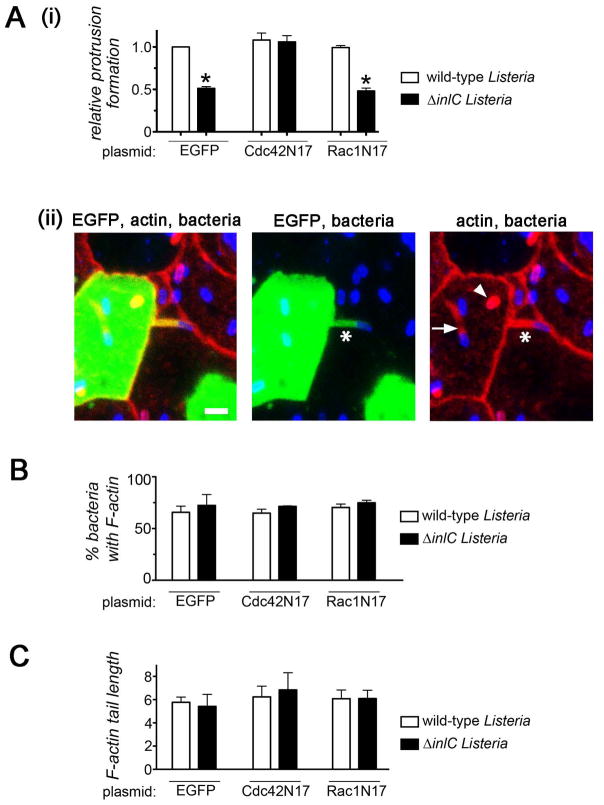
Given the known role of Tuba in Listeria protrusion formation (Rajabian et al., 2009) and the ability of Tuba to activate Cdc42 (Otani et al., 2006; Salazar et al., 2003; Fig. 1), we determined if Cdc42 controls bacterial protrusions. The dominant negative allele Cdc42N17 (Nobes and Hall, 1999) was used to inhibit Cdc42. We employed a previously described confocal microscopy-based approach (Rajabian et al., 2009) to assess bacterial protrusion formation in Caco-2 BBE1 cells transiently expressing EGFP-tagged Cdc42N17, dominant negative Rac1 (Rac1N17) (Ridley et al., 1992), or EGFP alone. Protrusions of both wild-type Listeria and an isogenic mutant strain deleted for the inlC gene (ΔinlC) were evaluated. As previously reported (Rajabian et al., 2009), the efficiency of protrusion formation of the ΔinlC mutant in control EGFP-expressing cells was about 50% of that of wild-type bacteria (Fig. 2Ai). These results indicate a role for InlC in the generation of protrusions. Importantly, in cells expressing EGFP-Cdc42N17, the frequency of protrusion formation by the ΔinlC mutant increased to a level similar to that of wild-type Listeria in EGFP-expressing cells. In contrast to the situation with Cdc42N17, expression of EGFP-Rac1N17 did not affect protrusions made by the ΔinlC mutant. Taken together, these findings indicate that inhibition of host Cdc42 restores normal protrusion generation to Listeria lacking InlC. The effect of Cdc42N17 on protrusions is virtually identical to that caused by RNAi-mediated depletion of Tuba (Rajabian et al., 2009), suggesting that Cdc42 and Tuba act on the same pathway to control Listeria spread.
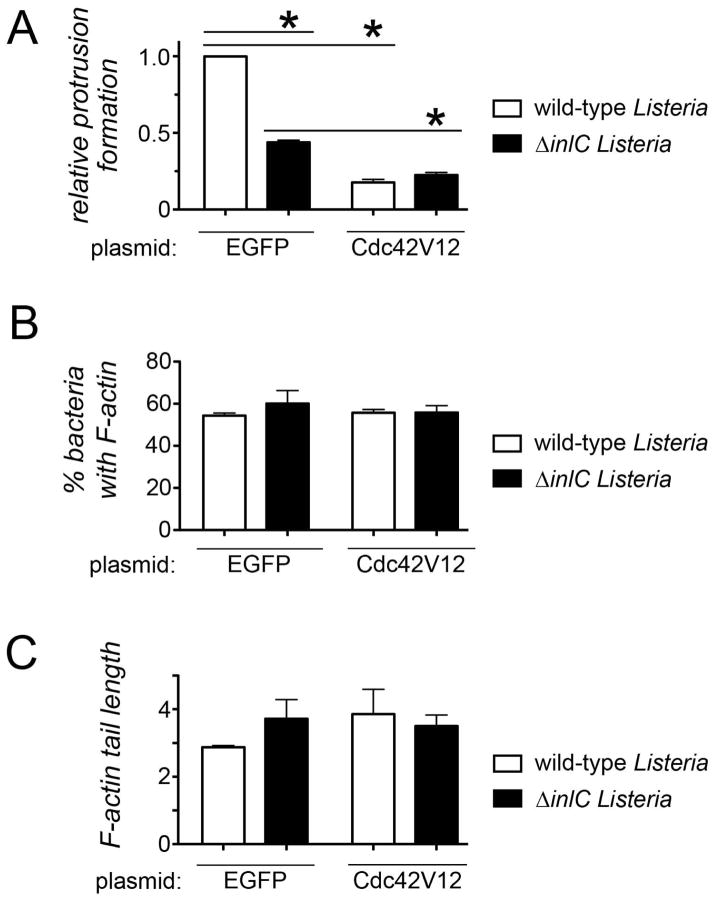
Figure 2. Inhibition of Cdc42 restores normal protrusion formation to Listeria lacking InlC.
A. Effect of Cdc42N17 on bacterial protrusion formation. Caco-2 BBE1 cells transiently expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 were infected with wild-type or ΔinlC strains of Listeria for 5.5 hours, and assessed for protrusion formation or F-actin assembly as described in the Experimental Procedures. (A). Protrusion formation (i). Bar graph displaying mean relative protrusion formation values +/− SEM. (ii). Confocal microscopy images illustrating how protrusions were quantified. Protrusions (*) were identified as EGFP- positive F-actin tails projecting from transfected cells into neighboring EGFP-negative cells. Bacteria with F-actin tails (arrow) and symmetric F-actin (arrowhead) within the EGFP-positive cell body were also scored. Protrusion formation efficiency is expressed as the percentage of total bacteria-associated F-actin structures that were in protrusions. Relative protrusion efficiencies were calculated by normalizing absolute percentage values to that of the WT strain in EGFP-expressing cells. The scale bar indicates 5 micrometers. (B). Bacteria recruitment of F-actin. The mean percentage of bacteria +/− SEM that assembled F-actin in comet tails or symmetric structures is shown. (C) F-actin tail assembly. The bar graph contains mean F-actin tail lengths +/− SEM.
Tuba appears to directly control the protrusion step of spreading, since depletion of Tuba does not alter Listeria F-actin tail formation (Rajabian et al., 2009). Similarly, genetic inhibition of Cdc42 did not affect the proportion of bacteria that recruited F-actin (Fig. 2B or the length of F-actin tails (Fig. 2C). These findings indicate that Cdc42 does not control actin-based motility of Listeria, and are consistent with previous reports (Suzuki et al., 2000).
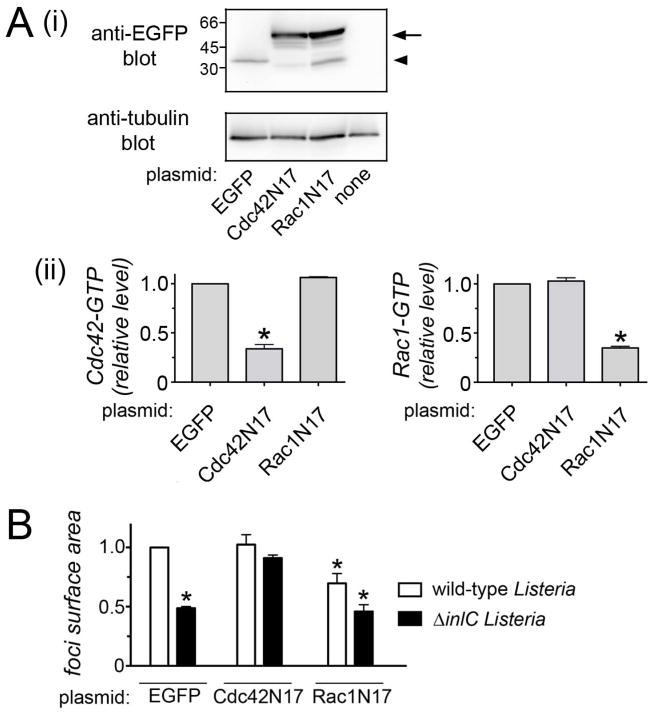
In addition to evaluating bacterial protrusions, we also determined the impact of Cdc42 on the final event of spreading. Cell-cell spread of Listeria was assessed in Caco-2 BBE1 cells stably expressing EGFP-tagged Cdc42N17, EGFP-Rac1N17, or EGFP alone (Figs. 3, S1). As expected, levels of Cdc42-GTP, but not Rac1-GTP, were reduced in the EGFP-Cdc42N17 cell line compared to the EGFP cell line (Fig. 3A). In the EGFP-Rac1N17 cell line, Rac1 activity was diminished and Cdc42 activity was unaffected. The efficiency of spreading of Listeria in these cell lines was measured by quantifying the size (surface area) of foci resulting from infection (Fig. S1). Similar to the situation observed with bacterial protrusions, Cdc42N17 restored normal spreading to the ΔinlC Listeria mutant (Fig. 3B). Interestingly, RacN17 caused an ~ 30% inhibition in spread of wild-type Listeria (Fig. 3B), despite the fact that this allele did not affect bacterial protrusions (Fig. 2Ai).
Figure 3. The Cdc42N17 allele restores normal cell-cell spread to ΔinlC mutant bacteria.
Caco-2 BBE1 cells stably expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 were constructed and used for assessment of cell-cell spread of wild-type and ΔinlC Listeria strains. (A) Western blotting data showing expression of EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 in the cell lines used for the spreading analysis. The arrow indicates EGFP-Cdc42N17 or EGFP-Rac1N17 proteins. The arrowhead indicates EGFP alone. The band the expected size of EGFP in the Rac1N17 cell line is likely a degradation product of the EGFP-Rac1N17 fusion. (B) Characterization of Cdc42 and Rac1 activity in the Caco-2 BBE1 cell lines. Cell lines stably expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 were grown in transwells for approximately 96 hours. Cell lysates were prepared and used for G-LISA assays to measure Cdc42 or Rac1-GTP levels. (C) Cell-cell spread of Listeria in cells stably expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17. The indicated cell lines were infected with wild-type or ΔinlC strains of Listeria for 12 hours followed by processing for immunofluorescence and analysis by confocal microscopy. (A). Quantification of cell-cell spread. Mean relative surface areas +/− SEM of foci produced by wild-type (wt) or ΔinlC bacterial strains. Data are from three experiments. *, P < 0.05 relative to EGFP-expressing cells infected with wild-type bacteria.
The collective results in Figures 2 and 3 indicate that host Cdc42 limits spreading of Listeria lacking InlC. By producing InlC, wild-type Listeria relieves Cdc42-mediated inhibition. The effect of Rac1N17 on bacterial spread could possibly reflect a minor role for Rac1 in events after protrusion formation, such as engulfment of protrusions by neighboring cells.
Listeria downregulates host Cdc42
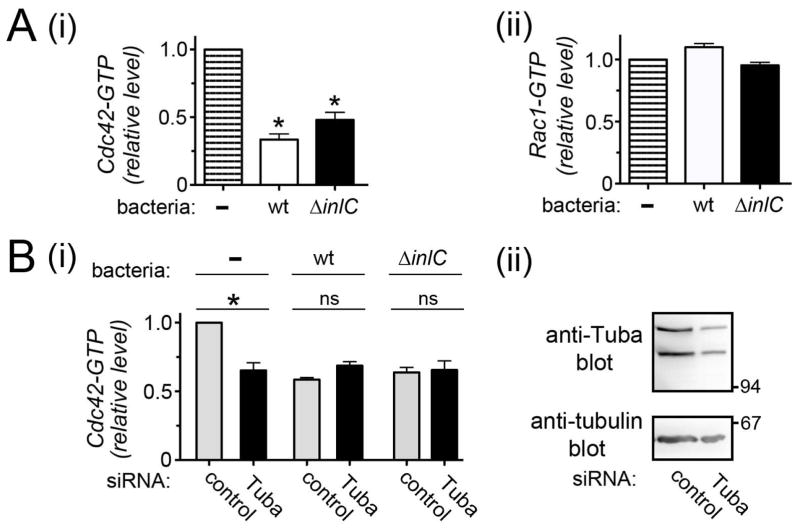
Given the role of Cdc42 in Listeria spread, we used an ELISA-based assay to examine if bacterial infection alters Cdc42 activity. Interestingly, infection of Caco-2 BBE1 cells with wild-type Listeria for 5.5 hours resulted in an approximately 65% decrease in Cdc42-GTP (Fig. 4Ai). Host Rac1-GTP levels were unaffected by bacterial infection (Fig. 4Aii). The ΔinlC mutant strain induced a decrease in Cdc42 activity that did not differ in a statistically significant fashion from the decrease caused by wild-type bacteria. These findings indicate that Listeria induces a downregulation in host Cdc42-GTP through a mechanism that does not require InlC.
Figure 4. Listeria impairs host Cdc42 activity.
A. Effect of bacterial infection on Cdc42 or Rac1 activity. Caco-2 BBE1 cells were either left uninfected (-) or infected with wild-type or ΔinlC bacterial strains for 5.5 hours. Cell lysates were used for measurement of Cdc42-GTP (i) or Rac1 (ii) levels using an ELISA-based method. Statistical analysis by ANOVA indicated P = 0.0001. *, P<0.05 relative to uninfected cells. B. Cdc42 activity in control or Tuba-depleted cells infected with wild-type or ΔinlC Listeria strains. Caco-2 BBE1 cells were treated with a non-targeting control siRNA or an siRNA directed against Tuba mRNA. Approximately 72 hours after transfection, cells were infected with the indicated Listeria strains for 5.5 hours and solubilized in G-LISA lysis buffer for determination of Cdc42-GTP levels (i). Statistical analysis by ANOVA indicated P = 0.0001. *, P<0.05 relative to uninfected cells treated with control siRNA (ii). In parallel with these infection experiments, a duplicate plate of transfected cells was solubilized in RipA buffer for confirmation of Tuba depletion. The GTPase activity data in A or B are mean relative Cdc42-GTP or Rac1-GTP amounts +/− SEM from three experiments. Relative Cdc42-GTP or Rac1-GTP values were obtained by normalizing absolute values to those from uninfected cells (A) or uninfected cells treated with control siRNA (B).
Interestingly, in Caco-2 BBE1 cells depleted for Tuba, Listeria infection failed to further reduce Cdc42 activity (Fig. 4B). The observation that Tuba knockdown and bacterial infection did not produce additive effects on Cdc42-GTP is consistent with the idea that Listeria impairs Cdc42 by acting through Tuba.
Constitutively activated Cdc42 impairs Listeria protrusion formation
In order to test if the ability of Listeria to downregulate host Cdc42 is important for spreading, we examined the effect of constitutively activated Cdc42 (Cdc42V12) on bacterial protrusions. Protrusions were assessed in Caco-2 BBE1 cells transiently expressing YFP-tagged Cdc42V12 (Nobes and Hall, 1995) or EGFP alone as a control. Importantly, Cdc42V12 inhibited protrusions made both by wild-type Listeria and also by the ΔinlC mutant (Figure 5A). Cdc42V12 did not affect the proportion of bacteria with F-actin or the length of F-actin tails (Fig. 5B, C), indicating that the allele likely directly inhibits protrusions. Two aspects of the relationship between inhibition by Cdc42V12 and Listeria genotype are worth mentioning. First, the fact that Cdc42V12 impairs protrusions by the ΔinlC bacterial strain indicates that Cdc42 affects InlC-independent spreading. Second, the observation that wild-type and ΔinlC Listeria have similar protrusion efficiencies in the presence of Cdc42V12 indicates that Cdc42 also affects InlC-dependent spread. The rationale behind this latter statement is that if Cdc42V12 inhibited only InlC-independent spread, then the Cdc42V12 and ΔinlC mutations would be expected to have additive effects on protrusions. However, additive effects were not observed, since in Cdc42V12-expressing host cells wild-type and ΔinlC Listeria strains spread similarly. The findings with Cdc42V12 in Figure 5 suggest that Cdc42 affects both InlC-mediated and InlC-independent dissemination. Taken together, the results in Figures 4 and 5 demonstrate that Listeria must inhibit host Cdc42 in order to spread efficiently.
Figure 5. Constitutively activated Cdc42 inhibits Listeria protrusion formation.
A. Effect of Cdc42V12 on protrusions. Caco-2 BBE1 cells transiently expressing EGFP or YFP-tagged Cdc42V12 were infected with the indicated Listeria strains for 5.5 hours, followed by fixation and immunolabeling. Confocal microscopy analysis was used to quantify efficiencies of protrusion formation (A), or bacterial F-actin recruitment (B), or F-actin tail length (C). Data are mean +/− SEM values from three experiments. Statistical analysis by ANOVA indicated P <0.0001. *, P < 0.05.
Cdc42 controls the structure of apical junctions
Our previous results indicate that cell-cell spread of Listeria involves perturbation of the structure of apical junctions in human cells (Rajabian et al., 2009; Polle et al., 2013). Specifically, infection of Caco-2 BBE1 cells with wild-type Listeria caused cell junctions to become curved, suggesting diminished cortical tension. The effect of Listeria on junctions appears to be due to inhibition of Tuba, since RNAi-mediated depletion of this host protein alters junctions similarly to bacterial infection (Otani et al., 2006; Rajabian et al., 2009). Given the involvement of Cdc42 in Listeria spread (Figs. 2A, 3A, and 5A) and the ability of bacteria to inhibit this GTPase (Fig. 4), we investigated if Cdc42 regulates host cell junctions.
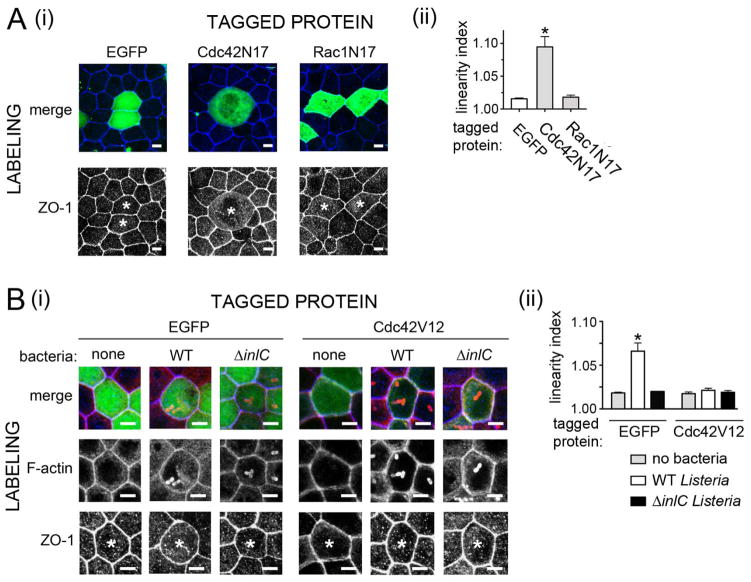
Importantly, expression of EGFP-tagged Cdc42N17 in Caco-2 BBE1 cells resulted in junctions with decreased linearity or increased curvature (Fig. 6A). Junction linearity was quantified through calculation of linear index values (Otani et al., 2006; Rajabian et al., 2009). A value of 1.0 indicates perfect linearity, whereas numbers greater than unity signify curvature. In contrast to the situation with Cdc42N17, EGFP-Rac1N17 did not detectably affect junction structure. The results with Cdc42 are consistent with previous data (Otani et al., 2006), and suggest a role for the GTPase in maintaining cortical tension. The similar effects of Cdc42N17 or Tuba depletion on junctions suggest that these two proteins may cooperate to control tension (Otani et al., 2006; Rajabian et al., 2009).
Figure 6. Inactivation of Cdc42 is needed for Listeria-induced alterations in apical junctions.
A. Effect of Cdc42N17 on apical junction structure. Caco-2 BBE1 cells transiently expressing EGFP or EGFP-tagged Cdc42N17, or Rac1N17 were fixed and labeled for the tight junction protein ZO-1. (i). Representative images of ZO1 labeling. In the top panels, both EGFP (green) and ZO-1 labeling (blue) are shown in merged images. In the bottom panels, only ZO-1 labeling is displayed. Asterisks indicate ZO1-labeling in cells expressing EGFP-tagged proteins. Scale bars are 5 micrometers. (ii). Quantification of linear index values. B. Effect of Cdc42V12 on apical junctions. Caco-2 BBE1 cells transiently expressing EGFP or YFP-tagged Cdc42V12 were either left uninfected or infected for 5.5 hours with the indicated Listeria strains. (i). Representative images of apical junctions as detected by ZO-1 labeling. In the top panels EGFP (green), F-actin (red), and ZO-1 labeling (blue) are shown in merged images. In the middle or bottom panels, only F-actin or ZO-1 labeling, respectively, is displayed. Asterisks indicate ZO-1 labeling in cells that express tagged proteins. In the case of samples subjected to bacterial infection, only host cells with intracellular bacteria decorated with F-actin are analyzed for ZO-1 distribution. Scale bars indicated 5 micrometers. (ii). Quantification of linear index values. Data in Aii and Bii are mean +/− SEM values from three experiments. Statistical analysis by ANOVA indicated P < 0.0001.*, P < 0.05 relative to EGFP-expressing cells.
In contrast to the situation observed with Cdc42N17, the constitutively activated allele Cdc42V12 suppressed junctional curvature normally caused by infection with wild-type Listeria (Fig. 6B). These findings indicate that Cdc42 inactivation is required for Listeria-induced alterations in apical junctions. Collectively, the results in Figure 6 indicate that Cdc42-GTP augments cortical tension. The correlation between effects of Cdc42 alleles on junction linearity and Listeria spread is consistent with previous data with Tuba (Rajabian et al., 2009), and supports the hypothesis that relief of cortical tension is important for generation of bacterial protrusions.
Discussion
In this work, we demonstrate an important role for the human GTPase Cdc42 in controlling cell-cell spread of Listeria. Activation of Cdc42 in the human enterocyte cell line Caco-2 BBE1 is partially dependent on Tuba, a protein known to regulate Listeria spreading (Rajabian et al., 2009). Experiments with a dominant negative Cdc42 allele indicate that the GTPase limits the formation of protrusions by a Listeria strain lacking InlC, but not by wild-type bacteria. These findings are similar to those observed with Tuba depletion (Rajabian et al., 2009), and indicate that Cdc42-GTP imposes a barrier to bacterial spread in the absence of InlC..
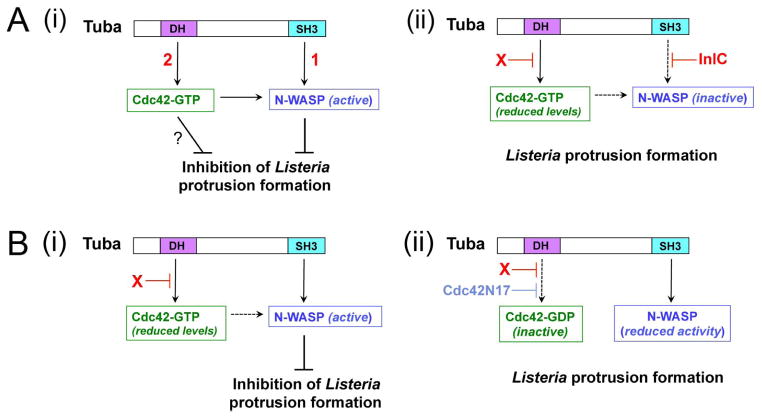
How does Listeria relieve Tuba- and Cdc42-mediated inhibition of spreading? There are two likely modes of action (Fig. 7A). One mechanism involves InlC antagonism of Tuba by binding to an SH3 domain in the host protein (Rajabian et al., 2009). This interaction displaces human N-WASP from Tuba. RNAi-based experiments demonstrate that both Tuba and N-WASP restrict bacterial protrusions in the absence of InlC. By preventing association of N-WASP with Tuba, InlC removes the inhibition in spread otherwise exerted by Tuba/N-WASP complexes. The second way that Listeria promotes protrusion formation, uncovered in the present study, involves downregulation of host Cdc42-GTP. Bacterial inhibition of Cdc42 occurred through a mechanism that did not require InlC. Experiments with a constitutively activated Cdc42 allele indicated that the ability to suppress Cdc42-GTP is critical for Listeria spread.
Figure 7. Mechanisms by which host Tuba and Cdc42 control Listeria protrusion formation.
(A). Model for how Listeria factors relieve Tuba- and Cdc42-mediated inhibition of Listeria spreading. A key feature of this model is that both Tuba and Cdc42 control N-WASP activity in an additive fashion. (i). In the absence of intervention by InlC or other bacterial proteins, Listeria protrusion formation is restrained by Tuba-mediated activation of N-WASP [1] and also by Cdc42-GTP [2]. Activated Cdc42 impairs protrusions by contributing to activation of N-WASP and also possibly through an unknown N-WASP-independent mechanism (?). (ii). InlC prevents binding of N-WASP to Tuba (Rajabian et al., 2009; Polle et al., 2013). An unknown bacterial factor ‘X’ impairs activation of Cdc42. Experiments involving depletion of Tuba in infected cells suggest that ‘X’ might attenuate Cdc42 activity by acting through Tuba. (B). Model explaining the spreading defect of ΔinlC mutant Listeria and the suppression of this defect by Cdc42N17. (i). In Caco-2 BBE1 cells infected with the ΔinlC mutant, N-WASP binds the Tuba SH3 domain. In the presence of reduced levels of Cdc42-GTP, interaction of Tuba with N-WASP produces sufficient N-WASP activity to impair bacterial protrusion formation. (ii). The Cdc42N17 mutant allele decreases Cdc42-GTP to such low levels that binding of the Tuba SH3 domain fails to activate N-WASP sufficiently to inhibit protrusions by the ΔinlC mutant.
In addition to affecting bacterial protrusion formation, dominant negative and constitutively activated alleles of Cdc42 altered the structure of apical junctions in human cells. Specifically, the Cdc42N17 allele elicited junctional curvature in uninfected cells, whereas Cdc42V12 abrogated curvature normally caused by infection with wild-type Listeria. These findings indicate that bacterial inhibition of Cdc42 is critical for perturbations in cell junctions that accompany cell-cell spread. The morphological changes in junctions are thought to reflect diminished cortical tension, which in turn may facilitate Listeria protrusion formation (Rajabian et al., 2009).
Although the work in this study demonstrates an important role for downregulation of Cdc42 in Listeria spread, a surprising aspect of the results merits explanation. The ΔinlC bacterial strain is nearly as effective as wild-type Listeria in downregulating host Cdc42 activity (Fig. 4A), and yet this mutant strain is still partly defective in cell-cell spread (Figs. 2A, 3A, 5A). A possible explanation for this apparent discrepancy is found in previous results indicating that the spreading defect of the ΔinlC mutant is at least partly due to the inability of this bacterial strain to interfere with Tuba/N-WASP complexes (Rajabian et al., 2009). N-WASP is activated by Cdc42 and also by interaction with SH3 domains in several cellular proteins (Carlier et al., 2000; Suetsugu and Gautreau, 2012). The effects of Cdc42-GTP and SH3 domains on N-WASP activity are additive (Carlier et al., 2000; Suetsugu and Gautreau, 2012). Binding of the Tuba SH3 domain may provide sufficient activation of N-WASP to inhibit spread of the ΔinlC mutant in the presence of low Cdc42 activity (Fig. 7Bi). Why then does the Cdc42N17 allele restore normal spreading to the ΔinlC mutant? A likely explanation is that Cdc42N17 attenuates endogenous Cdc42 activity to a greater extent than does infection with the ΔinlC mutant (Fig. 7Bii). Indeed, we have found that Cdc42-GTP levels in Caco-2 BBE1 cells expressing EGFP-Cdc42N17 are about 50% lower than that in control cells expressing EGFP only and infected with ΔinlC bacteria (Fig. S2). These findings suggest that in Cdc42N17-expressing cells, the contribution of Cdc42 to N-WASP activity may be so low that binding of the Tuba SH3 domain is insufficient to activate N-WASP.
There are other potential explanations for the ability of the Cdc42N17 allele to restore spread of ΔinlC mutant bacteria. First, InlC might interfere with host signaling downstream of Cdc42-GTP, possibly by acting on Cdc42 effectors. Alternatively, it is possible that InlC inhibits the activation of a pool of Cdc42 restricted to a particular subcellular locale. Our results with an ELISA-based assay indicated that InlC is not required for bacterial inhibition of the total cellular pool of Cdc42. However, this assay is not capable of detecting local effects on a minority pool of the GTPase. Future work is needed to determine if InlC disrupts signaling downstream of Cdc42-GTP or impairs activation of a subcellular fraction of Cdc42.
An interesting question arising from this work is what is the mechanism of Listeria downregulation of Cdc42? Genetic data support the idea that bacterial inhibition of Cdc42 may occur through Tuba. This interpretation is based on the observation that RNAi-mediated depletion of Tuba failed to further reduce Cdc42-GTP levels in cells infected with Listeria (Fig. 4B). These findings raise the possibility that Listeria dampens Tuba GEF activity. The mechanism by which this effect might occur is not known.
Another interesting question is what biological functions of Tuba and Cdc42 are disrupted by Listeria in order to enhance cell-cell spread? Cdc42 regulates many events in mammalian cells, including cell motility, endocytic and exocytic trafficking of vesicles, the formation and maintenance of cell junctions, and cell polarity (Bryant et al., 2010; Harris and Tepass, 2010; Jaffe and Hall, 2005; Otani, et al. 2006). There is evidence to suggest that Cdc42 controls cell junctions and polarity, in part, through its membrane trafficking activity (Harris and Tepass, 2010). As expected for a Cdc42 activator, Tuba participates in many of the cellular functions of Cdc42 (Bryant et al., 2010; Otani et al., 2006; Kovacs et al., 2006, Kovacs et al., 2011; Qin et al., 2010, Yap, 2011). Our results suggest that Tuba- and Cdc42-mediated control of apical junctions is important for Listeria spread. Regulation of junction structure by Tuba and Cdc42 may be direct, or instead could be a secondary consequence of other activities, such as membrane trafficking. Future work should elucidate if Tuba and Cdc42 control cell junctions and bacterial spread by regulating endocytic and/or exocytic membrane flow.
Experimental Procedures
Bacterial strains, mammalian cell lines, and media
The wild-type Listeria monocytogenes strain EGD and the isogenic ΔinlC strain with an in-frame deletion in the inlC gene were previously described (Engelbrecht et al., 1996; Rajabian et al., 2009). These strains were grown in brain heart infusion (BHI) broth and prepared for infection of cultured human cells as described (Ireton et al., 1999). The human enterocyte cell line Caco-2 BBE1 (ATTC; CRL-2102) was cultured in DMEM with 4.5 g/l glucose, 2 mM glutamine, 10% FBS, and 0.01 mg/ml human transferrin. Cells were grown on transwell permeable supports (Costar; 0.4 μm pore size) for 4–7 days, depending on the experiment. The passage number of cells was always between 17 and 20.
Antibodies, siRNAs, and plasmids
Rabbit polyclonal antibodies against the last SH3 domain in Tuba were previously described (Rajabian et al., 2009). Commercially available primary antibodies used were rabbit anti- Listeria monocytogenes (Becton Dickenson, 223021), mouse anti-GFP (Santa Cruz Biotechology, sc-9996), mouse anti-tubulin (Sigma; T5168), and mouse anti-ZO1 (Life Technologies 33-9100). Secondary antibodies coupled to Alexa Fluor 488 or Alexa Fluor 647, and phalloidin conjugated to Alexa Fluor 555 were purchased from Life Technologies. siRNA used to deplete human Tuba was siTuba-1 (5′-AUAUGCAGAUGGUGAUUAAUU-3′). The EGFPC1 plasmid was from Clontech. Plasmids expressing EGFP-Cdc42N17 or EGFP-Rac1N17 were obtained from G. Bokoch (Scripps Research Institute). The YFP-Cdc42V12 plasmid (Addgene catalog number 11399) was from J. Swanson (University of Michigan).
Plasmid DNA or siRNA transfection
Approximately 1×105 Caco-2 BBE1 cells were grown in transwells for ~ 24 hours prior to transfection. Plasmid DNA transfections were performed using 2.5 micrograms of DNA, and 3.5 microliters of Lipofectamine 2000 (LF2000) per transwell. siRNA transfections were done with 100 nM siRNA and 5 μl RNAiMAX (Life Technologies) per transwell. Transfections were carried out in Opti-MEM I (Life Technologies; catalog no. 31985-070) supplemented with 10% FBS and 0.01 mg/ml human transferrin. About 72 hours after transfection, cells were solubilized for analysis of target protein expression or infected with Listeria strains.
Construction of cell lines stably expressing EGFP-tagged proteins
Caco-2 BBE1 cell lines stably expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17, were constructed by limiting dilution, essentially as described (Basar et al., 2005). Clones were selected using G418 at 0.50 or 1.0 mg/ml. Expression of EGFP-tagged constructs was confirmed by Western blotting with anti-GFP antibodies.
Western blotting analysis
Caco-2 BBE1 cells were washed once in PBS, solubilized in RipA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.05% SDS, 0.25% sodium deoxycholate, 2 mM EDTA, 1mM sodium orthovanadate, 1 mM PMSF, 10 micrograms per ml each aprotinin and leupeptin), and supernatants remaining after centrifugation at 12,000 rpm were used for determination of protein concentrations using a BCA kit (Pierce). Equal protein quantities from each sample were migrated on SDS/polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. Incubation of membranes with primary antibodies and secondary antibodies coupled to horseradish peroxidase, and detection with ECL or ECL Plus reagents were performed as described (Shen et al., 2000). After detection of signals corresponding to Tuba, membranes were stripped and re-probed with antibodies against tubulin to confirm equivalent sample loading. Quantification of relative Tuba expression in samples was performed using Image J (version 1.45r) software. Briefly, integrated pixel densities in bands corresponding to Tuba or tubulin were quantified and background was subtracted. In order to correct for small differences in sample loading, the ratio of Tuba:tubulin values were determined. Ratio values were then normalized to that of the no siRNA control in order to obtain relative Tuba expression levels.
Measurement of Cdc42 and Rac1 activity
Levels of activated Cdc42 or Rac1 in uninfected or infected Caco-2 BBE1 cell lysates were measured using G-LISA kits (Cytoskeleton; catalog nos. BK127 or BK128) according to the manufacturer’s protocol. In control experiments in Fig. 4C, lysates from uninfected Caco-2 BBE1 cells were either left alone or loaded with 1 mM GDP or 1 mM GTPγS to modulate GTPase activity. Purified constitutively activated Cdc42 or Rac1 proteins (Cytoskeleton) served as positive controls in these experiments. A Tecan Infinite M200 plate reader was used to measure absorbance at 490 nm. For each condition, absorbance measurements were performed in duplicate or triplicate.
Quantification of bacterial protrusion formation
Protrusion efficiency was measured using a previously described assay involving the detection of protrusions projecting from human cells expressing EGFP-tagged proteins into surrounding EGFP-negative cells (Rajabian et al., 2009). Caco-2 BBE1 cells were transfected with plasmids expressing EGFP, EGFP-Cdc42N17, EGFP-Rac1N17, or YFP-Cdc42V12. Approximately 72 hours after transfection, cells were infected with wild-type or ΔinlC strains of Listeria for 1.5 h in the absence of gentamicin and 4.0 h in the presence of gentamicin. Cells were then washed in PBS, fixed in 3% paraformaldehyde (PFA), permeabilized, labeled, and mounted essentially as described (Sun et al., 2005). Samples were labeled with anti-Listeria antibodies and phalloidin Alexa Fluor 555 to detect F-actin. Images of optical sections 1.0 μM in thickness were acquired using a Zeiss LSM 710 confocal microscope equipped with diode (405 nm), multiline argon (458/488/514 nm), helium-neon 1 (543 nm), and helium-neon 2 (633 nm) lasers. Protrusions were identified as F-actin comet tails emanating from EGFP-positive cells into neighboring EGFP-negative cells. Protrusions, F-actin comet tails in the cell body, and bacteria decorated with symmetric F-actin in the cell body of each EGFP-positive cell were quantified. Approximately 50 protrusions were scored for the wild-type Listeria strain in control EGFP-expressing cells in each experiment. Corresponding numbers of total bacterial-associated actin structures were quantified for all other conditions. Protrusion efficiency was expressed as the percentage of total bacteria-associated F-actin structures in protrusions. Relative protrusion efficiencies were calculated by normalizing absolute percentage values to that of the wild-type strain in EGFP-expressing cells.
Analysis of F-actin recruitment and tail length
Analyses of bacterial F-actin recruitment and tail length were performed on the same samples used for evaluation of protrusion efficiencies. F-actin recruitment efficiencies were expressed as the percentage of total bacteria decorated with F-actin. Bacteria having symmetric F-actin or asymmetric comet tails were scored. In each experiment, 100–200 bacteria with F-actin were scored for the control condition of EGFP-expressing cells infected with wild-type Listeria. An equivalent number of bacteria were analyzed for the other conditions. Comet tail lengths were measured using Image J software as previously described (Rajabian et al., 2009). The lengths of approximately 40 comet tails were measured for each condition in each experiment.
Measurement of cell-cell spread
Caco-2 BBE1 cell lines stably expressing EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 were infected with wild-type or ΔinlC strains of Listeria for 1.5 h in the absence of gentamicin followed by 10.5 h in the presence of gentamicin. Cells were then washed in PBS and fixed in 3% PFA. Samples were permeabilized and labeled with rabbit anti-L. monocytogenes antibodies, anti-rabbit antibodies coupled to Alexa Fluor 647, and phalloidin conjugated to Alexa Fluor 555 to detect F-actin.
Spreading efficiencies of wild-type or ΔinlC bacterial strains were assessed by measuring the surface areas of foci containing bacteria decorated with F-actin. Images were acquired using a Zeiss LSM 710 confocal microscope using the 40x objective. Areas with contiguous infected human cells were identified. Image J software was used to draw regions of interest (ROIs) around foci. These ROIs were made so as to trace the borders of the outermost infected human cells of the foci. Cell borders were detected by F-actin labeling. Surface areas of ROIs were then measured. Approximately 20 foci were measured for each condition in each experiment. Absolute surface areas were converted to relative surface areas by normalization to absolute values for EGFP-expressing cells infected with the wild-type Listeria strain.
Analysis of cell junction structure
Caco-2 BBE1 cells were transfected with EGFP, EGFP-Cdc42N17, EGFP-Rac1N17, or YFP-Cdc42V12 plasmids. Approximately 72 hours after transfection, cells were either processed immediately for immunofluorescence labeling (Fig. 6A) or infected with wild-type or ΔinlC Listeria strains, followed by fixation in 3% PFA and antibody labeling (Fig. 6B). Bacterial infections were performed for 1.5 h in the absence of gentamicin and 4.0 h in the presence of gentamicin. Fixed cells in Fig. 6A were labeled with mouse anti-ZO1 and anti-mouse-Alexa Fluor 555 antibodies. Samples in Fig. 6B were labeled with phalloidin coupled to Alexa Fluor 555 and, mouse anti-ZO1, and anti-mouse-Alexa Fluor 647 antibodies. Apical junctions were detected by ZO1 staining. Images of optical sections 1.0 μM in thickness were obtained using a Zeiss LSM 710 confocal microscope. Only cells expressing EGFP- or YFP-tagged proteins were analyzed for cell junction structure. In the case of infected samples, only EGFP- or YFP-positive cells with intracellular bacteria decorated with F-actin were analyzed. Cell junction morphology was evaluated by measuring the degree of junction linearity, as described (Otani et al., 2006; Rajabian et al., 2009). This analysis involves the calculation of ‘linear index’ values, where an index of 1.0 indicates perfect linearity and values above 1.0 indicate curvature. Linear index values of approximately 100 cell junctions were calculated for each condition in each experiment.
Statistical analysis
Statistical analysis was performed using Prism (version 5.0a, GraphPad software). Analysis of variance (ANOVA) and the Tukey-Kramer posttest were employed. A P value of 0.05 or lower was considered significant.
Supplementary Material
(A). Images of foci containing intracellular bacteria. (i) Low magnification view of foci with borders demarcated with white lines. F-actin is labeled in red. Image J (version 1.43r) software was used to measure foci surface area, as described in the Experimental Procedures. (ii). Higher magnification of a selected region in the focus from EGFP-expressing cells infected with wild-type (WT) bacteria. Note the presence of intracellular bacteria decorated with F-actin inside the foci border. (B). Images of EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 green channel fluorescence from the same fields of view as those shown in part A. Scale bars in parts A and B indicate 20 micrometers.
Caco-2 BBE1 cell lines stably expressing EGFP or EGFP fused to Cdc42N17 were either left uninfected or infected with wild-type or ΔinlC strains of Listeria for 5.5 hours. Cell lysates were prepared and used to measure Cdc42-GTP levels using a G-LISA assay. Cdc42 activity data are mean relative Cdc42-GTP amounts +/− SEM from three experiments. Relative Cdc42-GTP values were obtained by normalizing absolute values to those from uninfected cells. Statistical analysis by ANOVA indicated P < 0.0001. *, P<0.05.
Acknowledgments
We thank Drs. J. Brumell and W.D. Schubert for reviewing the manuscript and Susie Verma for advice on siRNA transfection. This work was supported by National Institutes of Health grant R01AI085072 awarded to K.I.
References
- Basar T, Shen Y, Ireton K. Redundant roles for Met docking site tyrosines and the Gab1 pleckstrin homology domain in InlB-mediated entry of Listeria monocytogenes. Infection and Immunity. 2005;73:2061–2074. doi: 10.1128/IAI.73.4.2061-2074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Nioche P, Broutin-L’Hermite, Boujemaa R, Le Clainche C, Egile C, et al. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J Biol Chem. 2000;275:21946–21952. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol Membr Biol. 2011;28:427–444. doi: 10.3109/09687688.2011.603101. [DOI] [PubMed] [Google Scholar]
- Engelbrecht F, Chun SK, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovic Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secred protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- Gouin E, Welch MD, Cossart P. Actin-based motility of intracellular pathogens. Curr Opin Microbiol. 2005;8:35–45. doi: 10.1016/j.mib.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Haglund CM, Welch MD. Pathogens and polymers: Microbe-host interactions illuminate the cytoskeleton. J Cell Biol. 2011;195:7–17. doi: 10.1083/jcb.201103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–1279. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guiponi M, et al. Endocytic protein intersectin-1 regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Ireton K. Molecular mechanisms of cell-cell spread of intracellular bacterial pathogens. Open Biol. 2013;3:1130079. doi: 10.1098/rsob.130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Payrastre B, Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositude-3-kinase. J Biol Chem. 1999;274:17025–17032. doi: 10.1074/jbc.274.24.17025. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kodani A, Kristensen I, Huang L, Sutterlin C. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell. 2009;20:1192–1200. doi: 10.1091/mbc.E08-08-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASP-dependent actin assembly. J Cell Sci. 2006;119:2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Verma S, Thomas SG, Yap AS. Tuba and N-WASP function cooperatively to position the central lumen during epithelial cyst morphogenesis. Cell Adh Migr. 2011;5:344–3350. doi: 10.4161/cam.5.4.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N, Gianfelice A, Gray-Owen SD, Ireton K. Impact of the Listeria monocytogenes protein InlC on infection in mice. Infec Immun. 2013;81:1334–1340. doi: 10.1128/IAI.01377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Theriot JA. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 2001:633–647. doi: 10.1046/j.1462-5822.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle L, Rigano LA, Julian R, Ireton K, Schubert WD. Structural details of human Tuba recruitment by InlC of Listeria monocytogenes elucidate bacterial cell-cell spreading. Structure. 2013 doi: 10.1016/j.str.2013.10.017. Pii S0969-2126(13)00429-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Posfay-Barbe KM, Wald ER. Listeriosis. Semin Fetal Neonatal Med. 2009;14:228–233. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabian T, Gavicherla B, Heisig M, Muller-Altrock S, Goebel W, Gray-Owen SD, Ireton K. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol. 2009;11:1212–1218. doi: 10.1038/ncb1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The Interaction between N-WASP and the Arp2/3 Complex Links Cdc42-Dependent Signals to Actin Assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, et al. Tuba, a novel protein containing Bin/Amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Shen Y, Naujokas M, Park M, Ireton K. InlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Gautreau A. Synergistic BAR-NPF interactions in actin-driven membrane remodeling. Trends Cell Biol. 2012;22:141–150. doi: 10.1016/j.tcb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Sun H, Shen Y, Dokainish H, Holgado-Madruga M, Wong A, Ireton K. Host adaptor proteins Gab1 and CrkII promote InlB-dependent entry of Listeria monocytogenes. Cell Microbiol. 2005;7:443–457. doi: 10.1111/j.1462-5822.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mimuro H, Miki H, Takenawa T, Sasaki T, Nakanishi H, et al. Rho family GTPase Cdc42 is essential for the actin-based motility of Shigella in mammalian cells. J Exp Med. 2000;191:1905–1920. doi: 10.1084/jem.191.11.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A). Images of foci containing intracellular bacteria. (i) Low magnification view of foci with borders demarcated with white lines. F-actin is labeled in red. Image J (version 1.43r) software was used to measure foci surface area, as described in the Experimental Procedures. (ii). Higher magnification of a selected region in the focus from EGFP-expressing cells infected with wild-type (WT) bacteria. Note the presence of intracellular bacteria decorated with F-actin inside the foci border. (B). Images of EGFP, EGFP-Cdc42N17, or EGFP-Rac1N17 green channel fluorescence from the same fields of view as those shown in part A. Scale bars in parts A and B indicate 20 micrometers.
Caco-2 BBE1 cell lines stably expressing EGFP or EGFP fused to Cdc42N17 were either left uninfected or infected with wild-type or ΔinlC strains of Listeria for 5.5 hours. Cell lysates were prepared and used to measure Cdc42-GTP levels using a G-LISA assay. Cdc42 activity data are mean relative Cdc42-GTP amounts +/− SEM from three experiments. Relative Cdc42-GTP values were obtained by normalizing absolute values to those from uninfected cells. Statistical analysis by ANOVA indicated P < 0.0001. *, P<0.05.