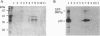Abstract
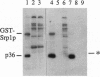
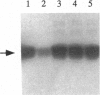
Srp1p, the protein encoded by SRP1 of Saccharomyces cerevisiae, is a nuclear-pore-associated protein. Its Xenopus homolog, importin, was recently shown to be an essential component required for nuclear localization signal (NLS)-dependent binding of karyophilic proteins to the nuclear envelope [Gorlich, D., Prehn, S., Laskey, R. A. & Hartman, E. (1994) Cell 79, 767-778]. We have discovered a protein kinase whose activity is stimulated by Srp1p (Srp1p fused to glutathione S-transferase and expressed in Escherichia coli) and is detected by phosphorylation of Srp1p and of a 36-kDa protein, a component of the protein kinase complex. The enzyme, called Srp1p kinase, is a protein-serine kinase and was found in extracts in two related complexes of approximately 180 kDa and 220 kDa. The second complex, when purified, contained four protein components including the 36-kDa protein. We observed that, upon purification of the kinase, phosphorylation of Srp1p became very weak, while activation of phosphorylation of the 36-kDa protein by Srp1p remained unaltered. Significantly, NLS peptides and the nuclear proteins we have tested greatly stimulated phosphorylation of Srp1p, suggesting that Srp1p, complexed with karyophilic proteins carrying an NLS, is the in vivo substrate of this protein kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Lobl T. J., Mitchell M. A., Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989 Jan 19;337(6204):276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K. D., Kenna M. A., Wei S., Davis L. I. Genetic and physical interactions between Srp1p and nuclear pore complex proteins Nup1p and Nup2p. J Cell Biol. 1994 Aug;126(3):619–630. doi: 10.1083/jcb.126.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Dasso M., Seki T., Azuma Y., Ohba T., Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 1994 Dec 1;13(23):5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M., Chavko M. Silver staining of native and denatured eucaryotic DNA in agarose gels. Anal Biochem. 1987 Aug 15;165(1):33–37. doi: 10.1016/0003-2697(87)90197-7. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Laskey R. A., Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994 Dec 2;79(5):767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Laskey R. A., Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994 Dec 2;79(5):767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N., Matsuoka Y., Kurihara T., Kohno K., Miyagi M., Sakiyama F., Okada Y., Tsunasawa S., Yoneda Y. Antibodies against 70-kD heat shock cognate protein inhibit mediated nuclear import of karyophilic proteins. J Cell Biol. 1992 Dec;119(5):1047–1061. doi: 10.1083/jcb.119.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys D. A., Vu L., Steffan J. S., Dodd J. A., Yamamoto R. T., Nogi Y., Nomura M. RRN6 and RRN7 encode subunits of a multiprotein complex essential for the initiation of rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae. Genes Dev. 1994 Oct 1;8(19):2349–2362. doi: 10.1101/gad.8.19.2349. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Dasso M., Newport J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J Cell Biol. 1994 May;125(4):705–719. doi: 10.1083/jcb.125.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M. A., Silver P. A. Nucleocytoplasmic transport in the yeast Saccharomyces cerevisiae. Annu Rev Biochem. 1993;62:219–254. doi: 10.1146/annurev.bi.62.070193.001251. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Saavedra C., Loeb J. D., Cole C. N., Silver P. A. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Thomas J. O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992 May;12(5):2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U., Osborne M., Kurihara T., Silver P. A yeast protein that binds nuclear localization signals: purification localization, and antibody inhibition of binding activity. J Cell Biol. 1991 Jun;113(6):1243–1254. doi: 10.1083/jcb.113.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U., Silver P. A. A conserved phosphoprotein that specifically binds nuclear localization sequences is involved in nuclear import. J Cell Biol. 1992 May;117(3):473–482. doi: 10.1083/jcb.117.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Oakes M. L., Tabb M. M., Nomura M. Yeast Srp1p has homology to armadillo/plakoglobin/beta-catenin and participates in apparently multiple nuclear functions including the maintenance of the nucleolar structure. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6880–6884. doi: 10.1073/pnas.91.15.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Oakes M., Yamaghishi M., Dodd J. A., Nomura M. Cloning and characterization of SRP1, a suppressor of temperature-sensitive RNA polymerase I mutations, in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Dec;12(12):5640–5651. doi: 10.1128/mcb.12.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]