Abstract
B-lymphocyte activation is a common characteristic of chronic hepatitis B virus (HBV) infection. B cell-activating factor (BAFF) plays a crucial role in the development and activation of B lymphocytes. This study investigated serum BAFF levels in 232 patients with different clinical diseases of chronic HBV infection [33 chronic asymptomatic HBV carrier (ASC), 53 chronic hepatitis (CH), 72 liver cirrhosis (LC), and 74 hepatocellular carcinoma (HCC)] and 61 gender- and age-matched healthy controls. Serum BAFF levels in HBV patients were significantly elevated compared with healthy controls (P<0.001). HCC patients had significantly higher levels of serum BAFF than ASC, CH, and LC (all P<0.001). Serum levels of BAFF in LC were significantly higher than in ASC (P<0.001) and CH (P=0.002). Serum level of BAFF was an independent variable associated with the presence of HCC in comparison with other disease groups in multivariate analysis. The area under receiver-operating characteristic curve (AUC) value of BAFF levels was 0.914 for HCC versus ASC, 0.825 for HCC versus CH, and 0.607 for HCC versus LC, respectively. The AUC value of BAFF levels was 0.854 for LC versus ASC and 0.748 for LC versus CH, respectively. The AUC value of BAFF (0.888) for HCC was higher than that of alpha-fetoprotein (0.776). We first demonstrate that serum BAFF levels in chronic HBV infection are elevated, correlated with clinical diseases, and could be used as a biomarker for indicating disease mechanisms, activity, and diagnosis.
Introduction
Hepatitis B virus (HBV) infection is a global public health problem, with 350–400 million people chronically infected worldwide, and estimated deaths due to HBV-related diseases of more than 0.5–1 million every year (European Association for the Study of the Liver 2012). Chronic HBV infection is associated with a variety of diseases, including asymptomatic carrier state, chronic hepatitis (CH), liver cirrhosis (LC), and hepatocellular carcinoma (HCC) (European Association for the Study of the Liver 2012).
The disease course of chronic HBV infection is a very heterogeneous dynamic process that is characterized by several sero-virological and hepatic biochemical and histological profiles reflecting a complex interaction between the virus and host immune response (Liaw and Chu 2009; European Association for the Study of the Liver 2012). The immune response, especially the T cell-mediated immune response, is considered a key factor in determining the outcome of HBV infection and it has been intensively studied, showing that dysfunctional or exhausted T-cell immune responses may affect the viral control and cause progressively more severe stages of HBV infection (Webster and others 2004; Bertoletti and Gehring 2006; Boni and others 2007; Lopes and others 2008; Maini and Schurich 2010; Schurich and others 2011). However, B cell-mediated immune response in HBV infection is relatively less addressed, whereas studies suggest that B cells also play an important role in the process of HBV infection. It is evidenced that HBV is present in B lymphocytes (Pontisso and others 1984; Pasquinelli and others 1986; Bouffard and others 1990) and this may affect the function of the cells. The HBV core antigen (HBcAg) is highly immunogenic and plays a pivotal role in inducing antiviral immune response against HBV. The HBcAg has the ability to bind a high frequency of naïve B cells and to activate B cells (Lazdina and others 2001), and this functional process takes place in a T-cell-independent fashion (Cao and others 2001). B cells, instead of dendritic cells (DC), were also demonstrated to be the primary antigen-presenting cells for HBcAg (Milich and others 1997). The priming of specific cytotoxic T lymphocytes (CTLs) by exogenous HBcAg particles was shown to be B-cell dependent (Lazdina and others 2003) and the B cell-CTL interactions during infection were regarded as a characteristic of HBV infection (Matter and others 2005). B-cell activation, possibly by T cells reacting with viral antigens on the surface of infected hepatocytes, is considered important in the initiation of the disease process of HBV infection (Williams 1975). The elevated B-cell activation in HBV patients may also contribute to the presence of circulating immune complexes and various autoantibodies often observed in patients with chronic hepatitis B (Budillon and others 1983; Kim and others 1990). A recent study revealed that interleukin-10-producing B cells were enriched in patients with chronic hepatitis B, and the frequency of these B cells correlated temporally with hepatic flares, suggesting that this subset of B cells may regulate T-cell immunity in chronic hepatitis B (Das and others 2012). Another recent study showed that B-cell activation is common in chronic hepatitis B with a significantly higher proportion of B cells from HBV-infected patients expressing activation markers (Oliviero and others 2011). Further, the levels of B-cell activation markers CD69 and CD86 and the expression of Fc receptor-like 1, an intrinsic activation molecule of B cells, were found to be increased in the acute and chronic hepatitis B patients (Wang and others 2012). These studies indicate that B cells per se and/or their interactions with T cells and HBV viral antigens play a critical role in the process of HBV infection.
B-cell activating factor (BAFF), also known as B cell-activating factor belonging to the tumor necrosis factor family or B lymphocyte stimulator (BLyS), plays a unique role in regulating peripheral B-cell survival and homeostasis and the antibody response (Moore and others 1999; Do and Chen-Kiang 2002; Rahman and Manser 2004). It enhances both T cell-independent and T cell-dependent humoral immune responses primarily by the attenuation of apoptosis (Do and others 2000). BAFF is also indicated to augment both T-cell and B-cell responses, particularly Th1-type responses (Sutherland and others 2005). Various types of cells, mostly immuno-inflammatory response-associated cells, including monocytes, macrophages, neutrophils, DC, T lymphocytes, and B lymphocytes, have been demonstrated to be able to produce BAFF (Moore and others 1999; Schneider and others 1999; Nardelli and others 2001; Scapini and others 2003).
Since B-cell activation is involved in chronic HBV infection, BAFF plays an important role in B-cell proliferation and function and interacts with T cells in response to viral infections, and, as a critical regulator for B cells, the change of BAFF in chronic HBV infection has not been yet explored systematically. We aimed at assessing the serum BAFF levels in different cross-sectional diseases of chronic HBV infection, in order to define the relationship between serum BAFF levels and the HBV infection process, and, in particular, the association with clinical diseases.
Patients and Methods
Patients and controls
Patients with chronic HBV infection from the First Affiliated Hospital, School of Medicine, Xi'an Jiaotong University, a tertiary hospital in northwest China, were included in the study. Patients who had a history of HBV infection for more than 6 months and had not been treated with nucleos(t)ide analogues or interferon (IFN)-α at study entry were eligible for inclusion. All patients had no evidence of infection with hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Coexistence of autoimmune, alcoholic, metabolic, or drug-induced liver disease was excluded.
A total of 232 patients [male/female, 172/60; age, 40.84±12.43 (17–72) years] with chronic HBV infection, which was determined by the evidence of HBV infection for more than 6 months, were enrolled. Patients were classified into the clinical diseases as chronic asymptomatic HBV carrier (ASC), CH, LC, and HCC. The clinical diagnosis of the patients was determined by the history of HBV infection, hepatitis B surface antigen (HBsAg)/anti-HBs, hepatitis B e antigen (HBeAg)/anti-HBe and anti-HBc serostatus, serum HBV DNA levels, biochemical liver function, alpha-fetoprotein (AFP) levels, ultrasonography, computerized tomography (CT), and/or magnetic resonance imaging (MRI). Briefly, ASC was determined based on the positivity of HBsAg, HBeAg, and anti-HBc, high level of HBV DNA, and persistently normal serum ALT level. CH was diagnosed on the base of the positivity of HBsAg and anti-HBc with HBeAg or anti-HBe, moderate to high level of HBV DNA, elevated [≥2× upper limit of normal] or significantly fluctuated ALT, and no evidence of LC and HCC. LC was diagnosed according to the positivity of HBsAg and anti-HBc with HBeAg or anti-HBe, detectable HBV DNA, abnormal liver function, the evidence of LC on liver biopsy and/or on ultrasonography and/or CT/MRI and gastroesophageal varices by endoscopy, and no evidence of HCC. HCC was defined based on the positivity of HBsAg and anti-HBc and HBeAg or anti-HBe, detectable HBV DNA, abnormal liver function, and the evidence of HCC on liver biopsy and/or ultrasonography and/or CT/MRI with or without elevated AFP. A total of 61 healthy controls [male/female, 42/19; age, 38.85±10.99 (21–74) years] were those who had normal liver biochemistries, without a history of hepatitis B and other liver diseases, and with seropositivity for anti-HBs due to hepatitis B vaccination or seronegativity for HBV markers without a history of hepatitis B vaccination. The study was approved by the Institutional Ethics Committee and conducted in accordance with the Declaration of Helsinki.
Determination of serum BAFF levels, biochemical liver function, AFP levels, and HBV markers
Serum levels of BAFF were analyzed by a commercially available Quantikine Human BAFF/BLyS enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions. The sensitivity, minimum detectable dose (MDD) of the assay ranged from 1.01–6.44 pg/mL and the mean MDD was 2.68 pg/mL. The intra-assay coefficient of variation ranged from 3.4% to 7.2% and the inter-assay coefficient of variation ranged from 9.9% to 11.6%. The people who determined the serum levels of BAFF were unaware of the case-control status and the patients' clinical characteristics when they read the BAFF ELISA. Biochemical liver function was assayed using the Olympus AU5400 automatic biochemical analyzer (Olympus Corporation, Mishama, Japan). Serum AFP levels (ng/mL) were determined by automated Eleceyes (Roche Diagnostics, Mannheim, Germany). Serum HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc were determined using ELISA provided by Beijing Wantai Biological Pharmacy (Beijing, China). Serum HBV DNA levels (IU/mL) were quantitatively determined using HBV fluorescence polymerase chain reaction diagnostic kit (Da An Gene Co., Ltd. of Sun Yat-Sen University, Guangzhou, China) according to the manufacturer's instructions.
Statistical analysis
Data were expressed as the mean±SD or median (range). Statistical analysis was performed by SPSS software version 16.0 (SPSS, Inc., Chicago, IL). Continuous and categorical variables were compared between groups using the Mann–Whitney test or Kruskall–Wallis rank-sum test. Correlation coefficients between serum BAFF levels and other parameters were calculated using Spearman's rank tests. Multivariate analysis was performed using logistic regression with the presence of HCC as the binary-dependent variable and gender, age, and the levels of serum laboratory parameters and BAFF as the predictor variables. Receiver-operating characteristic (ROC) curve analysis was carried out to evaluate the diagnostic performances of BAFF for LC or HCC from other disease conditions. The diagnostic performances of BAFF and AFP for diagnosing HCC were compared. A P value<0.05 was considered statistically significant.
Results
Characteristics of the study subjects
The male/female ratio and age of the healthy controls were compatible with those of the patients with chronic HBV infection (both P>0.05). The clinical diagnoses of the patients were as follows: ASC, 33; CH, 53; LC, 72; and HCC, 74. Demographics and laboratory parameters of the patients according to clinical diagnosis are summarized in Table 1.
Table 1.
Demographics and Laboratory Parameters in Hepatitis B Virus Patients with Different Clinical Diagnosis
| ASC (n=33) | CH (n=53) | LC (n=72) | HCC (n=74) | P value | |
|---|---|---|---|---|---|
| Gender (M/F) | 20/13 | 36/17 | 50/22 | 66/8 | 0.003 |
| Age [years, mean±SD (range)] | 24.88±6.84 (17–53) | 33.58±10.63 (18–57) | 44.71±9.55 (23–72) | 49.39±9.24 (33–67) | <0.001 |
| HBV DNA (IU/mL, log) | 7.39±1.09 | 5.97±1.75 | 5.29±1.68 | 4.68±1.48 | 0.001 |
| ALT (IU/L) | 27 (12–39) | 146.0 (10–1,248) | 50.0 (9–504) | 49.50 (7–3,629) | 0.045 |
| AST (IU/L) | 27 (13–45) | 84.0 (16.8–873) | 56.5 (19–474) | 70.50 (15–4,082) | <0.001 |
| Tbil (μM) | 13.4 (3.12–24.4) | 23.1 (2.0–577.6) | 21.6 (6.5–153.7) | 30.14 (1.5–727.2) | <0.001 |
| Albumin (g/L) | 41.0 (34.7–50) | 37.1 (20.9–48.7) | 31.3 (18.8–50.7) | 33.9 (21.1–51.3) | <0.001 |
ALT, alanine aminotransferase; ASC, chronic asymptomatic HBV carrier; AST, aspartate aminotransferase; CH, chronic hepatitis; LC, liver cirrhosis; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; Tbil, total bilirubin.
Associations of serum BAFF levels with clinical diseases
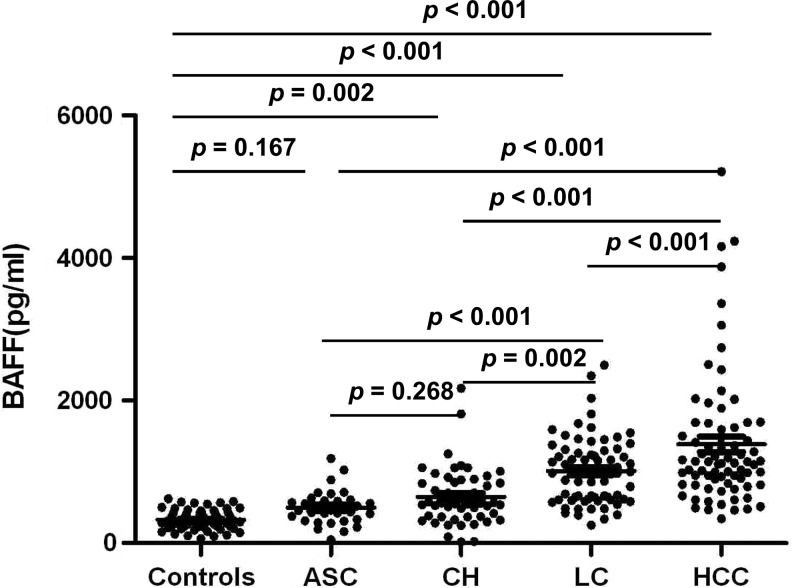
Serum BAFF levels in HBV patients [median 842.06 (18.24–5210.00) pg/mL] were significantly elevated compared with those in healthy controls [median 312.04 (61.62–624.79) pg/mL, P<0.001].
Although not significant between ASC [median 463.33 (48.44–1188.33) pg/mL] and healthy controls (P=0.167), serum levels of BAFF in CH [median 563.83 (18.24–2173.69) pg/mL], LC [median 1024.5 (250.26–2497.42) pg/mL], and HCC [median 1119.97 (340.67–5210.00) pg/mL] were significantly elevated compared with healthy controls (P=0.002, P<0.001, and P<0.001, respectively, Fig. 1). Serum levels of BAFF exhibited a stepwise increase from ASC and CH to LC and HCC (Fig. 1). HCC had significantly higher levels of BAFF compared with ASC, CH, and LC (all P<0.001, Fig. 1). Serum levels of BAFF in LC were significantly higher than in ASC (P<0.001) and CH (P=0.002) but lower than in HCC (P<0.001, Fig. 1). The levels of BAFF in CH were lower than in LC (P=0.002) and HCC (P<0.001) but were not significantly different from those in ASC (P=0.268, Fig. 1).
FIG. 1.
Serum B cell-activating factor (BAFF) levels in healthy controls and in patients according to clinical diseases of chronic hepatitis B virus (HBV) infection. ASC, chronic asymptomatic HBV carrier; CH, chronic hepatitis; LC, liver cirrhosis; HCC, hepatocellular carcinoma.
Correlations between serum BAFF levels and clinical indices in different clinical diseases
Correlations of serum BAFF levels with other clinical indices in patients with different clinical diseases were analyzed. In each group, serum BAFF levels were not correlated with the gender, age, and HBV DNA. However, in ASC, serum BAFF levels were positively correlated with ALT (r=0.526, P=0.002) and AST (r=0.348, P=0.047). In CH, there were positive correlations between the levels of BAFF and ALT (r=0.433, P=0.001), AST (r=0.545, P<0.001), and total bilirubin (r=0.383, P=0.005). In LC, serum levels of BAFF were not significantly correlated with any clinical indices included. In HCC, there was a positive correlation between the levels of BAFF and total bilirubin (r=0.329, P=0.004) and a negative correlation between the levels of BAFF and albumin (r=−0.422, P<0.001). Serum levels of BAFF in HCC were not significantly correlated with AFP (Table 2). There were positive correlations between the levels of BAFF and age (r=0.361, P<0.001) and total bilirubin (r=0.237, P<0.001) and a negative correlation between the levels of BAFF and albumin (r=−0.371, P<0.001) when all 232 subjects were analyzed regardless of clinical diseases (Table 2).
Table 2.
Correlation of Serum B Cell-Activating Factor Levels with Other Parameters According to Clinical Diseases of Chronic Hepatitis B Virus Infection
| Clinical disease (n) | Parameter | r | P value |
|---|---|---|---|
| ASC (33) | Gender (M/F) | −0.087 | 0.632 |
| Age | 0.208 | 0.245 | |
| HBV DNA | −0.229 | 0.199 | |
| ALT | 0.526 | 0.002 | |
| AST | 0.348 | 0.047 | |
| Tbil | 0.200 | 0.266 | |
| Albumin | 0.101 | 0.577 | |
| CH (53) | Gender (M/F) | −0.141 | 0.314 |
| Age | 0.220 | 0.113 | |
| HBV DNA | 0.212 | 0.127 | |
| ALT | 0.433 | 0.001 | |
| AST | 0.545 | <0.001 | |
| Tbil | 0.383 | 0.005 | |
| Albumin | −0.083 | 0.556 | |
| LC (72) | Gender (M/F) | 0.197 | 0.097 |
| Age | 0.008 | 0.946 | |
| HBV DNA | 0.098 | 0.413 | |
| ALT | 0.085 | 0.479 | |
| AST | 0.208 | 0.079 | |
| Tbil | 0.186 | 0.118 | |
| Albumin | −0.191 | 0.107 | |
| HCC (74) | Gender (M/F) | 0.171 | 0.144 |
| Age | 0.070 | 0.555 | |
| HBV DNA | −0.069 | 0.558 | |
| ALT | −0.011 | 0.924 | |
| AST | 0.023 | 0.845 | |
| Tbil | 0.329 | 0.004 | |
| Albumin | −0.422 | <0.001 | |
| AFP | 0.061 | 0.604 | |
| All (232) | Gender (M/F) | −0.038 | 0.569 |
| Age | 0.361 | <0.001 | |
| HBV DNA | −0.004 | 0.948 | |
| ALT | 0.037 | 0.576 | |
| AST | 0.117 | 0.076 | |
| Tbil | 0.237 | <0.001 | |
| Albumin | −0.371 | <0.001 |
AFP, alpha-fetoprotein; BAFF, B cell activating factor.
Multivariate analysis of parameters associated with HCC in comparison with other clinical diseases
Given that HCC patients had the highest levels of BAFF, gender, age, and the serum levels of HBV DNA, AST, ALT, total bilirubin, albumin and BAFF were used as predictor variables for discriminating HCC from ASC, CH, and LC by multivariate analysis. The results showed that the serum level of BAFF, in addition to age, was an independent variable associated with the presence of HCC in comparison with all other disease groups (Table 3).
Table 3.
Multivariate Analysis Using Logistic Regression for Discriminating HCC from ASC, CH, and LC in Chronic HBV Infection
| HCC (n=74) vs. ASC (n=33) | HCC (n=74) vs. CH (n=53) | HCC (n=74) vs. LC (n=72) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | SE | P value | OR | SE | P value | OR | SE | P value |
| Gender | 7.923 | 2.179 | 0.062 | 4.791 | 0.826 | 0.058 | 6.218 | 0.522 | <0.001 |
| Age (years) | 1.536 | 0.139 | 0.002 | 1.156 | 0.032 | <0.001 | 1.065 | 0.020 | 0.002 |
| HBV DNA (IU/mL, log) | 0.313 | 0.771 | 0.131 | 0.505 | 0.196 | 0.001 | 0.701 | 0.124 | 0.004 |
| ALT (IU/L) | 1.004 | 0.015 | 0.816 | 1.000 | 0.001 | 0.550 | 0.999 | 0.001 | 0.432 |
| AST (IU/L) | 1.015 | 0.022 | 0.503 | 0.953 | 0.001 | 0.953 | 1.001 | 0.001 | 0.349 |
| Tbil (μM) | 1.071 | 0.079 | 0.385 | 0.993 | 0.003 | 0.021 | 1.003 | 0.004 | 0.448 |
| Albumin (g/L) | 0.903 | 0.143 | 0.474 | 0.928 | 0.049 | 0.128 | 1.082 | 0.031 | 0.012 |
| BAFF (pg/mL) | 1.006 | 0.003 | 0.028 | 1.002 | 0.001 | 0.001 | 1.001 | 0.001 | 0.004 |
OR, odds ratio; SE, standard error.
Diagnostic performance of serum BAFF levels for LC or HCC
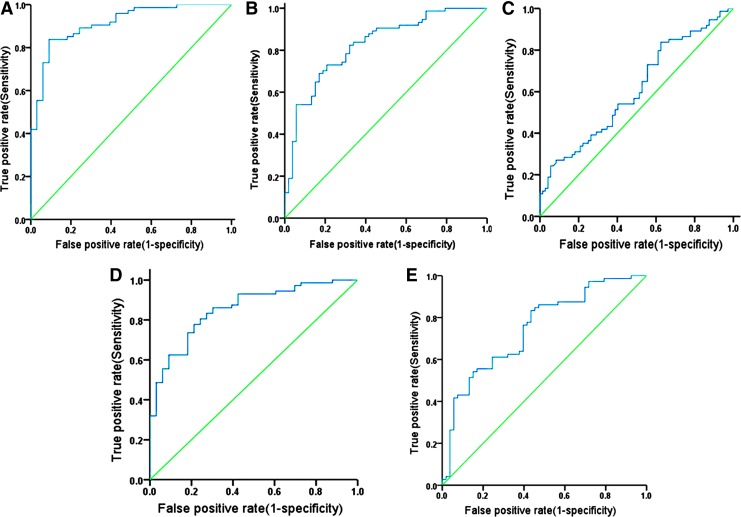
Given that HCC and LC patients had significantly higher levels of BAFF in comparison with CH and ASC, the ROC curves were used to evaluate the performance of serum BAFF in predicting HCC and LC versus other clinical diagnoses in the patients with chronic HBV infection. The area under ROC curve (AUC) value for BAFF levels was 0.914 [95% confidence interval (CI)=0.858–0.969, P<0.001, Fig. 2A] for HCC versus ASC, 0.825 (95% CI=0.752–0.897, P<0.001, Fig. 2B) for HCC versus CH and 0.607 (95% CI=0.516–0.699, P=0.025, Fig. 2C) for HCC versus LC, respectively. The AUC value for BAFF levels was 0.854 (95% CI=0.779–0.928, P<0.001, Fig. 2D) for LC versus ASC and 0.748 (95% CI=0.662–0.835, P<0.001, Fig. 2E) for LC versus CH, respectively.
FIG. 2.
Receiver-operating characteristic (ROC) curve analysis of serum BAFF levels for predicting the presence of LC and HCC in chronic HBV infection. Area under ROC curve (AUC) value: (A) 0.914 [95% confidence interval (CI)=0.858–0.969, P<0.001] for HCC versus ASC, (B) 0.825 (95% CI=0.752–0.897, P<0.001) for HCC versus CH, (C) 0.607 (95% CI=0.516–0.699, P=0.025) for HCC versus LC, (D) 0.854 (95% CI=0.779–0.928, P<0.001) for LC versus ASC and (E) 0.748 (95% CI=0.662–0.835, P<0.001) for LC versus CH, respectively.
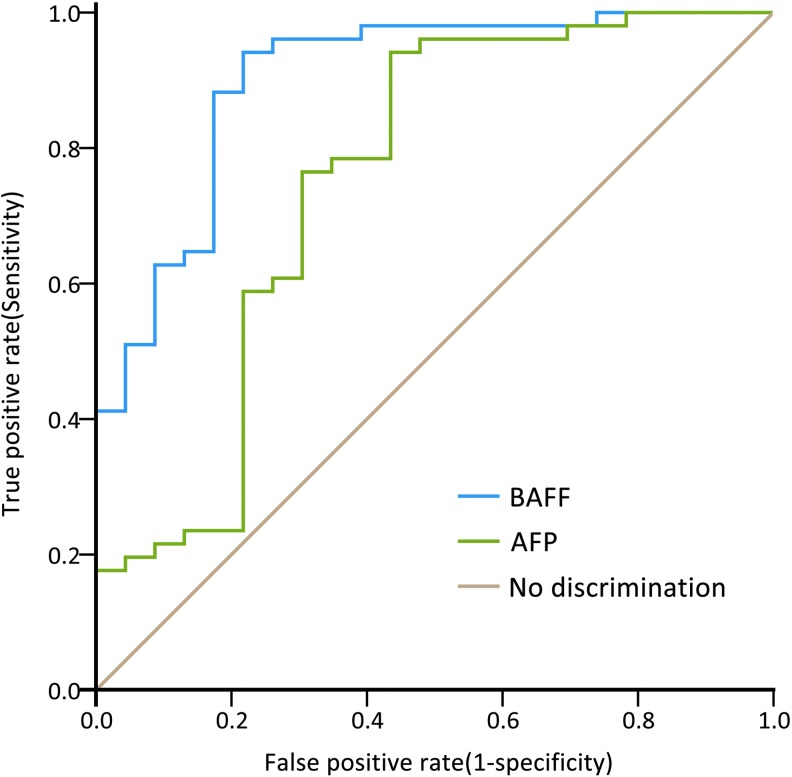
As indicated in the ROC curves of serum BAFF and AFP for HCC, the AUC value of BAFF (0.888, 95% CI=0.805–0.972, P<0.001, Fig. 3) was higher than that of AFP (0.776, 95% CI=0.644–0.908, P<0.001, Fig. 3].
FIG. 3.
Diagnostic performances of the serum BAFF levels and alpha-fetoprotein (AFP) for indicating HCC in HBV infection. Area under receiver-operating characteristic curve value of serum BAFF levels was 0.888 (95% CI=0.805–0.972, P<0.001), which was greater than that of AFP (0.776, 95% CI=0.644–0.908, P<0.001).
Discussion
Several studies which included a very small number of HBV patients as controls showed that BAFF levels in HBV infection were not much higher than those in HCV infection and normal controls (Toubi and others 2006; Lake-Bakaar and others 2012). However, these studies included a very limited number of HBV-infected patients only for the purpose of control and did not correlate the BAFF levels with the clinical diseases. We enrolled a relatively large number of patients with chronic HBV infection, classified the patients according to the disease conditions, and analyzed the associations with BAFF levels. For the first time, our study evidenced that serum BAFF levels were elevated in patients with chronic HBV infection compared with healthy controls and that the levels varied along with clinical diseases. Obviously, serum BAFF levels had a significant stepwise increase from ASC and CH to LC and HCC. These findings suggest that BAFF is involved in the process of chronic HBV infection and is associated with the disease progression. BAFF plays a unique role in B-cell development, maturation, survival, and activation (Do and Chen-Kiang 2002; Schneider and Tschopp 2003). B-cell activation and its interaction with T cells have been revealed to be involved in the disease process of HBV infection (Williams 1975; Budillon and others 1983; Kim and others 1990; Milich and others 1997; Cao and others 2001; Lazdina and others 2001; Lazdina and others 2003; Matter and others 2005; Oliviero and others 2011; Das and others 2012; Wang and others 2012). Moreover, viral infections such as HCV, HIV and respiratory syncytial virus infections have been shown to lead to the induction of BAFF (Rodriguez and others 2003; Toubi and others 2006; McNamara and others 2013), and, thus, induction of BAFF by viral infection is considered a general phenomenon although the mechanisms may vary (Ittah and others 2011). In line with these studies, our study added novel evidence that HBV infection may also result in the induction of BAFF which may influence the role of B cells in HBV infection.
Our correlation study showed that serum BAFF levels were significantly associated with ALT and AST levels in ASC and CH, with total bilirubin and albumin in HCC and with age, total bilirubin, and albumin when all the patients were included in the analysis. These results may indicate the relationships between the immuno-inflammatory and pathophysiological reaction and the BAFF induction, reflecting the disease activity and liver damage in various disease conditions of chronic HBV infection. In primary biliary cirrhosis patients, serum BAFF levels were shown to be significantly correlated with AST or total bilirubin, and the elevated serum BAFF levels were correlated with advanced interface hepatitis (Migita and others 2010).
BAFF was also assumed as a primarily pathogenic player in microenvironments of both solid and hematological tumors. So far, BAFF has been shown to promote the invasive migration activity in hypoxic breast cancer cell line (Zhu and others 2012). Elevated serum BAFF levels derived from neutrophils have been noted in oral cavity cancers (Jablonska and others 2011). Serum BAFF levels have been found to be related to angiogenesis and prognosis in multiple myeloma (Fragioudaki and others 2012). Recently, BAFF was revealed to promote tumor invasion and metastasis in human pancreatic cancer (Koizumi and others 2013). However, the involvement of BAFF in the carcinogenesis of HCC has not yet been explored. We showed that serum BAFF levels in HCC patients were the highest across all the clinical diseases of chronic HBV infection, including LC, CH, and ASC, suggesting the involvement of BAFF in the development of HCC. Serum BAFF levels were not found to be correlated with AFP levels in HCC patients in our study, possibly suggesting the differential associations of BAFF and AFP with HCC.
BAFF up-regulation in viral infection was shown to be IFN dependent, and IFN can stimulate the release of BAFF (Nardelli and others 2001; Xu and Shu 2002; Schneider and Tschopp 2003). In patients with acute HIV-1 infection, the addition of pegylated-IFN-α 2b to antiretroviral therapy was found to be able to induce higher anti-HIV antibody titers with a broader specificity compared with patients treated with antiretroviral therapy alone (Adalid-Peralta and others 2008). The addition of IFN results in higher levels of BAFF, and the success of the treatment was attributed partially to increased BAFF signaling (Adalid-Peralta and others 2008). On the other hand, inhibition of BAFF alone or plus standard therapy has been demonstrated to significantly improve response rate, reduce disease activity, and flares in systemic lupus erythematosus (Furie and others 2011; Navarra and others 2011). Whether the rationale by stimulating or inhibiting BAFF may also be employed to design therapeutic approaches for HBV-related diseases is an interesting issue of future studies.
In this study, BAFF levels in LC and HCC patients were found to be much higher than in CH and ASC patients. The AUC value of BAFF was 0.914 for HCC versus ASC, 0.825 for HCC versus CH, and 0.607 for HCC versus LC, respectively. The AUC value for BAFF levels was 0.854 for LC versus ASC and 0.748 for LC versus CH, respectively. These data suggest that BAFF could be a useful biomarker for HBV infection, particularly for LC and HCC. Furthermore, the AUC value of serum BAFF for HCC (0.888) was higher than that of AFP (0.776). It seems that BAFF has an advantage over AFP for diagnosing or differentially diagnosing HCC in chronic HBV infection. It is worth mentioning that the prognostic use of BAFF levels has already shown promise in follicular lymphoma (Li and others 2012) and in chronic lymphocytic leukemia when combined with CD38, ZAP70 expression, and the mutational status (Molica and others 2010). Whether serum BAFF may be used to monitor and predict the outcome of HBV infection, the response to antiviral treatment and the prognosis of HBV-associated HCC deserves investigation in future studies.
In conclusion, to the best of our knowledge, for the first time, this study shows that serum BAFF levels are increased in patients with chronic HBV infection. The elevated BAFF levels are associated with clinical diseases, especially LC and HCC. The serum level of BAFF is an independent variable that is associated with HCC in comparison with other HBV-related diseases. BAFF level has a higher AUC value for HCC compared with AFP level. These findings may suggest that serum BAFF levels in chronic HBV infection could be linked with the disease mechanisms and activity and the assay of serum BAFF levels could be used as a biomarker for the diagnosis and differential diagnosis of HBV-related clinical diseases. Large prospective studies are needed to evaluate the longitudinal changes of serum BAFF levels in the disease course of HBV infection and the potential of serum BAFF levels in predicting and monitoring the disease prognosis and the treatment response to immune-modulatory therapy such as IFN-α or antiviral therapy such as oral nucleos(t)ide analogues. Further studies are also warranted to elucidate the mechanisms of BAFF in the progression of HBV infection and the development of HBV-associated diseases.
Acknowledgments
This work was supported in part by funding from the National Natural Science Foundation of China (Grant no. 81371798). The authors are indebted to Guoyu Zhang and Zhu Li for their help in this study.
Author Disclosure Statement
The authors have no competing financial interests to declare.
References
- Adalid-Peralta L, Godot V, Colin C, Krzysiek R, Tran T, Poignard P, Venet A, Hosmalin A, Lebon P, Rouzioux C, Chene G, Emilie D; Interprim ANRS 112 Study Group. 2008. Stimulation of the primary anti-HIV antibody response by IFN-alpha in patients with acute HIV-1 infection. J Leukoc Biol 83:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Gehring AJ. 2006. The immune response during hepatitis B virus infection. J Gen Virol 87:1439–1449 [DOI] [PubMed] [Google Scholar]
- Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 81(8):4215–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard P, Lamelin JP, Zoulim F, Pichoud C, Trepo C. 1990. Different forms of hepatitis B virus DNA and expression of HBV antigens in peripheral blood mononuclear cells in chronic hepatitis B. J Med Virol 31:312–317 [DOI] [PubMed] [Google Scholar]
- Budillon G, Scala G, D'Onofrio C, Cassano S, De Ritis F. 1983. Diminished active T rosette levels and increased spontaneous B lymphocyte blastogenesis in hepatitis B virus positive chronic active hepatitis. Clin Exp Immunol 52:472–476 [PMC free article] [PubMed] [Google Scholar]
- Cao T, Lazdina U, Desombere I, Vanlandschoot P, Milich DR, Sällberg M, Leroux-Roels G. 2001. Hepatitis B virus core antigen binds and activates naive human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J Virol 75:6359–6366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. 2012. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 189:3925–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do RK, Chen-Kiang S. 2002. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev 13:19–25 [DOI] [PubMed] [Google Scholar]
- Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 192:953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. 2012. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 57:167–185 [DOI] [PubMed] [Google Scholar]
- Fragioudaki M, Tsirakis G, Pappa CA, Aristeidou I, Tsioutis C, Alegakis A, Kyriakou DS, Stathopoulos EN, Alexandrakis MG. 2012. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk Res 36:1004–1008 [DOI] [PubMed] [Google Scholar]
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF; BLISS-76 Study Group. 2011. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 63:3918–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittah M, Miceli-Richard C, Lebon P, Pallier C, Lepajolec C, Mariette X. 2011. Induction of B cell-activating factor by viral infection is a general phenomenon, but the types of viruses and mechanisms depend on cell type. J Innate Immun 3:200–207 [DOI] [PubMed] [Google Scholar]
- Jablonska E, Slodczyk B, Wawrusiewicz-Kurylonek N, Garley M, Dziemianczyk D, Kretowski A, Grabowska SZ. 2011. Overexpression of B cell-activating factor (BAFF) in neutrophils of oral cavity cancer patients—preliminary study. Neoplasma 58:211–216 [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee SI, Choi IH, Shin JS, Uhm JR, Kim SJ, Choi HJ. 1990. Circulating immune complexes and cell-mediated immunity in patients with hepatitis B virus associated liver diseases. Yonsei Med J 31:347–358 [DOI] [PubMed] [Google Scholar]
- Koizumi M, Hiasa Y, Kumagi T, Yamanishi H, Azemoto N, Kobata T, Matsuura B, Abe M, Onji M. 2013. Increased B cell-activating factor promotes tumor invasion and metastasis in human pancreatic cancer. PLoS One 8:e71367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake-Bakaar G, Jacobson I, Talal A. 2012. B cell activating factor (BAFF) in the natural history of chronic hepatitis C virus liver disease and mixed cryoglobulinaemia. Clin Exp Immunol 170:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdina U, Alheim M, Nyström J, Hultgren C, Borisova G, Sominskaya I, Pumpens P, Peterson DL, Milich DR, Sällberg M. 2003. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol 84:139–146 [DOI] [PubMed] [Google Scholar]
- Lazdina U, Cao T, Steinbergs J, Alheim M, Pumpens P, Peterson DL, Milich DR, Leroux-Roels G, Sällberg M. 2001. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J Virol 75:6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Jiang WQ, Rao HL, Huang JJ, Xia Y, Huang HQ, Lin TY, Xia ZJ, Li S, Li ZM. 2012. Expression of BAFF and BAFF-R in follicular lymphoma: correlation with clinicopathologic characteristics and survival outcomes. PLoS One 7:e50936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw YF, Chu CM. 2009. Hepatitis B virus infection. Lancet 373:582–592 [DOI] [PubMed] [Google Scholar]
- Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A, Maini MK. 2008. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest 118:1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini MK, Schurich A. 2010. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol 52:616–619 [DOI] [PubMed] [Google Scholar]
- Matter M, Mumprecht S, Pinschewer DD, Pavelic V, Yagita H, Krautwald S, Borst J, Ochsenbein AF. 2005. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur J Immunol 35:3229–3239 [DOI] [PubMed] [Google Scholar]
- McNamara PS, Fonceca AM, Howarth D, Correia JB, Slupsky JR, Trinick RE, Al Turaiki W, Smyth RL, Flanagan BF. 2013. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 68:76–81 [DOI] [PubMed] [Google Scholar]
- Migita K, Ilyassova B, Kovzel EF, Nersesov A, Abiru S, Maeda Y, Komori A, Ito M, Yano K, Yatsuhashi H, Shimoda S, Ishibashi H, Nakamura M. 2010. Serum BAFF and APRIL levels in patients with PBC. Clin Immunol 134:217–225 [DOI] [PubMed] [Google Scholar]
- Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, Hughes JL. 1997. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A 94:14648–14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molica S, Digiesi G, Battaglia C, Cutrona G, Antenucci A, Molica M, Giannarelli D, Sperduti I, Gentile M, Morabito F, Ferrarini M. 2010. Baff serum level predicts time to first treatment in early chronic lymphocytic leukemia. Eur J Haematol 85:314–320 [DOI] [PubMed] [Google Scholar]
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. 1999. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285:260–263 [DOI] [PubMed] [Google Scholar]
- Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97:198–204 [DOI] [PubMed] [Google Scholar]
- Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA; BLISS-52 Study Group. 2011. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377:721–731 [DOI] [PubMed] [Google Scholar]
- Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F, Barnaba V, Mondelli MU. 2011. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol 55:53–60 [DOI] [PubMed] [Google Scholar]
- Pasquinelli C, Lauré F, Chatenoud L, Beaurin G, Gazengel C, Bismuth H, Degos F, Tiollais P, Bach JF, Bréchot C. 1986. Hepatitis B virus DNA in mononuclear blood cells. A frequent event in hepatitis B surface antigen-positive and -negative patients with acute and chronic liver disease. J Hepatol 3:95–103 [DOI] [PubMed] [Google Scholar]
- Pontisso P, Poon MC, Tiollais P, Brechot C. 1984. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J (Clin Res Ed) 288:1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ZS, Manser T. 2004. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J Immunol 173:6179–6188 [DOI] [PubMed] [Google Scholar]
- Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. 2003. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS 17:1983–1985 [DOI] [PubMed] [Google Scholar]
- Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. 2003. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med 197:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. 1999. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189(11):1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Tschopp J. 2003. BAFF and the regulation of B cell survival. Immunol Lett 88:57–62 [DOI] [PubMed] [Google Scholar]
- Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, Maini MK. 2011. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 53:1494–1503 [DOI] [PubMed] [Google Scholar]
- Sutherland AP, Ng LG, Fletcher CA, Shum B, Newton RA, Grey ST, Rolph MS, Mackay F, Mackay CR. 2005. BAFF augments certain Th1-associated inflammatory responses. J Immunol 174:5537–5544 [DOI] [PubMed] [Google Scholar]
- Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, Zuckerman E. 2006. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun 27:134–139 [DOI] [PubMed] [Google Scholar]
- Wang K, Pei H, Huang B, Yang RL, Wu HY, Zhu X, Zhu L. 2012. Overexpression of Fc receptor-like 1 associated with B-cell activation during hepatitis B virus infection. Braz J Med Biol Res 45:1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol 78:5707–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. 1975. Immunopathology of hepatitis B antigen positive and negative active chronic hepatitis. Dev Biol Stand 30:341–349 [PubMed] [Google Scholar]
- Xu LG, Shu HB. 2002. TNFR-associated factor-3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-kappa B activation and IL-10 production. J Immunol 169:6883–6889 [DOI] [PubMed] [Google Scholar]
- Zhu J, Sun L, Lin S, Zhao R, Zhou L, Fang D, Chen L, Liu J, Shi W, Zhang L, Yuan S. 2012. BlyS is up-regulated by hypoxia and promotes migration of human breast cancer cells. J Exp Clin Cancer Res 31:31. [DOI] [PMC free article] [PubMed] [Google Scholar]