Abstract
Wound infection presents a challenging and growing problem. With the increased prevalence and growth of multidrug-resistant bacteria, there is a mounting need to reduce and eliminate wound infections using methodologies that limit the ability of bacteria to evolve into further drug-resistant strains. A well-known strategy for combating bacterial infection and preventing wound sepsis is through the delivery of silver ions to the wound site. High surface area silver nanoparticles (AgNPs) allowing extensive silver ion release have therefore been explored in different wound dressings and/or skin substitutes. However, it has been recently shown that AgNPs can penetrate into the stratum corneum of skin or diffuse into the cellular plasma membrane, and may interfere with a variety of cellular mechanisms. The goal of this study was to introduce and evaluate a new type of high surface area metallic silver in the form of highly porous silver microparticles (AgMPs). Polylactic acid (PLA) nanofibers were successfully loaded with either highly porous AgMPs or AgNPs and the antimicrobial efficacy and cytotoxicity of the two silver-based wound dressings were assessed and compared. To better mimic the physiological environment in vivo where both human cells and bacteria are present, a novel coculture system combining human epidermal keratinocytes and Staphylococcus aureus bacteria was designed to simultaneously evaluate human skin cell cytotoxicity with antimicrobial efficacy in a three-dimensional environment. We found that highly porous AgMPs could be successfully incorporated in nanofibrous wound dressings, and exhibited comparable antimicrobial efficacy and cytotoxicity to AgNPs. Further, PLA nanofibers containing highly porous AgMPs exhibited steady silver ion release, at a greater rate of release, than nanofibers containing AgNPs. The replacement of AgNPs with the newly introduced AgMPs overcomes concerns regarding the use of nanoparticles and holds great promise as skin substitutes or wound dressings for infected wound sites.
Introduction
Wound infection presents a challenging and growing problem, in particular, for those individuals with compromised immune systems. Wound infection delays wound closure,1,2 diminishes tensile strength of the healing wound tissue, increases the length of hospital stay and costs, and increases the patient's risk of bacteremia, sepsis, multisystem organ failure, and death.3 With the increased prevalence and growth of multidrug-resistant bacteria, there is mounting need to reduce and eliminate wound infection using methodologies that limit the ability of bacteria to evolve into further drug-resistant strains. A well-known strategy for combating bacterial infection and preventing wound sepsis is through delivery of silver ions to the wound site.4 Silver ions can be introduced to infected wound sites by a number of different mechanisms. A well-known technique is utilization of silver salt solutions, the most effective of which is silver nitrate (AgNO3).5 Aqueous silver nitrate at a concentration of 0.5% has been shown effective as a topical treatment for burn patients.5 Silver nitrate is usually applied to the wound site by wetting cotton gauze dressings with silver nitrate solution every 3–4 h per day.6 Other forms of silver have been investigated, in particular, silver sulfadiazine. Silver sulfadiazine is also delivered topically to burn wounds, typically in a 1% cream or suspension.7 Silver sulfadiazine provides a sufficient concentration of silver and its residual activity is higher than silver nitrate, allowing for application only twice per day.
Whereas both these approaches have proven successful for their antimicrobial and antibacterial attributes, they lack the ability for continued release of silver ions, requiring constant reapplication of the compound to painful wound sites. The large, rapid release of silver ions from silver nitrate and silver sulfadiazine can also have negative effects on the host cells, as explained later in this article. Therefore, interest has grown in methods to deliver silver ions continuously, without constant reapplication of the silver-containing compound. A recent technique to address this challenge is to incorporate metallic silver in wound dressings.8 When the wound dressings are exposed to fluids in the wound bed and tissue exudates, a sustained release of silver ions occurs.9 The total amount of silver ions that can be released from metallic silver depends on the available surface area.10,11 High surface area silver nanoparticles (AgNPs) allowing extensive silver ion release have therefore been explored in different wound dressings and/or skin substitutes.8 Their excellent antimicrobial efficacy has been shown; however, concern has grown regarding the release of AgNPs from different products into the environment. It is now known that AgNPs can penetrate into the stratum corneum of skin12,13 or even diffuse into the cellular plasma membrane, and interfere with a variety of cellular mechanisms.14,15 The ability to harness the antimicrobial benefits of high surface area metallic silver in wound dressings, without the use of AgNPs, remains a significant challenge. A solution has yet to be determined.
When implementing silver in wound dressings, the cytotoxicity of the dressings to the host cells is as important a component of their fabrication as their antimicrobial properties. Although it has been traditionally reported that silver causes low mammalian cell toxicity,16,17 silver ions do have cytotoxic effects on some mammalian cells. It has been shown that epidermal keratinocytes and dermal fibroblasts, the two dominant cell types in human skin, are susceptible to lethal damage when exposed to high concentrations of silver.18 However, since cytotoxicity is directly proportional to the silver concentration,19 this problem can potentially be addressed by using metallic silver dressings with controlled release capabilities, allowing for complete wound healing to occur.20 Ultimately, the desired goal is to achieve an optimized release profile that results in antimicrobial/antibacterial efficacy without mammalian cell cytotoxicity.20
The goal of this study was to introduce and evaluate a new type of high surface area metallic silver in the form of highly porous silver microparticles (AgMPs). The AgMPs are composed of AgNP aggregates, resulting in the desired high surface area without undesired detachment and release of AgNPs in the environment. These high surface area AgMPs were incorporated within polylactic acid (PLA) nanofibrous dressings. Biodegradable, biocompatible PLA nanofibers, with their morphological similarities to the extracellular matrix of skin, have great potential to be used as skin substitutes and wound dressings.21 PLA nanofibers were loaded with either highly porous AgMPs or AgNPs and the antimicrobial efficacy and cytotoxicity of the two silver-based wound dressings were assessed and compared. To better mimic the physiological environment in vivo where both human cells and bacteria are present, a novel coculture system combining human epidermal keratinocytes and Staphylococcus aureus bacteria was designed to simultaneously evaluate cytotoxicity and antimicrobial efficacy in a three-dimensional (3D) culture environment. This new coculture system could also be used to evaluate any other antimicrobial dressings.
Materials and Methods
Scaffold Fabrication
PLA (molecular weight=70,000 g/mol) was dissolved in chloroform and dimethylformamide (Sigma) at a ratio of 3:1 to create a 12% solution. AgNPs (20 nm Citrate Biopure™ Silver) (Nanocomposix Company) or highly porous AgMPs (average diameter=6.9 μm, surface area=3.9 m2/g) (Bio-Gate AG Company) (Fig. 1) were added to the PLA solution to obtain a 0.5% concentration of silver to polymer ratio. Mixtures were stirred on a magnetic stirrer plate for 4 h at 80°C and then sonicated for 30 min to further ensure particle dispersion. Polymer solutions were used immediately after sonication to eliminate particle precipitation (of particular importance for AgMPs) and prevent evaporative loss of solvent and the consequent change in solution concentrations. The PLA solution was electrospun in a custom electrospinning system22 for 2 h using 15 kV voltage, feed rate of 0.7 μL/h, and spinning distance of 13–15 cm. Electrospun scaffolds were kept under a fume hood overnight to fully evaporate residual solvents. Scaffolds were then removed from the fiber collector and cut into circles using a punch (d=1.6 cm). Scaffolds were sterilized with 70% ethanol for 10 min, then rinsed three times with phosphate-buffered saline (PBS), and soaked in the keratinocyte growth medium (KGM) (KGM-Gold; Lonza) without antibiotics for 12 h to allow proteins to attach to the scaffolds. Pure PLA scaffolds containing no silver were used as controls.
FIG. 1.

SEM micrograph of the surface of highly porous AgMPs. AgMPs, silver microparticles; SEM, scanning electron microscopy.
Silver release studies
Scaffolds were soaked in deionized water and incubated at 37°C and 5% CO2 for 1 week. At specific time points, 3, 6, 18, 30, 42, 66, 120, and 168 h, half of the water was removed and replaced with fresh deionized water. The concentration of silver ions released at each time point was quantified using the removed water by means of a Perkin-Elmer AA300 atomic adsorption spectrophotometer (PerkinElmer, Inc.).
Coculture system of human epidermal keratinocytes with S. aureus
To evaluate the antimicrobial efficacy of AgNP- or AgMP-loaded scaffolds when seeded with cells, a coculture system and an experimental process were designed (Fig. 2). Separate experiments with only human epidermal keratinocytes or S. aureus bacteria were also performed to better understand and elucidate the results of the coculture system (Table 1). PLA scaffolds loaded with AgMPs, AgNPs, or neither (pure PLA, controls) were seeded with human epidermal keratinocytes derived from adult skin (2nd passage; Lonza) at a density of 4×104 cells per scaffold and incubated for 24 h. The cell-seeded scaffolds were then inoculated with 10 CFU/mL S. aureus bacteria (AATCC# 43300™) dispersed in 1 mL of keratinocyte growth medium without antibiotics. Epidermal keratinocyte- and S. aureus-seeded scaffolds were then placed and maintained in an incubator (37°C, 5% CO2) for 72 h on a rotating plate. Rotation was incorporated to better mimic body fluid circulation in the wound site and assist with the gradual movement of bacteria and prevention of their rapid suspension on the nanofibers.23
FIG. 2.
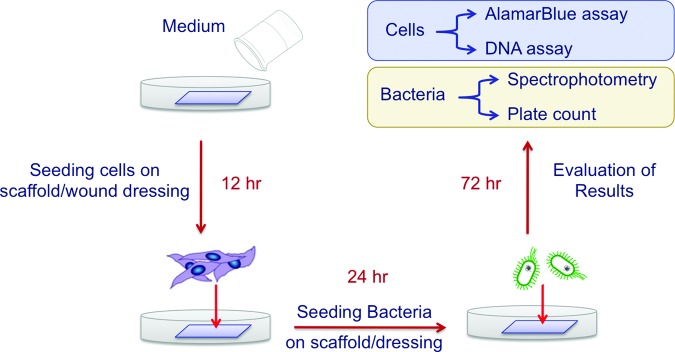
Three-dimensional coculture system to evaluate human skin and/or other mammalian cells in combination with bacteria on scaffolds/wound dressings. Color images available online at www.liebertpub.com/tec
Table 1.
Design of Experiment for Antimicrobial and Cytotoxicity Evaluation of Scaffolds Either Separately or in a Coculture System
| Scaffold | Replicates | Analyses | |
|---|---|---|---|
| Cytotoxicity experiment | Pure PLA | 3 | AlamarBlue assay |
| PLA+AgNPs | 3 | DNA assay | |
| PLA+AgMPs | 3 | ||
| Antimicrobial experiment | Pure PLA | 3 | Spectrophotometry |
| PLA+AgNPs | 3 | Plate count | |
| PLA+AgMPs | 3 | ||
| Coculture experiment | Pure PLA | 3 | Spectrophotometry |
| PLA+AgNPs | 3 | Plate count | |
| PLA+AgMPs | 3 | DNA assay |
PLA, polylactic acid; AgMPs, silver microparticles; AgNPs, silver nanoparticles.
Monitoring S. aureus growth in coculture system
At specific time points (12, 24, 36, 42, and 72 h), 100 μL of culture medium was taken (after pipetting up and down several times to ensure bacteria were well suspended in the medium), diluted multiple times, and spread on Mueller Hinton agar plates (Thermo Scientific). Agar plates were incubated overnight to allow bacterial colonies to grow and become visible to the naked eye for counting and monitoring bacterial growth in each well. After 72 h, bacterial counts were confirmed by the measurement of the optical density of the bacterial suspension, taken from each sample, using a UV-Vis spectrophotometer (Biomate3; Thermo Electron Corporation) at a wavelength of 600 nm.
Human epidermal keratinocyte viability and proliferation in coculture system
Viability analyses were performed at day 1 (24 h after keratinocyte seeding on the scaffolds and before addition of bacteria) and day 3 (72 h after addition of bacteria) using a fluorescent method (Live-Dead Assay Cytotoxicity Kit for mammalian cells; Molecular Probes). Specifically, keratinocyte-seeded scaffolds were placed in KGM with the addition of 4 mM calceinacetoxymethyl ester-AM (staining the cytoplasm of live cells green) and 4 mM ethidiumhomodimer (staining the nuclei of dead cells red). The samples were then incubated for 20 min while protected from light. Cell proliferation, in cultures without bacteria, was determined with a cell viability assay (alamarBlue; AbDSerotec) at different time points after seeding of cells on scaffolds (days 1, 2, and 3). AlamarBlue, at a volume of 10% of the culture medium, was added to each well, 7 h before each measurement. After incubation with alamarBlue, 200 μL of each sample was taken in triplicate and the absorbency read at 600 nm using a microplate reader (TecanGENios; Tecan). A greater alamarBlue reduction% indicated greater cell proliferation. Since the alamarBlue assay is not an accurate method to evaluate the proliferation of cells in coculture systems (the presence of bacteria changes the reduction%), the number of cells on the scaffolds was quantified by measuring the DNA content in each scaffold after 72 h. The scaffolds were washed at least three times with PBS to confirm that the bacteria were detached from the scaffolds. To verify that all bacteria were washed out from the scaffolds, the PBS solution from the last wash was used for bacterial analysis to confirm no bacteria were present in the PBS from the last wash. Once this was confirmed, the amount of DNA in each nanofibrous scaffold was then measured with the DNA binding dye Hoechst 33258 in a microplate format after an overnight digestion at 60°C in 2.5 U/mL papain in PBS with 5 mM ethylenediaminetetraacetic acid and 5 mM cysteine HCl (all reagents from Sigma).
Microscopic analyses
Keratinocyte morphology on the scaffolds was evaluated using scanning electron microscopy (SEM) (FESEM JEOL 6400 F) at 15 kV accelerating voltage. At each time point, nanofibrous scaffolds were fixed in 10% buffered formalin for 30 min and then dehydrated with a graded concentration (50–100% v/v) of ethanol. Dehydrated scaffolds were immersed in hexamethyldisilazane for 15 min and dried overnight in a fume hood. Dried samples were sputter coated with gold to observe the morphology of cells using SEM. The transmission electron microscope (TEM) (Hitachi HF2000) was used to further characterize longitudinal dispersion of particles within ultrafine electrospun fibers at 200 kV accelerating voltage.
Statistical analyses
Statistical analyses were performed using SPSS 14.0. Data were analyzed using the Duncan test with p-values less than 0.05 considered statistically significant.
Results
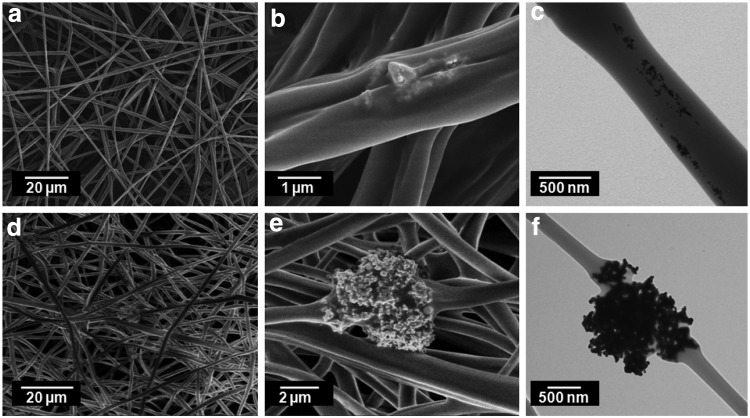
Electrospun PLA fibers containing either AgNPs or highly porous AgMPs formed in a uniform manner on the fiber collector (Fig. 3a, d). However, as expected, incorporation of the two particles within the fibers varied. SEM images indicated that AgNPs were not present on the surface of fibers and there were very few locations where AgNPs were close to the fiber surface (Fig. 3b). TEM analysis confirmed the presence of well-dispersed AgNPs inside the fibers, closer to the core (Fig. 3c). Highly porous AgMPs, on the other hand, were too large to be encapsulated inside the nanofibers. SEM (Fig. 3e) and TEM (Fig. 3f) images showed that these AgMPs were present on the surface of fibers.
FIG. 3.
PLA nanofibers containing AgNPs (a–c) or highly porous AgMPs (d–f). AgNPs, silver nanoparticles; PLA, polylactic acid.
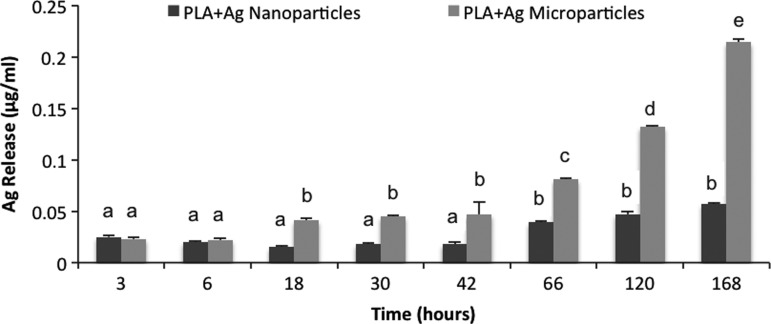
Release profiles revealed that silver ions gradually release from both scaffolds, doped with AgNPs or highly porous AgMPs, over a 1-week (168 h) period (Fig. 4). Silver ion release was slow at the first two time points (3 and 6 h) for both scaffolds, then significantly accelerated at the 18-h time point for nanofibers doped with highly porous AgMPs (Fig. 4). The rate of release and cumulative release remained greater for scaffolds doped with highly porous AgMPs at all remaining time points.
FIG. 4.
Release profiles of silver ions from PLA scaffolds loaded with silver nano- or microparticles. Different letters indicate significant differences between groups (p<0.05).
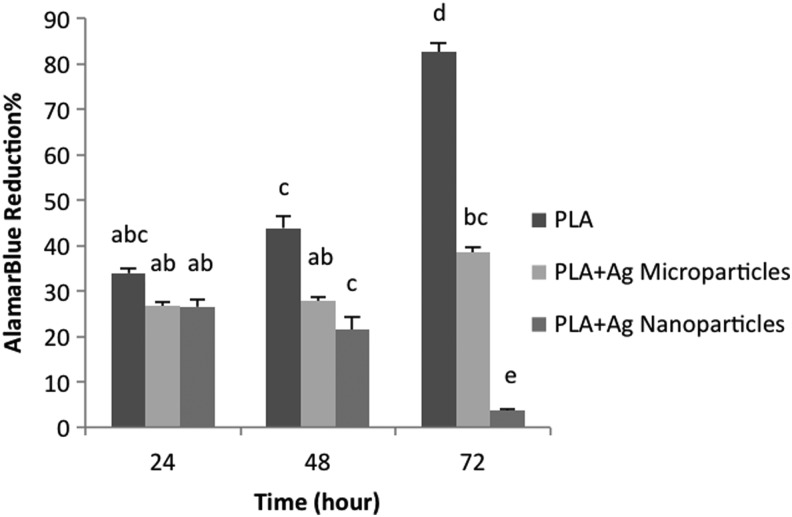
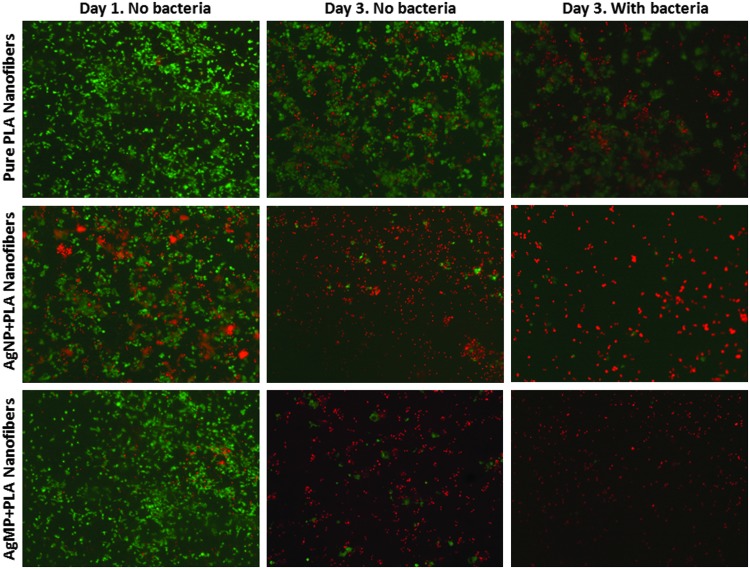
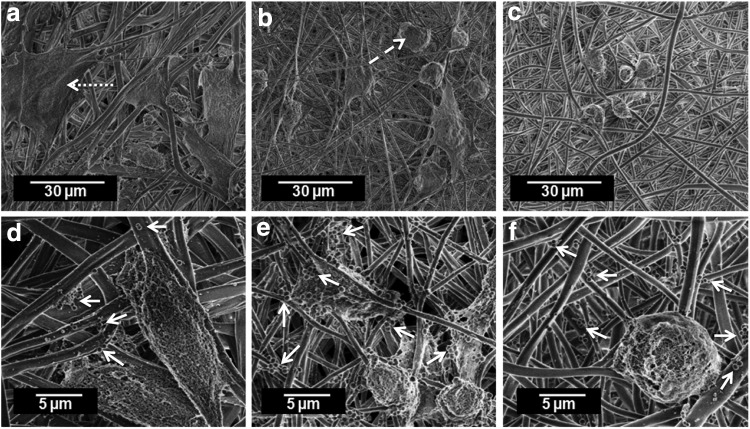
AlamarBlue findings indicated that proliferation of keratinocytes was diminished on PLA scaffolds loaded with AgNPs or AgMPs compared with pure PLA scaffolds, with lowest proliferation observed for AgNP-loaded scaffolds (Fig. 5). Similarly, viability analyses indicated that neither AgNP- nor AgMP-loaded scaffolds supported human epidermal keratinocyte viability after 3 days (Fig. 6). Live/dead images showed no obvious difference between the viability of keratinocytes on nanofibers containing either AgNPs or AgMPs at day 3, but a greater viability of AgMP-loaded scaffolds relative to AgNP-loaded scaffolds at day 1. Addition of bacteria to the culture medium further decreased cell viability for all three keratinocyte-seeded scaffolds (Fig. 6). SEM micrographs indicated that keratinocytes adhered and spread throughout the pure PLA scaffolds. However, keratinocyte morphology was clearly different on PLA scaffolds loaded with AgNPs or AgMPs. On those scaffolds, the cell number was reduced and cells were rounded, representing the morphology of dead cells (Fig. 7). SEM images showed no obvious difference between cells on nanofibers containing AgNPs or AgMPs. Consistent with qualitative viability analyses (Fig. 6), SEM images represented that addition of bacteria to the culture medium further reduced the number of viable cells on pure PLA scaffolds. SEM images also confirmed the presence of S. aureus bacteria on all three scaffolds.
FIG. 5.
Human epidermal keratinocyte proliferation on scaffolds without bacteria in the culture medium. Different letters indicate significant difference (p<0.05).
FIG. 6.
Viability of human epidermal keratinocytes on scaffolds (live cells=green; dead cells=red). Addition of AgNPs and AgMPs to nanofibers reduced viability of keratinocytes on these samples. The presence of bacteria in cultures also diminished the number of viable cells on all three nanofibrous dressings. Color images available online at www.liebertpub.com/tec
FIG. 7.
SEM micrographs of human epidermal keratinocytes seeded on scaffolds (a, d: pure PLA; b, e: AgNP loaded; c, f: AgMP loaded) with (d–f) and without (a–c) the presence of Staphylococcus aureus at day 3. Dotted arrow shows the morphology of live cell, dashed arrow shows the morphology of dead cell, and solid arrows point to the bacteria.
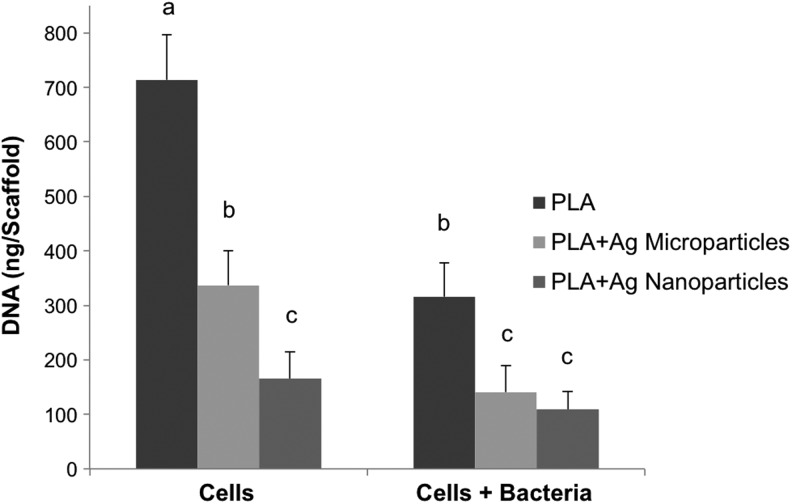
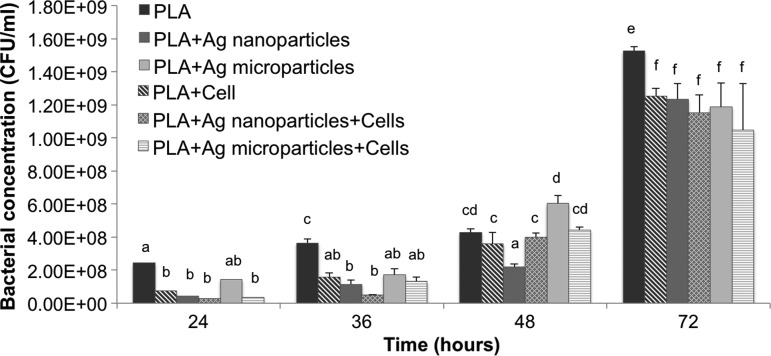
DNA quantitation (Fig. 8) indicated that pure PLA scaffolds supported the greatest keratinocyte viability relative to either AgNP- or AgMP-loaded scaffolds in the presence or absence of bacteria consistent with viability (Fig. 6) and SEM (Fig. 7) analyses. Similar to the alamarBlue findings (Fig. 5), DNA content analysis showed higher proliferation of keratinocytes in the absence of bacteria on AgMP-loaded nanofibers compared with AgNP-loaded nanofibers. The DNA content significantly dropped for pure PLA scaffolds and scaffolds loaded with AgMPs with the addition of bacteria to the culture medium. Bacterial analyses indicated that S. aureus proliferated on all three scaffolds, with the highest rate of growth for pure PLA scaffolds without keratinocytes (Fig. 9). Although the addition of AgNPs and AgMPs to the PLA scaffolds reduced the bacterial growth rate, no significant difference was observed between the antimicrobial properties of nanofibers containing either AgNPs or AgMPs. The presence of keratinocytes in coculture systems with S. aureus further diminished bacterial growth for cultures with pure PLA nanofibers after 72 h (Fig. 9).
FIG. 8.
Human epidermal keratinocyte DNA quantitation on scaffolds both in the presence and absence of S. aureus bacteria at day 3. Different letters indicate significant differences between groups (p<0.05).
FIG. 9.
S. aureus bacterial growth on acellular or human epidermal keratinocyte-seeded scaffolds. Different letters indicate significant differences between groups (p<0.05).
Discussion
In this study, a new metallic silver compound comprising highly porous AgMPs was successfully incorporated within PLA electrospun nanofibers using a conventional electrospinning approach. Gradual, sustained release of silver ions from these scaffolds was observed over a 7-day experimental period. Interestingly, within 18 h and for all time points thereafter, the rate of silver ion release was significantly higher for AgMP-loaded scaffolds relative to AgNP-loaded scaffolds. This can be related to the different cross-sectional locations of the two particles within the fibers. Dopants closer to the outer layer of fibers release faster than those well incorporated inside the core (Mohiti-Asli et al., Submitted). AgNPs located inside the fiber therefore release silver ions slower than highly porous AgMPs that, by function of their size, are present at the surface of fibers. As expected, cytotoxicity analyses of the AgNP- and AgMP-loaded scaffolds indicated that keratinocytes do not proliferate as well on either of these scaffolds relative to their proliferation on pure PLA scaffolds. Our findings are consistent with previous investigators who have reported diminished proliferation of keratinocytes in the presence of silver ions.24–26
In the absence of bacteria in the culture medium, alamarBlue and DNA content findings indicated that keratinocytes exhibited a significantly greater proliferation on AgMP-loaded scaffolds relative to AgNP-loaded scaffolds. However, with the inclusion of S. aureus bacteria in the coculture system, there was no significant difference in keratinocyte cytotoxicity between AgMP or AgNP scaffolds according to DNA content analyses. These results indicate that even though the AgMP-loaded scaffolds exhibited a higher silver ion release rate, their cytotoxicity to keratinocytes was less than AgNP-loaded scaffolds. A limitation of this experiment was the possibility that, even after multiple washes and confirmation of no bacteria in the solution, some residual bacteria remained adhered to the scaffolds. However, even if this did occur, the same washing approach was used for all samples and samples were compared with each other. Therefore, any effects were likely normalized and not expected to affect conclusions regarding the differences between the scaffold types.
As expected, antimicrobial analyses revealed a delay in the growth of S. aureus bacteria on silver ion release scaffolds relative to pure PLA controls. However, the amount of silver ions released from the two scaffolds was not adequate to kill all bacteria within the solution with the initial concentration of 10 CFU/mL S. aureus bacteria that was evaluated. Although increasing the initial concentration of silver particles in nanofibers can increase the release of silver ions (Mohiti-Asli et al., Submitted) and thereby increase their antimicrobial properties, we would not recommend such an approach except for severely infected wounds given the cytotoxic effects of increased silver concentration on human epidermal keratinocytes that we have found and reported here. The rate of bacterial growth over 72 h was not significantly different for scaffolds containing AgNPs or AgMPs. This finding was not unexpected given that while the difference in silver ion release was statistically significant at most time points (Fig. 4), quantitatively the difference in silver release was very slight between the two scaffolds before the 120-h evaluation point. The maximum difference in silver ion release between the two scaffolds occurred at the 66-h time point and was equal to 0.042 ug/mL (AgMP-loaded scaffold silver release=0.081; nanoparticle-loaded scaffold release=0.039; difference=0.042 μg/mL). This difference was apparently not high enough to result in a significant difference in antibacterial efficacy between the AgMP- or AgNP-loaded scaffolds.
To avoid complex interactions between mammalian cells and bacteria, a majority of previous studies have evaluated the antimicrobial and cytotoxicity of antimicrobial agents separately. Such an approach may not appropriately address the in vivo environment. To address this, some recent studies have tried to implement two-dimensional, monolayer coculture systems of cells and bacteria to test antimicrobial agents that can be added to the culture medium.27–30 To date, there is no published method on evaluating antimicrobial dressings in coculture systems. Therefore, we designed a novel 3D coculture system to assess the cytotoxicity and antimicrobial properties of antimicrobial scaffolds/wound dressings in one experiment. The presence of S. aureus reduced the viability and proliferation of human epidermal keratinocytes on all three scaffolds (pure PLA, AgNP loaded, and AgMP loaded). This has been reported previously by other researchers who studied the interaction of cells and bacteria in the absence of dressings.30,31 In addition, the growth of S. aureus bacteria was diminished on scaffolds seeded with epidermal keratinocytes relative to acellular scaffolds. This phenomenon is likely attributed to the release of antimicrobial peptides when the bacteria come in contact with the keratinocytes.32,33
In conclusion, we have shown that highly porous AgMPs can be successfully incorporated into nanofibrous wound dressings, and exhibit comparable antimicrobial efficacy and cytotoxicity to nanofibrous wound dressings incorporating AgNPs. Further, PLA nanofibers containing highly porous AgMPs exhibited steady silver ion release, at a greater rate of release, than nanofibers containing AgNPs. We propose that highly porous AgMPs can be used as an alternative to AgNPs in skin substitutes or wound dressings for infected wound sites. Future studies should investigate the cytotoxicity of nanofibers containing different concentrations of AgMPs both in vitro using skin equivalents,34 which have many morphologic and phenotypic properties of human skin, and in vivo and assess the degradation of particles over extended durations.
Acknowledgments
This research was supported by NIH/NIBIB 1R03EB008790 (E.G.L.), NSF/CBET 1133427 (E.G.L.), Nonwovens Cooperative Research Center (Project 10–128, E.G.L.), Chancellor's Innovation Fund (E.G.L.), and a North Carolina Biotechnology Center Collaborative Funding Grant (E.G.L.). The authors thank Dr. Susan Bernacki for microbiology assistance and all other members of the Cell Mechanics Laboratory.
Disclosure Statement
No competing financial interests exist.
References
- 1.Madsen S.M., Westh H., Danielsen L., and Rosdahl V.T.Bacterial colonization and healing of venous leg ulcers. APMIS 104,895, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Robson M.C.Wound infection: a failure of wound healing caused by an imbalance of bacteria. The surgical clinics of North America 77,637, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Campton-Johnston S.M., and Wilson J.A.Infected wound management: advanced technologies, moisture-retentive dressings, and die-hard methods. Crit Care Nurs Q 24,64, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wright J.B., Hansen D.L., and Burrell R.E.The comparative efficacy of two antimicrobial barrier dressings: in-vitro examination of two controlled release of silver dressings. Wounds 10,179, 1998 [Google Scholar]
- 5.Moyer C.A., Brentano L., Gravens D.L., Margraf H.W., and Monafo W.W., Jr.Treatment of large human burns with 0.5% silver nitrate solution. Arch Surg 90,812, 1965 [DOI] [PubMed] [Google Scholar]
- 6.Klasen H.J.A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26,131, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Fox C.L., Jr.Silver sulfadiazine—a new topical therapy for pseudomonas in burns: therapy of pseudomonas infection in burns. Arch Surg 96,184, 1968 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson L.J., White R.J., and Chipman J.K.Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J Wound Care 20,543, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Mooney E.K., Lippitt C., and Friedman J.Silver dressings. Plast Reconstr Surg 117,666, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramirez J.T., et al. The bactericidal effect of silver nanoparticles. Nanotechnology 16,2346, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Raimondi F., Scherer G.G., Kotz R., and Wokaun A.Nanoparticles in energy technology: examples from electrochemistry and catalysis. Angew Chem Int Ed Engl 44,2190, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Larese F.F., D'Agostin F., Crosera M., Adami G., Renzi N., Bovenzi M., et al. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 255,33, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Samberg M.E., Oldenburg S.J., and Monteiro-Riviere N.A.Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect 118,407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samberg M.E., Loboa E.G., Oldenburg S.J., and Monteiro-Riviere N.A.Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine (Lond) 7,1197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S., Jain J., Rajwade J.M., and Paknikar K.M.Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett 179,93, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kirsner R.S., Orsted H., and Wright J.B.Matrix metalloproteinases in normal and impaired wound healing: a potential role for nanocrystalline silver. Wounds 13,5, 2001 [Google Scholar]
- 17.Wright J.B., Lam K., Buret A.G., Olson M.E., and Burrell R.E.Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen 10,141, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Poon V.K., and Burd A.In vitro cytotoxity of silver: implication for clinical wound care. Burns 30,140, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lansdown A.Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 33,17, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ziegler K., Görl R., Effing J., Ellermann J., Mappes M., Otten S., et al. Reduced cellular toxicity of a new silver-containing antimicrobial dressing and clinical performance in non-healing wounds. Skin Pharmacol Physiol 19,140, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Mohiti-Asli M., Pourdeyhimi B., Loboa E.G.Novel, silver ion releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater pii:, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohiti Asli M., Pourdeyhimi B., and Loboa E.G.Release profiles of tricalcium phosphate nanoparticles from poly(L-lactic acid) electrospun scaffolds with single component, core-sheath, or porous fiber morphologies: effects on hASC viability and osteogenic differentiation. Macromol Biosci 12,893, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Farrer D.Advanced Wound Repair Therapies. Cambridge, UK: Woodhead Publishing Series in Biomaterials, 2011 [Google Scholar]
- 24.Paddle-Ledinek J.E., Nasa Z., and Cleland H.J.Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg 117,110S; discussion 9S, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Du Toit D.F., and Page B.J.An in vitro evaluation of the cell toxicity of honey and silver dressings. J Wound Care 18,383, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Orlowski P., Krzyzowska M., Winnicka A., Chwalibog A., and Sawosz E.Experimental immunology toxicity of silver nanoparticles in monocytes and keratinocytes: potential to induce inflammatory reactions. Cent Eur J Immunol 37,123, 2012 [Google Scholar]
- 27.Hidalgo E., Bartolome R., Barroso C., Moreno A., and Dominguez C.Silver nitrate: antimicrobial activity related to cytotoxicity in cultured human fibroblasts. Skin Pharmacol Physiol 11,140, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Muller G., and Kramer A.Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 61,1281, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kloth L.C., Berman J.E., Laatsch L.J., and Kirchner P.A.Bactericidal and cytotoxic effects of chloramine-T on wound pathogens and human fibroblasts in vitro. Adv Skin Wound Care 20,331, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Wiegand C., Abel M., Ruth P., and Hipler U.C.HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen 17,730, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Mempel M., Schnopp C., Hojka M., Fesq H., Weidinger S., Schaller M., et al. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br J Dermatol 146,943, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Menzies B.E., and Kenoyer A.Staphylococcus aureus infection of epidermal keratinocytes promotes expression of innate antimicrobial peptides. Infec Immun 73,5241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisich K.O., Howell M.D., Boguniewicz M., Heizer H.R., Watson N.U., and Leung D.Y.The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol 127,2368, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Gay R., Swiderek M., Nelson D., and Ernesti A.The living skin equivalent as a model in vitro for ranking the toxic potential of dermal irritants. Toxicol In Vitro 6,303, 1992 [DOI] [PubMed] [Google Scholar]