Abstract
Initial descriptions of the HIV engagement continuum are limited by short-term follow-up and incomplete data. We evaluated engagement in a newly HIV-diagnosed cohort. Our goals were to assess long-term engagement-in-care, evaluate the effects of out-of-state migration on engagement estimates, and determine whether engagement has improved in more recently diagnosed individuals. This is a retrospective cohort study of individuals newly HIV-diagnosed at two large HIV care centers in the Denver metropolitan area from 2005 to 2009. Clinical data were obtained from three public HIV providers and two clinical trial groups. For statewide evaluation, we used mandated laboratory reporting databases for CD4 lymphocyte counts and HIV-1 RNA levels. From 2005 to 2009, 615 individuals were diagnosed with HIV. By 18 months after HIV diagnosis, 84% of the cohort had linked to care, 73% were retained in care, 49% were prescribed antiretroviral therapy, and 36% had viral suppression. By 5 years after HIV diagnosis, 55% of the cohort were retained in care, 37% had viral suppression, 15% had moved out of state, and 3% were deceased. When censoring for outmigration and death, 66% of the cohort were retained in care and 45% of the cohort had viral suppression 5 years after HIV diagnosis. Engagement-in-care 18 months after diagnosis was better in individuals diagnosed more recently. Retention in care declined while viral suppression increased over time after HIV diagnosis. Accounting for outmigration and death significantly increased estimates of engagement-in-care. Performance in the engagement continuum 18 months after diagnosis improved significantly in individuals more recently diagnosed with HIV.
Introduction
For HIV-infected individuals, suppressed HIV-1 RNA levels are associated with markedly improved health outcomes and decreased risk of HIV transmission to others.1,2 The engagement-in-care continuum is a method for evaluating the success of HIV care, from diagnosis through achievement of viral suppression. Previous estimates suggest only 19–25% of HIV-infected individuals in the United States have complete viral suppression, but the analytic methods for describing the engagement-in-care continuum are still being developed.3–8 Studies from single institutions are limited by the frequency with which patients change their HIV care sites.9–14 Though analyses of jurisdictional databases containing mandatory reports of CD4 lymphocyte counts and HIV-1 RNA levels can track patients who transfer care within the same jurisdiction, such analyses may fail to account for out-of-state migration, may be missing laboratory reports from some providers of HIV care (clinical trial participants and persons in care in federal institutions such as the Veteran's Administration), and commonly have no metric to estimate the completeness of reporting in their jurisdiction.12,15–17 Finally, engagement-in-care is a dynamic process and likely varies over time.18
We used clinical record review and statewide HIV laboratory reporting databases to describe engagement-in-care over 5 years following the initial HIV diagnosis. Our goals were to assess (1) variations in the engagement-in-care continuum over time; (2) the impact of outmigration and death on engagement-in-care estimates, and (3) whether engagement-in-care is better in more recently diagnosed individuals. These analyses and considerations may be helpful as HIV treatment centers and municipalities use estimates of the engagement-in-care continuum as the basis for system-wide quality improvement.
Methods
Study population
We evaluated patients diagnosed with HIV in two large publically funded HIV care programs in the Denver metropolitan area. Denver Health (DH) is an integrated safety-net health care system and includes the public health department (which has the largest HIV testing program in the area, including outreach testing, and a large HIV clinic), community-based clinics caring for ∼170,000 individuals, and a 500-bed hospital. The University of Colorado Denver (UCD) includes a tertiary academic medical center that has the largest HIV care clinic in the state. Individuals diagnosed with HIV through DH or the UCD hospitals, clinics, or their associated outreach programs from 2005 to 2009 were identified through a combination of laboratory records, sexually transmitted diseases (STD) clinic data, outreach testing data, and DH linkage-to-care program data.
Individuals with a positive HIV enzyme-linked immunosorbent assay (ELISA) and a positive HIV Western blot were included in the study. Once identified, records were cross-checked with Colorado Department of Public Health and Environment HIV diagnosis data to identify persons who had tested positive for HIV prior to 2005 or tested at a location other than those associated with DH and UCD. Those persons were excluded from the review. Children younger than 13 years of age and individuals who tested anonymously were also excluded. Pregnant women were included in the study if they were HIV-diagnosed at DH or UCD. Individuals diagnosed with HIV while incarcerated were excluded as they were not diagnosed with HIV at DH or UCD. Individuals who were diagnosed at one of the two study institutions, then later incarcerated, remained in the study as their labs were available through the statewide database.
The study was approved by the Colorado Multiple Institutional Review Board and the Colorado Department of Public Health and Environment Institutional Review Board, and reviewed by the Federal Office of Human Research Protection.
Data abstraction and definitions
A complete medical record review was performed for individuals who received care through DH, UCD, the Children's Hospital Colorado, or the local sites of two national clinical trial units. Information abstracted included demographics, testing locations, follow-up locations, countries of birth, preferred language for health care visits, HIV acquisition risk factors, outpatient HIV visits, antiretroviral therapy prescriptions, CD4 lymphocyte counts, HIV-1 RNA levels, out-of-state migration, and death. During the study period, all CD4 lymphocyte counts less than 500 cells/μL, CD4 lymphocyte percentages less than 29%, and detectable HIV-1 RNA levels that were drawn in the state of Colorado were reported to CDPHE. Despite the regulations, most clinical laboratories operating in the state of Colorado during this study reported all HIV-1 RNA levels, including suppressed viral loads.13 Laboratory results were reviewed for all individuals included in the study and these results were used as a proxy for engagement-in-care in Colorado at sites other than those with complete record review.
To estimate the total number of HIV-infected individuals in our testing catchment area, we assumed that the individuals who were newly diagnosed in our study period represented 80% of all HIV-infected individuals in the community. This estimate was based on national data from the Centers for Disease Control and Prevention for the years of diagnosis, as well as from Denver-specific data from the National HIV Behavioral Surveillance System (NHBS) MSM cycle, both reporting 20% of HIV-infected individuals were undiagnosed.19–21
Linkage to care is the process of engaging newly diagnosed HIV-infected individuals into HIV primary care. We considered the first HIV visit or lab documented in the medical record or statewide surveillance database as evidence of linkage to care, excluding labs drawn on or near the day of HIV diagnosis to which we had complete access during institutional data review. In the literature, retention in care has not been consistently defined, but generally refers to attendance at medically necessary visits with an HIV primary care provider. In our study, patients were considered retained in care if they had a visit or lab in the prior 6- or 12-month interval (depending on the specific analysis), excluding the initial linkage to care visit.
Information regarding initiation of antiretroviral therapy (ART) was obtained via chart review. For those individuals engaged in care outside of the institutions with full chart review, a two log drop in HIV-1 RNA levels was considered evidence of probable antiretroviral initiation. For the data analyses in this article we considered viral suppression as an HIV-1 RNA <200 copies/mL. As we present engagement-in-care over time, we considered the most recent HIV-1 RNA level within the past 12 months as a marker of current virologic status. If there were no HIV-1 RNA levels reported during the past 12 months, we considered the HIV-1 RNA level to be unsuppressed (missing=failure).
Individuals were considered to have moved out of state if a request for records from an out-of-state clinic was included in the medical record or if an individual told his or her provider he or she was moving out of state and had no subsequent visits. Death records were obtained from CDPHE and chart review. The data collection included the first 5 years after HIV diagnosis or until July 1, 2011, whichever came first.
Data analyses
We first present the characteristics of the cohort, and then describe the proportions engaged in each step of the HIV care continuum at 18 months after HIV diagnosis using basic descriptive statistics. We used the Chi-square test and Wilcoxon rank-sum test to compare characteristics of individuals with suppressed viral loads to those with unsuppressed viral loads at a given time point. Factors associated with viral suppression at 18 months were evaluated by bivariate and multivariate logistic regression. The multivariate model utilized forward selection. To evaluate engagement-in-care by year of diagnosis, we calculated Pearson correlation coefficients between year of diagnosis and engagement-in-care categories. We compared characteristics of out-of-state migrators with those of nonmigrators using the Chi-square test and Wilcoxon rank-sum test. We used SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC) for all analyses.
Results
Demographics
From 2005 to 2009, 615 individuals were diagnosed with HIV infection through DH and UCD. Most were male (559, 90%) and had an HIV acquisition risk factor of having sex with other men (498, 78%) (Table 1). Significant proportions were Hispanic (205, 33%) or black (96, 16%). The median age at the time of diagnosis was 34 years [interquartile range (IQR) 27–42 years]. Nine women were known to be pregnant at the time of HIV diagnosis. No other women had documentation of pregnancy during the study follow-up period. One-hundred twenty individuals (20%) were foreign-born, 80 of whom were born in Mexico (13% of the total newly HIV-diagnosed cohort). Spanish was the preferred language for health care visits for 76 individuals (12%). The median CD4 lymphocyte count and log10 HIV-1 RNA level at diagnosis were 401 cells/μL (IQR 221–603) and 4.6 copies/mL (IQR 3.9–5.1).
Table 1.
Demographic Characteristics for 615 Newly HIV-Diagnosed Individuals from 2005 Through 2009 at Two Large Public Institutions in Denver, Colorado, USA
| Demographic | Total newly HIV-diagnosed cohort, n=615 |
|---|---|
| Median age at diagnosis | 34 years (IQR 27–42 years) |
| Gender | 9% female |
| 91% male | |
| Race | 81% White (47% Non-Hispanic White) |
| 16% Black | |
| 2% Asian/Pacific Islander | |
| 1% American Indian/Alaskan Native | |
| Ethnicity | 33% Hispanic |
| 64% Non-Hispanic | |
| Place of birth | 20% Foreign-born |
| 80% US-born | |
| Place of diagnosis | 46% STD clinic |
| 24% Outreach | |
| 11% Primary or specialty clinic | |
| 10% Emergency department | |
| 9% Inpatient | |
| HIV acquisition risk factor | 73% MSM |
| 15% Heterosexual | |
| 4% IDU | |
| 5% IDU/MSM | |
| 4% Unknown/other | |
| Median initial CD4 count (cells/mm3) | 40 (IQR 221–603) |
| Median initial log10 HIV-1 RNA (copies/mL) | 4.6 (IQR 3.9–5.1) |
IDU, injection drug user; MSM, men who have sex with men; STD, sexually transmitted diseases.
Two-hundred eighty-two individuals (46%) were diagnosed in the DH STD clinic, ninety-nine individuals (16%) were diagnosed at gay bathhouses, and the rest of the cohort were diagnosed through other outreach services, the emergency departments, inpatient wards, or affiliated clinics of the two hospitals. Two-thirds of individuals in the cohort (n=408) followed-up at the HIV care clinics of the three hospitals with complete record review. One hundred forty-nine individuals (24%) followed-up at other clinics including 20 individuals (3%) with initial follow-up out of state, 63 individuals (10%) participated in clinical trials, and 59 individuals (10%) had no evidence of linkage to HIV care. The median duration of follow-up was 33 months (IQR 16–53).
Engagement-in-care continuum 18 months after HIV diagnosis
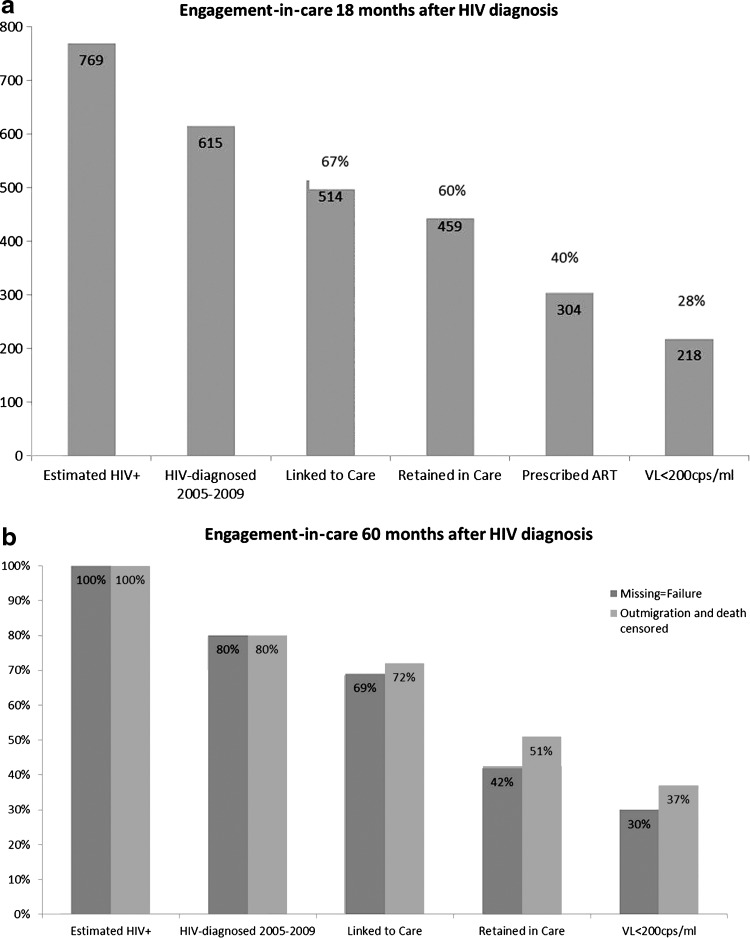
To estimate the total number of HIV-infected individuals in our testing catchment area, we assumed that the 615 individuals who were newly diagnosed in our study period represented 80% of the total HIV-infected individuals in the community. Therefore, we estimated that 769 individuals in our HIV testing area were either newly HIV-diagnosed or HIV-infected but undiagnosed from 2005 to 2009 (Fig. 1a).
FIG. 1.
The engagement-in-care continuum 18 months (a) and 60 months (b) after HIV diagnosis for a cohort of 615 individuals diagnosed with HIV from 2005 through 2009. ART, antiretroviral therapy.
Linkage to care was 70% at 3 months and increased to 84% at 18 months. We evaluated retention in care using several of the definitions that have been proposed. Depending on the definition used, 63–75% of newly-diagnosed individuals were retained in care 18 months after initial HIV diagnosis (Supplementary Table S1; supplementary material is available online at www.liebertpub.com/apc).
Three hundred and three individuals (49% of the newly-diagnosed cohort) were prescribed ART 18 months after HIV diagnosis, and 218 individuals (28% of estimated HIV-infected individuals, 36% of the newly-diagnosed cohort, 72% of those on ART) were virologically suppressed 18 months after diagnosis.
Factors associated with achievement of viral suppression
In bivariate analyses, more recent year of HIV diagnosis, lower initial CD4 lymphocyte count, older age, female gender, and heterosexual acquisition of HIV were associated with greater odds of viral suppression 18 months after HIV diagnosis.
In multivariate logistic regression analyses (Table 2), full engagement-in-care as evidenced by suppressed HIV-1 RNA levels 18 months after diagnosis was associated with more recent year of diagnosis and lower initial CD4 lymphocyte count. Of the 257 individuals with initial CD4 counts ≤350 cells/μL, 140 (54%) had viral suppression 18 months after HIV diagnosis, and of the 137 individuals with an initial CD4 count <200 cells/μL, 85 (62%) were virologically suppressed by 18 months. Older age and female gender were also independently associated with viral suppression 18 months after diagnosis.
Table 2.
Factors Associated with Likelihood of Achieving Suppressed HIV-1 RNA Levels 18 Months After HIV Diagnosis in Cohort of 615 Individuals Diagnosed with HIV from 2005 through 2009
| Variable | Number (%) HIV-1 RNA <200 cps/m | Bivariate OR (95% CI) | Bivariate p value | Multivariate OR (95% CI) | Multivariate p value |
|---|---|---|---|---|---|
| Gender (female vs. male) | Female: 27 (47%) Male: 191 (34%) |
1.7 (1.0–3.0) | 0.05 | 2.0 (1.0–3.7) | 0.04 |
| Age at diagnosis (per 10 year increase) | 16–25: 29 (26%) 26–35: 67 (31%) 36–45: 60 (37%) 46–55: 46 (52%) >55: 16(50%) |
1.5 (1.2–1.7) | <0.001 | 1.4 (1.2–1.7) | <0.001 |
| Race (black vs. non-black) | Black: 41 (43%) Non-black: 177 (34%) |
1.5 (0.9–2.3) | 0.09 | ||
| Risk factor (heterosexual vs. non-heterosexual | Heterosexual: 45 (48%) Non-heterosexual: 173 (33%) |
1.8 (1.2–2.9) | 0.007 | ||
| Baseline CD4 level (per 100 cell increase) | 0–100: 62 (68%) 101–200: 23 (50%) 201–300: 38 (54%) 301–400: 32 (35%) 401–500: 16 (21%) >500: 45 (20%) |
0.7 (0.7–0.8) | <0.001 | 0.8 (0.7–0.8) | <0.001 |
| Foreign-born (yes vs. no) | FB: 51 (41%) Non-FB: 167 (34%) |
1.4 (0.9–2.0) | 0.14 | ||
| Diagnosis year (per 1 year advance 2005–2009) | 2005: 37 (27%) 2006: 38 (30%) 2007: 41 (39%) 2008: 52 (39%) 2009: 50 (45%) |
1.2 (1.1–1.4) | <0.001 | 1.3 (1.1–1.4) | <0.001 |
We evaluated the correlations between linkage to care and viral suppression 18 months after diagnosis among subgroups who received care exclusively at the institutions with complete medical record review. Excluding individuals who never linked to care or who linked more than 18 months after diagnosis, we found that those who linked to care within 3 months were more likely to achieve viral suppression than those who linked between 3 and 18 months after diagnosis (45% vs. 29%, p=0.005). Of those who linked to care after 90 days but within a year of diagnosis, the percentage of individuals with viral suppression at 18 months remained statistically significantly lower than those who linked within 90 days (32% vs. 45%, p=0.03).
Correlation between retention in care and viral suppression by 18 months after diagnosis was limited by the short time interval for these analyses. Among those who linked to care within 6 months of HIV diagnosis (473 individuals), 398 had good early retention as defined by a lab or visit in months 6 through 12 after HIV diagnosis. Of those with good early retention, 52% were virally suppressed by 18 months after HIV diagnosis compared to 5% of those with poor early retention (p<0.001). When censoring the group with poor early retention for outmigration and death, 12% were virally suppressed by 18 months after HIV diagnosis (p<0.001).
Engagement-in-care 5 years after HIV diagnosis
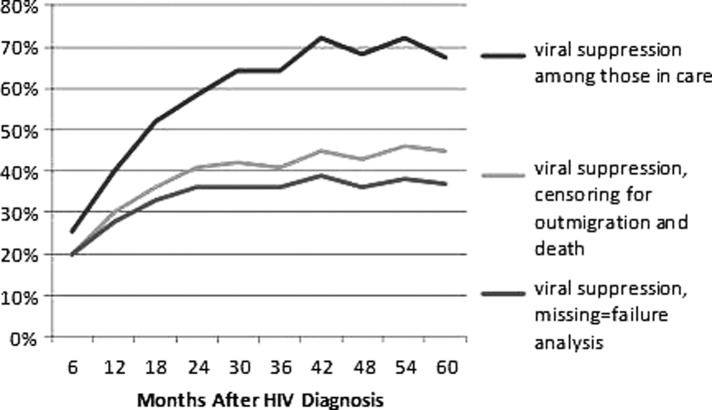
The proportion of individuals engaged in HIV care changed with follow-up beyond 18 months. By 24 months after HIV diagnosis, 65% of the newly-diagnosed cohort were known to be retained in care, and that percentage did not differ significantly in the subsequent years of follow-up (Supplementary Fig. S1). Similarly, the percentage of HIV-diagnosed individuals with suppressed HIV-1 RNA levels increased over the first 2 years and then stabilized at 42–45% (Fig. 2).
FIG. 2.
Percentages of newly HIV-diagnosed individuals with viral suppression in the 5 years following HIV diagnosis, comparing results when censoring for outmigration and death.
Outmigration and death
By 5 years after HIV diagnosis, 15% of the original cohort had moved out of state and 3% had died. Of the HIV-diagnosed individuals alive and residing in the state of Colorado 5 years after diagnosis, 66% were engaged in care and 45% were virologically suppressed (Supplementary Fig. S1). Figure 1b displays the care continuum at 5 years after diagnosis for individuals in the cohort, and accounts for the additional 20% of individuals presumed to be HIV-infected but undiagnosed. Figures 1b and 2 show the effect of censoring for outmigration and death on the estimate of effective engagement in care, as evidenced by viral suppression.
The demographic characteristics of the migrators did not vary significantly from those who did not outmigrate. Specifically, there were no significant differences between migrators and nonmigrators by gender, median age at time of diagnosis, race, ethnicity, foreign- versus US-born, test location, median initial CD4 lymphocyte level, or median initial HIV-1 RNA level (data not shown).
Changes in the engagement continuum by year of diagnosis
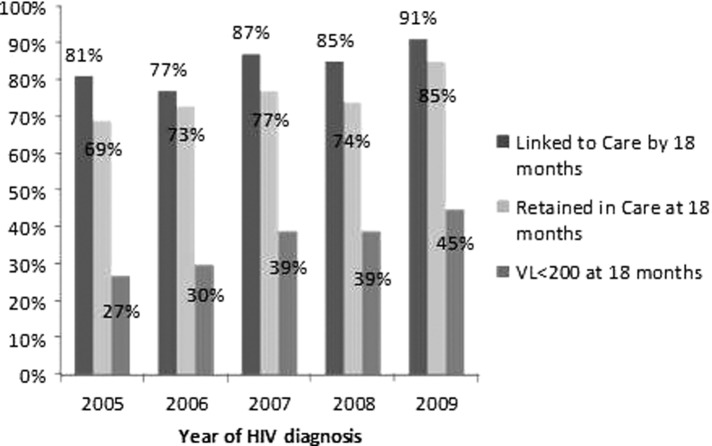
Linkage to care, retention in care, and attainment of viral suppression 18 months after HIV diagnosis all improved significantly with each subsequent year of HIV diagnosis from 2005 through 2009 (Fig. 3). Of those diagnosed in 2005, 81% linked to care in 18 months, 39% were on antiretroviral therapy at 18 months, and 27% had suppressed HIV-1 RNA levels 18 months after diagnosis. Of those diagnosed in 2009, 90% linked to HIV care within 18 months of diagnosis, 58% were prescribed antiretroviral therapy, and 45% had suppressed HIV-1 RNA levels 18 months after diagnosis.
FIG. 3.
Proportions of newly HIV-diagnosed individuals engaged in care 18 months after HIV diagnosis, by year of HIV diagnosis, for a cohort of individuals newly HIV-diagnosed from 2005 through 2009.
Discussion
To monitor progress toward achieving the goals of the National HIV/AIDS Strategy, accurate estimates of engagement in HIV care are crucial. Using clinical follow-up through three major HIV care providers, two research sites, and a statewide surveillance data, we evaluated long-term engagement-in-care for a large cohort of newly HIV-diagnosed individuals in an urban setting in the United States. Our analysis demonstrates that estimates of engagement-in-care vary markedly depending on the completeness of available data. Using clinic records, we found that outmigration, either at the time of initial diagnosis or later, was common (15% of the cohort). Similarly, surveillance laboratory data showed that movement of care to sites other than the three large clinics included in the study was also common (24% of the cohort). We also accessed clinical trial records for the 10% of the cohort who participated in clinical trials. These records are not included in mandated state databases in Colorado but revealed additional evidence of engagement-in-care. Without these three sources of data, we would have markedly underestimated engagement-in-care. Finally, we showed that all engagement parameters improved steadily for individuals newly HIV-diagnosed over the years of the study (2005–2009).
Linkage to care over time
Linkage to care occurred within 3 months for 70% of the newly-diagnosed cohort, but had increased to 79% by the last year of the study (2009). These linkage rates are consistent with meta-analyses reported by Marks et al., in which an average of 72% of HIV-infected individuals linked to care within 4 months of diagnosis.18 However, they fall short of the National HIV/AIDS Strategy goal of linkage to care for 85% of newly-diagnosed HIV-infected individuals within 3 months,22 a goal already met by some HIV care centers.23
Retention in care over time
Our calculations of retention in care varied greatly with time since diagnosis and when censoring for outmigration and death. We calculated proportions of newly HIV-diagnosed individuals retained in care as low as 52% 4 years after HIV diagnosis in a missing-equal-failure analysis and when we required a visit in the prior 6 months to be considered “retained.” When we liberalized the definition of retention in care to one visit in the prior 12 months and censored the cohort for outmigration and death, the proportion of individuals retained in care 4 years after HIV diagnosis was 71%, nearly 20% higher. This difference in the estimates of retention in care likely underlies some of the wide variation (45–81%) in published estimates of retention. The higher estimates required only one visit in the prior 1 year and/or censored the denominators for outmigration, while lower estimates were derived from definitions requiring at least one visit in multiple 6-month intervals or from state databases alone.4,5,17,18,23–25
Out-of-state migration
In the entire US population, rates of out of state migration are approximately 2% per year.26 Hence, in a 5-year interval, we could expect that up to 10% of the overall US population moved between states. Estimates of outmigration among HIV-infected cohorts report 5–25% of the cohort outmigrated from the study jurisdiction, though not necessarily the state during the study interval.10–12,14,23,27,28 To our knowledge, outmigration data obtained through chart review has not been previously described for a cohort of newly HIV-diagnosed individuals. By 5 years after HIV diagnosis, we determined that 15% of the original cohort had moved out of state, 8% in the first year after diagnosis and between 2% and 3% in each subsequent year. This is comparable to the findings reported by Buskin and colleagues in which 1196 individuals of a cohort of 8484 people living with HIV/AIDS (14%) were determined to have relocated, leading to a reduction in percentage of individuals thought to be out of care from 27% to 16%.12 We believe that our figures underestimate true outmigration as documentation of outmigration may not be present in the medical record for all individuals who moved out of state. Although our data may differ from the migration patterns of HIV-infected individuals in other geographic areas, we hope it underscores the impact of outmigration on retention in care estimates. We believe a rigorous evaluation of the impact of migration on HIV engagement-in-care estimates at the national level is necessary to help further clarify our findings.
Attainment of suppressed HIV-1 RNA levels
We also noted significant variations in proportions of the cohort with suppressed HIV-1 RNA levels based on time since diagnosis and denominator for the population evaluated. Although only 36% of the newly-diagnosed cohort were virologically suppressed 18 months after diagnosis (28% of the estimated total HIV-infected population), 45% of the newly-diagnosed cohort were virologically suppressed 5 years after HIV diagnosis (adjusted for outmigration and death), which was 37% of the estimated total HIV-infected population at that time interval. This is significantly higher than national estimates of suppressed HIV-1 RNA levels which range from 19% to 25% of HIV-infected individuals.4,5,7 To better determine the true percentage of HIV-infected individuals with suppressed HIV-1 RNA levels, a process that considers outmigration, death, and changes in ART use over time should be employed.
We attribute improved performance in the engagement continuum by year of diagnosis (Fig. 3) to better linkage to care programs, more aggressive retention efforts at the clinic level, and changes to the HIV treatment guidelines in the past few years. Studies by Dombrowski and colleagues8 and Montaner and colleagues29 have demonstrated higher rates of viral suppression in more recent years. Our analysis of viral suppression by year of diagnosis suggests that trends of engagement-in-care and ART use among more recently diagnosed individuals may be impacting the higher rates of viral suppression overall, consistent with findings by Axelrad and colleagues,30 in which individuals diagnosed in 2010 and retained in care were more likely to achieve viral suppression 1 year after HIV diagnosis than individuals diagnosed in 2000 and retained in care.
This study had three significant limitations. First, this was a retrospective study that relied on documentation in medical records, clinical trials records, and statewide databases. Second, because our estimate of migration outside the state of Colorado was based on medical records at just three clinics, it is likely that we underestimated outmigration. Third, though the state laboratory surveillance system provides a valuable tool for monitoring engagement in care, reporting of CD4 lymphocyte counts above 500 cells/μL was not mandatory until January 2014, and mandatory reporting of suppressed HIV-1 RNA levels did not start until April 2010. Therefore, we may have underestimated engagement-in-care among persons receiving care at clinics other than the three with complete medical record review in the study. However, of the 129 individuals who followed-up at sites in-state for whom we did not have complete medical record review, fewer than 10% of these individuals were seen at sites that did not report all viral load and CD4 lymphocyte results.13
In conclusion, our findings underscore the variability in engagement-in-care calculations when actual patient data is used for analyses. While large cross-sectional studies provide snapshots of the HIV epidemic in the US, following patient data simultaneously through state databases and chart review over longer periods of time may elicit more precise and nuanced information. For example, knowing that retention in care decreases after the first 2 years after HIV diagnosis may help target efforts to improve engagement-in-care in that time interval, ultimately improving proportions of individuals retained in care in later years. Because retention in care is associated with increased likelihood of viral suppression, efforts at this stage would likely have downstream effects.15,31,32 Similarly, as proportions of individuals with suppressed viral loads are lower in the first 2 years after diagnosis, starting antiretroviral therapy sooner after HIV diagnosis could improve rates of viral suppression earlier, possibly decreasing new infections, particularly from those recently infected.2,33
A means to facilitate better data collection and help states to account for outmigration in their HIV-infected populations is needed to prevent underestimates of engagement-in-care at the state level. A real-time, updated database of current addresses shared between states could address this problem. Additionally, further investigation of outmigration effects in other jurisdictions would corroborate our findings and help define reasonable estimates for modifications to engagement estimates when outmigration data are not available. Finally, we demonstrate that substantial improvements in estimates of engagement-in-care are possible with accurate data collection and we document actual improvements along the continuum in more recent years.
Supplementary Material
Acknowledgments
Dr. Rowan compiled and analyzed the database for this study. She prepared the article. Dr. Burman assisted with study design, analyses, and critical review and revision of the article. Dr. Johnson assisted with study design, reviewed and revised the article. Dr. Connick oversees the clinical trials unit at University of Colorado Denver, one of the sites from which data for the study was obtained. She also reviewed the article. Dr. Reirden assisted with collection of data from Children's Hospital Colorado and reviewed the article. Dr. Daniloff provided data from the statewide laboratory reporting database for individuals included in the study. She also reviewed the article. Dr. Gardner assisted with data collection and study design. He performed statistical analyses and provided critical review and revision of the article.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases T32 AI07447 (SR), K01 AI067063 (EG), and P01 AI55356 (EC), as well as through UL1 TR00015 and UM1 A069450.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bhaskaran K, Hamouda O, Sannes M, et al. . Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 2008;300:51–59 [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buscher A, Mugavero M, Westfall AO, et al. . The association of clinical follow-up intervals in HIV-infected persons with viral suppression on subsequent viral suppression. AIDS Patient Care STDs 2013;27:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks G, Gardner LI, Craw J, et al. . The spectrum of engagement in HIV care: Do more than 19% of HIV-infected persons in the US have undetectable viral load? Clin Infect Dis 2011;53:1168–1169; author's reply 1169–1170. [DOI] [PubMed] [Google Scholar]
- 6.CDC Fact Sheet: HIV in the United States: The Stages of Care. 2012
- 7.Mahle Gray K, Tang T, Shouse L, Li J, Mermin J, Hall HI. Using the HIV surveillance system to monitor the National HIV/AIDS Strategy. Am J Public Health 2013;103:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombrowski JC, Kitahata MM, Van Rompaey SE, et al. . High levels of antiretroviral use and viral suppression among persons in HIV care in the United States, 2010. J Acq Immune Defic Synd 2013;63:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieb S, Friedman SR, Zeni MB, et al. . An HIV prevalence-based model for estimating urban risk populations of injection drug users and men who have sex with men. J Urban Health: Bull NY Acad Med 2004;81:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agee BS, Funkhouser E, Roseman JM, Fawal H, Holmberg SD, Vermund SH. Migration patterns following HIV diagnosis among adults residing in the nonurban Deep South. AIDS Care 2006;18:S51–S58 [DOI] [PubMed] [Google Scholar]
- 11.Berk ML, Schur CL, Dunbar JL, Bozzette S, Shapiro M. Short report: Migration among persons living with HIV. Soc Sci Med 2003;57:1091–1097 [DOI] [PubMed] [Google Scholar]
- 12.Buskin SE, Kent JB, Dombrowski JC, Golden MR. Migration distorts surveillance estimates of engagement in care: Results of public health investigations of persons who appear to be out of HIV care. Sex Trans Dis 2014;41:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner EM, Daniloff E, Thrun MW, et al. . Initial linkage and dubsequent tetention in HIV vare for a newly fiagnosed HIV-infected vohort in Denver, Colorado. J Intl Assoc Providers AIDS Care 2013;12:384–390 [DOI] [PubMed] [Google Scholar]
- 14.Cohn SE, Klein JD, Mohr JE, van der Horst CM, Weber DJ. The geography of AIDS: Patterns of urban and rural migration. South Med J 1994;87:599–606 [DOI] [PubMed] [Google Scholar]
- 15.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: A statewide study. AIDS Res Human Retroviruses 2011;27:751–758 [DOI] [PubMed] [Google Scholar]
- 16.Tripathi A, Gardner LI, Ogbuanu I, et al. . Predictors of time to enter medical care after a new HIV diagnosis: A statewide population-based study. AIDS Care 2011;23:1366–1373 [DOI] [PubMed] [Google Scholar]
- 17.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acq Immune Defic Syndr 2012;60:77–82 [DOI] [PubMed] [Google Scholar]
- 18.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: A meta-analysis. AIDS 2010;24:2665–2678 [DOI] [PubMed] [Google Scholar]
- 19.CfDC, Prevention. HIV surveillance–United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011;60:689–693 [PubMed] [Google Scholar]
- 20.Centers for Disease C, Prevention. Prevalence and awareness of HIV infection among men who have sex with men—21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep 2010;59:1201–1207 [PubMed] [Google Scholar]
- 21.Prevention CfDCa. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data-United States and 6 U.S. Dependent Areas-2010. 2013
- 22.Federal Implementation Plan In: Policy WHOoNA, ed2010 [Google Scholar]
- 23.Dombrowski JC, Kent JB, Buskin SE, Stekler JD, Golden MR. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS 2012;26:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005–2009: Do patients who initiate care stay in care? AIDS Patient Care STDs 2011;25:79–88 [DOI] [PubMed] [Google Scholar]
- 25.Ulett KB, Willig JH, Lin HY, et al. . The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs 2009;23:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geographic Mobility/Migration. 2012. http://www.census.gov/hhes/migration/
- 27.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost—or just not following up?: Public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS 2013;27:2271–2279 [DOI] [PubMed] [Google Scholar]
- 28.Harris NS, Dean HD, Fleming PL. Characteristics of adults and adolescents who have migrated from place of AIDS diagnosis to place of death, United States, 1993–2001. AIDS Educ Prev 2005;17:39–48 [DOI] [PubMed] [Google Scholar]
- 29.Montaner JS, Lima VD, Harrigan PR, et al. . Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV Treatment as Prevention” experience in a Canadian setting. PloS One 2014;9:e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axelrad JE, Mimiaga MJ, Grasso C, Mayer KH. Trends in the spectrum of engagement in HIV care and subsequent clinical outcomes among men who have sex with men (MSM) at a Boston community health center. AIDS Patient Care STDs 2013;27:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano TP, Gifford AL, White AC Jr., et al. . Retention in care: A challenge to survival with HIV infection. Clin Infect Dis 2007;44:1493–1499 [DOI] [PubMed] [Google Scholar]
- 32.Mugavero MJ, Amico KR, Westfall AO, et al. . Early retention in HIV care and viral load suppression: Implications for a test and treat approach to HIV prevention. J Acq Immune Defic Syndr 2012;59:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner BG, Roger M, Routy JP, et al. . High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 2007;195:951–959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.