Diagnostic nano- and microparticles are favorably suited for labeling the mononuclear phagocytic system (eg, liver, spleen, lymph nodes), and the use of diagnostic nanoparticles is indicated for ex vivo labeling of cells, scaffolds, and other kinds of tissue-engineered implants that are to be visualized in vivo.
Abstract
Nanoparticles are frequently suggested as diagnostic agents. However, except for iron oxide nanoparticles, diagnostic nanoparticles have been barely incorporated into clinical use so far. This is predominantly due to difficulties in achieving acceptable pharmacokinetic properties and reproducible particle uniformity as well as to concerns about toxicity, biodegradation, and elimination. Reasonable indications for the clinical utilization of nanoparticles should consider their biologic behavior. For example, many nanoparticles are taken up by macrophages and accumulate in macrophage-rich tissues. Thus, they can be used to provide contrast in liver, spleen, lymph nodes, and inflammatory lesions (eg, atherosclerotic plaques). Furthermore, cells can be efficiently labeled with nanoparticles, enabling the localization of implanted (stem) cells and tissue-engineered grafts as well as in vivo migration studies of cells. The potential of using nanoparticles for molecular imaging is compromised because their pharmacokinetic properties are difficult to control. Ideal targets for nanoparticles are localized on the endothelial luminal surface, whereas targeted nanoparticle delivery to extravascular structures is often limited and difficult to separate from an underlying enhanced permeability and retention (EPR) effect. The majority of clinically used nanoparticle-based drug delivery systems are based on the EPR effect, and, for their more personalized use, imaging markers can be incorporated to monitor biodistribution, target site accumulation, drug release, and treatment efficacy. In conclusion, although nanoparticles are not always the right choice for molecular imaging (because smaller or larger molecules might provide more specific information), there are other diagnostic and theranostic applications for which nanoparticles hold substantial clinical potential.
Introduction
Nano- and microparticles based on polymers, proteins, lipids, lipoproteins, metals, and silica, as well as fullerenes, carbon nanotubes, selenium-cadmium nanocrystals (ie, quantum dots), and microbubbles, are frequently suggested as diagnostic and theranostic agents. In general, one can distinguish between nanodiagnostics (1 nm to <1 µm) and microdiagnostics (1 µm to <1 mm; however, most microdiagnostics are 1–5 µm) on the basis of their size. Theranostic nanomedicines are nanoparticles that act as both diagnostic and therapeutic agents. In this context, depending on the particle design, the obtained diagnostic information can be different, for example, providing insights into the availability of a molecular target in the tissue, the vascular permeability and retention of the molecule, the drug release from the particle, and the response of the target tissue. Several review articles about these materials, their synthesis, and their functionalization are available (1–6), and it would go beyond the scope of this review article to discuss these issues in detail. Thus, much of the pioneering work from the 1970s, 1980s, and 1990s on lipoproteins and numerous other key particulate-based contrast agents could not be considered.
Rather, the aim of this article is to discuss for which clinical applications diagnostic and theranostic nano- and microparticles are promising and which chemical, pharmacologic, and medical demands must be considered during their conception, preclinical evaluation, and clinical translation.
Nanoparticles
Many different types of nanoparticles have been designed and evaluated over the years. Initially, systems such as liposomes, polymers, proteins, dendrimers, and micelles were primarily used for therapeutic purposes, that is, for more efficient delivery of (chemo-)therapeutic drugs to pathologic sites, while reducing their accumulation in potentially endangered healthy tissues. Currently, several different therapeutic nanoparticles are routinely used in the clinic, including, for example, Doxil (Janssen, Horsham, Pa), Ambisome (Gilead, Foster City, Calif), and Abraxane (Celgene, Summit, NJ), and ever more of these so-called nanomedicine formulations are being evaluated in preclinical and clinical trials (7–10).
Progress in the use of nanoparticles for diagnostic purposes has been somewhat slower. It took approximately 2 decades longer before the first nanodiagnostics were described, and their clinical translation has been somewhat less successful, with only a few formulations finding their way into routine clinical practice. As will be outlined below, despite substantial progress in synthesizing state-of-the-art nano- and microdiagnostics, there are a number of reasons why the bench-to-bedside translation of these advanced materials has been limited (9).
At first, it might be helpful to consider some key differences between nanotherapeutics and nanodiagnostics. Although the former are intended to have pharmacologic activity, the latter should not generate physiologic or pathophysiologic effects. Depending on the disease to be treated, it is acceptable for the former to stay around in the body for prolonged periods of time and to have certain side effects because their main purpose is therapy of a possibly life-threatening disease. For the latter, on the other hand, unless being applied in low, pharmacologically inactive doses (as is possible for several positron emission tomography [PET] agents), rapid biodegradation and/or elimination are strongly preferred, and the incidence and intensity of side effects must be minimal, because these are potentially also administered to patients who may be disease-free, including children. Consequently, nanodiagnostics must be excreted or metabolized relatively quickly after local, oral, or parenteral administration and they must be completely devoid of pharmacologic and/or toxicologic activity.
The intrinsic properties of nanoparticles, however, do not comply well with these pharmacokinetic and pharmacodynamic requirements. Given their size, which ranges from a few nanometers for small polymers and dendrimers to more than 1000 nm for microbubbles (Fig 1), the biodistribution of nanoparticles tends to be confined to the compartment in which they are injected. If administered locally, they tend to stay around at the site of injection and are then slowly cleared via lymphatic drainage. If given orally, nanoparticles generally remain exclusively within the gastrointestinal tract and are excreted via the feces. And if given intravenously, depending on their size, charge, and surface properties (eg, stealth coating), the biodistribution of nanoparticles tends to be restricted to the vascular system and to organs with a fenestrated endothelium, such as liver and spleen, as well as to tumors and sites of inflammation, which are also characterized by fenestrated endothelium and enhanced vascular leakiness. For intravenously administered nanotherapeutics, these features generally are beneficial. The larger size of nanoparticles generally results in longer time spent in the circulation system, and the usually intact vascular endothelium limits their accumulation in the majority of healthy tissues, thereby potentially reducing side effects. At the same time, the abnormal and leaky neovasculature of tumors and inflammation allows the particles’ extravasation by means of the enhanced permeability and retention (EPR) effect, thereby potentially increasing therapeutic efficacy (7–9,11–13).
Figure 1:
Representative examples of nanoparticles (NP) frequently used for diagnostic and theranostic purposes. USPIO = ultrasmall superparamagnetic iron oxide.
Conversely, these properties are not necessarily beneficial for intravenously administered nanodiagnostics (Fig 2). They do, on the one hand, enable semispecific EPR-mediated accumulation in tumors and inflammatory lesions over time. EPR, however, is based on the long-circulating properties of nanoparticles and therefore gradually increases over time, peaking somewhere between 12 and 48 hours. Consequently, nanoparticles are unable to achieve the rapid and highly site-specific contrast enhancement that is necessary for efficient disease diagnosis, and they are routinely outperformed by “standard” low-molecular-weight imaging agents when it comes to generating high signal-to-noise levels within a reasonably short period of time. These small molecules are retained at pathologic sites to a lesser extent than nanoparticles but are also excreted from the vascular system and the body much more rapidly. Additional issues limiting the applicability of systemically administered nanoparticles for diagnostic purposes relate to (a) their poor tissue-penetrating properties (because of their relatively large size, thereby constraining their access to target cells located farther away from blood vessels), (b) their prolonged residence times in certain organs (in particular in liver and spleen, potentially giving rise to long-term side effects upon repetitive administration), and (c) their composition (heavy metal–based quantum dots, for instance, will always raise concerns about biocompatibility). There are, however, a number of applications for which nano- and micrometer-sized diagnostics do hold substantial potential. As will be discussed in more detail below, these include their use as blood pool contrast agents and their use in imaging of the MPS, labeling and tracking of (stem) cells, monitoring of (tissue-engineered) implant materials, molecular imaging of vascular targets, facilitating interventional radiologic treatments, and theranostic approaches that aim to personalize nanomedicine treatments by means of the combination of drug targeting and imaging.
Figure 2:
Blood half-life, extravasation, renal clearance, mononuclear phagocytic system (MPS) uptake, immunologic response, and flexibility to functionalize are important characteristics of imaging probes and theranostic compounds. Diagram illustrates how these properties usually change depending on probe size. Note that an absolute generalization of probe behavior based on size is not possible, because its composition, surface charge, and functionalization can also have a substantial influence on these properties. * = Strongly depends on surface properties of particles. ** = Option to increase detection sensitivity by attaching multiple imaging markers per molecule or strong endogenous imaging properties of the particle (eg, USPIO, microbubble).
Nanoparticles as Diagnostic Probes
Nanoparticles as Blood Pool Contrast Agents
The visualization of small and larger blood vessels is of high diagnostic relevance for many diseases, including atherosclerosis, thrombosis, venous insufficiency, vascular inflammation, and cancer. In general, there are two major strategies for achieving good delineation of vessels with contrast agents. First, low-molecular-weight clinical contrast agents (eg, gadolinium chelates or iodinated molecules) can be used if the imaging time is short enough to image the region of interest during the first pass of the contrast agent, which is mostly intravascular in this early phase. However, this approach requires optimal timing between contrast agent injection and image execution and often needs compromises to achieve the required spatial resolution and to optimize the contrast-to-noise ratio. Alternatively, contrast agents with long blood half-lives can be used, and these do not require complex and timely imaging strategies. In particular, in small animal imaging, the latter concept is frequently applied because of the demand for imaging with high spatial resolution and an excellent contrast-to-noise ratio in imaging units requiring longer imaging times (eg, most small animal computed tomographic [CT] units do not yet provide spiral acquisitions). In addition, circulation times of contrast agents in mice are much shorter than those in humans, making first-pass measurements more difficult.
For magnetic resonance (MR) imaging, a multitude of positive (T1) and negative (T2) nano-sized contrast agents have been developed, and few of them have entered clinical evaluation. As positive MR contrast agents (bright at T1-weighted imaging), gadolinium-loaded polymers (eg, poly-lysine dendrimers, N-[2-hydroxypropyl]methacrylamide copolymers) (4) and paramagnetic liposomes (6), as well as very small superparamagnetic iron oxide (SPIO) nanoparticles such as citrate-coated very small SPIO nanoparticles (14), were successfully applied in preclinical and/or clinical trials. Unfortunately, none of these agents was finally approved for clinical use. Reasons for the lacking final clinical translation are as follows: (a) pharmacologic concerns, (b) the competition from MR angiography methods that do not require contrast agent injection (eg, time of flight and hybrid of opposite-contrast [15] techniques), (c) competition with low-molecular-weight agents with high plasma protein binding affinity (eg, gadofosveset) (16), and (d) the small return of investment of the costs for getting the U.S. Food and Drug Administration approval considering the considerably small market of the designated applications.
The lack of clinical implementation of macromolecular MR contrast agents is unfortunate because, in preclinical studies, at least for the assessment of functional vascular information in tumors with dynamic contrast material—enhanced MR imaging, these agents have shown substantial added value over most clinically available low-molecular-weight agents because they show slower and more “tumor-specific” extravasation, decreasing the required temporal resolution on dynamic images, and facilitating the differentiation of perfusion and permeability effects (4,17). This is primarily because of their slower extravasation and relatively stable relaxivities. Small protein-binding gadolinium chelates such as gadofosvoset and gadocoletic acid (4) change their relaxivity significantly when interacting with proteins, making it difficult to quantify their concentration in tissues.
Negative MR contrast agents (strong darkening on T2- and T2*-weighted images) with blood pool properties are frequently used for blood volume mapping in tumors (18,19) and for steady-state “vessel size imaging,” with the latter being an MR imaging method to determine the mean vessel diameter in tissues (20,21). Usually, these are USPIO nanoparticles like Sinerem, Feridex, Feraheme, and Supravist (22). Because these nanoparticles are predominantly used for MPS imaging, more details will be given in the next paragraph.
Nano-sized blood pool contrast agents have also been developed and commercialized for preclinical CT imaging. Most of them are “soft” nanoparticles based on iodinated liposomes (23–26), polymers (27,28), and micelles (29,30), with hydrodynamic diameters of approximately 50–150 nm. Iodine concentrations of 50–160 mg of iodine per milliliter in the injection solution provide good intravascular contrast for more than 30 minutes until the agents are predominantly metabolized in liver and spleen. Unfortunately, some of these agents are not well tolerated by animals, which is particularly true at higher doses and in animals receiving a cholesterol-rich diet.
In addition to soft nanoparticles, metal-based nanoparticles can also generate excellent contrast for CT imaging. For example, polyvinylpyrrolidone-coated bismuth nanoparticles (31), bismuth oxide–human serum albumin core-shell nanoparticles (32), barium-ytterbium-fluor nanoparticles with silica and polyethylene glycol coating (33), and different gold nanoparticles with organic and inorganic coatings have been suggested (2). Today, long-circulating gold nanoparticles with organic coatings and good biotolerability are commercially available for preclinical CT under the trade name AuroVist (Nanoprobe, Yaphank, NY). Side effects and the long intracorporal persistence may be reasons why macromolecular CT contrast agents have not yet been developed for clinical use so far. In addition, image acquisition times of current clinical CT scanners have become so short that first-pass examinations with clinical low-molecular-weight contrast agents (with injected iodine concentrations of 300–400 mg of iodine per milliliter) can easily be performed over large regions of the body, rendering blood pool CT contrast agents superfluous for most clinical applications.
Microbubbles are clinically established as blood pool contrast agents for ultrasonography (US) and consist of 1–3-μm large gas bubbles stabilized by phospholipids, polymers, sugars, or denaturated proteins. Microbubbles can be detected with US with high sensitivity and specificity, and they are used to visualize microvessels and to quantify perfusion with destruction replenishment techniques (34).
Imaging the MPS
Most nanoparticles are ideal candidates for imaging of the MPS (formerly reticuloendothelial system), which consists of liver, spleen, and the lymphatics, because these are the main tissues most particles are being taken up in (Fig 3). The reason for this natural targeting of particles to the MPS lies in the high content of macrophages (Kupffer cells in the liver) in these organs. A wide variety of particles end up in the MPS, and nanoparticle-based MPS imaging agents have been suggested for various modalities, including MR imaging (35–41), CT (24–26,28,30–32), single photon emission CT (SPECT) (42), optical imaging (43), photoacoustic imaging (44), and even US (45,46). In particular for sentinel lymph node detection, where the diagnostic probes are injected into the area of the tumor and then accumulate in the draining lymph node(s) by means of passive or active transport, micro- and nanoparticles proved to be highly suitable. This holds true for iron oxide nanoparticles (MR imaging [41]), quantum dots (optical imaging [43]), gold nanocages (photoacoustic imaging [44]), and microbubbles (US [45,46]).
Figure 3:
Use of nanoparticles for MPS imaging. A, MR image shows that liver macrophages strongly incorporate SPIO, leading to decrease in signal intensity of normal liver tissue and improving delineation of hepatocellular carcinoma (arrow). (Reprinted, with permission, from reference 35.) B, CT scan shows that iodinated polymeric 2-methacryloyloxyethyl(2,3,5-triiodobenzoate) nanoparticles accumulate strongly in liver macrophages, generating positive contrast. Thus, liver metastases in a metastatic mouse model can be differentiated as unenhanced areas (arrows). (Reprinted, with permission, from reference 28.) C, MR image shows that USPIO nanoparticles can help differentiate metastatic (arrows) and healthy (arrowheads) lymph nodes in patient with tonsillar carcinoma. (Reprinted, with permission, from reference 36.) D, T2*-weighted MR image of inflammatory lesions after USPIO injection shows that macrophage-rich atherosclerotic plaque (arrow) can be detected owing to its strong decrease in signal intensity. (Reprinted, with permission, from reference 37.)
However, only iron oxide nanoparticles (eg, USPIO nanoparticles) have so far been used for clinical MPS imaging. These particles allow for MR imaging owing to their superparamagnetic nature. Their polymer-coated (often a dextran variant) iron cores induce proton dephasing and thus decrease the signal intensity on T2-weighted images in addition to creating local heterogeneities in the magnetic field, generating local susceptibility changes and thus decreases in the T2* signal, which allows their use as an MPS-specific MR imaging contrast agent (47). A few SPIO and USPIO nanoparticles have transitioned into clinical trials and have also entered for some time in routine use, especially in liver imaging. To our knowledge, however, it appears that—except for the oral iron oxide contrast agent Lumirem or Gastromark, which was approved by the U.S. Food and Drug Administration in 1996, and the iron replacement agent Feraheme, which was approved in 2009— none of these nanoparticles are available on the European and U.S. market. Resovist is, as of writing this review, only sold in Japan, and Feridex, Sinerem, and Clariscan have been completely removed from the market or discontinued for various reasons, with most of those reasons being regulatory and marketing issues.
Like virtually all other nanoparticles larger than approximately 5–10 nm, SPIO and USPIO nanoparticles are cleared from the blood by means of phagocytosis through macrophages (eg, Kupffer cells), resulting in uptake in liver, spleen, and lymph nodes (48) (Fig 3, C). In the liver, this uptake only occurs in areas of normal liver tissue hosting Kupffer cells. Most abnormalities (eg, scarring, regeneration, and shunting in cirrhotic liver, as well as other inflammatory or infectious processes) reduce uptake of SPIO and leave a bright defect in the otherwise signal-decreased liver parenchyma on T2-weighted images. In malignant liver tumors, Kupffer cells are displaced by tumor cells; thus, these lesions lack T2 decrease on MR images, which helps clearly delineate them from the surrounding normal liver with decreased T2 (35,49) (Fig 3, A). In particular for the detection of liver metastasis (eg, of colorectal cancer), SPIO-enhanced liver MR imaging has been shown to be highly sensitive (50,51). Kupffer cells still present in early stages of hepatocellular carcinoma can sometimes cause particle uptake similar to that seen in hepatic adenomas (52). All iron oxide nanoparticles essentially increase tumor-to-liver contrast in malignant lesions and allow detection of more lesions then at unenhanced MR imaging on T2-weighted images (53) because the nanoparticles cause a signal decrease in benign lesions with either Kupffer cells or a substantial blood pool. However, the degree of uptake in focal nodular hyperplasia, regenerating nodules, and adenomas may vary depending on the number of Kupffer cells present in these lesions (54). In this context, it is worth mentioning that the positive contrast of SPIO during its blood pool phase can support diagnosis finding by depicting, for example, early arterial feeding in small hepatomas or a gradual centripetal fill-in pattern in hemangiomas.
Iron oxide particles are also rapidly taken up by lymph nodes as part of the MPS (Fig 3, C). In particular, particles with long circulation times slowly traverse into the lymphatics, either directly or via phagocytosing mononuclear cells, to ultimately end up in the lymph nodes (55). The presence of the iron oxide particles causes a decrease in signal intensity on T2- and T2*-weighted MR images. If parts (micrometastases) or entire lymph nodes are replaced by tumor cells, then the phagocytozing capability of the node is severely reduced in this region of the node and the expected signal intensity decrease is absent. Thus, normal lymph nodes are void of signal on T2- or T2*-weighted images, whereas metastases in the nodes are highlighted by a nondecreased, bright T2 signal (Fig 3, C). Contrast-enhanced MR lymphography with these agents has been shown to improve the detection of lymph node metastasis and the differentiation between reactive and metastatically infiltrated lymph nodes in patients with prostate cancer (38,56), breast cancer (57), head and neck tumors (36), and renal cancer (58). However, all of these studies were on off-label use of these agents.
The strong uptake of USPIO by MPS cells can also be used to delineate and characterize inflammatory lesions such as atherosclerotic plaques (37) (Fig 3, D). Here, the uptake of particles mostly in lesional macrophages and monocytes leads to good contrast at MR imaging (59). However, for applications like these (imaging inflammation, plaques) it is important to know that various monocyte subsets internalize not all particles with the same avidity, therefore resulting in variable loading efficacy and thus a signal intensity change at MR imaging, possibly also reflecting different biologic properties (60). Successful preclinical imaging with iron oxide nanoparticles has also been achieved to image transplant rejection (61) and tissue repair processes such as postischemic lesions in the brain (39) and heart (62).
MR imaging with iron oxide nanoparticles has been used in animals and ultimately in patients to visualize insulitis as early manifestation of type I diabetes, where an autoimmune process leads to an inflammatory destruction of the islet cells. The accompanying inflammatory reaction is accompanied by microvascular changes, particularly vessel leakiness (63). Long-circulating nanoparticles extravasate out of the leaky vessels into the surrounding tissue and are taken up by residing macrophages. MR imaging quantification of particle accumulation in the pancreas could help differentiate patients with recent-onset diabetes from nondiabetic control subjects (64).
Cell Labeling and Tracking
Cell tracking can be performed by means of either direct or indirect cell labeling (65–67). In direct cell labeling approaches, the cells are labeled ex vivo with the imaging markers and then transplanted. Indirect cell-labeling methods take advantage of reporter genes that make the cells produce or accumulate imaging markers after their transplantation in vivo (65). Because of the need for genetic modification, indirect cell labeling is not suited for fast-tracked clinical translation even though approaches with nanoparticles have been described in this context as well (68,69). However, direct cell labeling is simply achieved by incubating the cells with the labeling nanoparticle (Fig 4) (65). In phagocytosing cells, these are then usually labeled relatively quickly (70,71). Although these direct cell-labeling methods appear highly suitable for assessing cell migration over a short period of time, there are also limitations that should be considered: With direct labeling methods, the nanoparticles become diluted in an expanding cell population through distribution over daughter cells, who then carry approximately 50% of the original labeling density if distributed symmetrically, or the label can be lost by the cells, as has been shown, for example, for indium 111 (111In) oxine (72,73). Importantly, direct labeling methods also do not allow for the evaluation of the viability, proliferation, activation, or death of the cells and, in the worst case, the label can be incorporated by other cells (eg, macrophages) that metabolize the remnants of the dead cellular transplants, which can lead to severe misinterpretation of the imaging findings.
Figure 4:
Principles of direct labeling methods for cell tracking. For direct labeling of cells, exogenous markers are coupled to either a transfection agent or a positively charged peptide and then incubated with cells. Primary cells, such as lymphocytes, are harvested from a donor first. Once labeled, cells are introduced into the recipient for repeated imaging. Because the label diffuses out of cells and is diluted during cell division, imaging is only feasible over a limited period of time. (Reprinted, with permission, from reference 65.)
For cell labeling, primarily nanoparticles suitable for MR imaging have been used. There are occasional reports about the use of other particles, for example, gold nanoparticles for CT (74), manganese particles for MR imaging (75), or positron-labeled particles for PET as in the case of copper 64–labeled chitosan nanoparticles (76), but the most widely used particles have been either gadolinium (paramagnetic) or iron oxide (superparamagnetic) nanoparticles for MR imaging (65). A weakness of using standard paramagnetic agents is that they do not cause a change in MR signal that is large enough to allow visualization of relatively small numbers of cells. Therefore, nanomaterials have been found to be ideally suited for carrying multiple individual gadolinium chelates to increase the signal change. Most widely used have been liposomes, dendrimers, and carbon nanotubes; however, except for liposomes, these are not approved for clinical use.
Certain SPIO formulations, however, have been approved and provide a significantly larger effect on MR relaxivity. The iron oxide core usually contains several thousands of iron atoms, significantly increasing the local iron concentration and enabling the detection of lower concentrations of particles. Therefore, these particles have been used to label and image multiple cell types in vitro (eg, monocytes [10,77] and T cells [78]). The relatively small intracellular particle concentration results in a relaxivity change sufficient for the detection of locally concentrated cells in vitro. Cells that are more actively phagocytosing, such as dendritic cells (79) or pancreatic islet cells (80), can accumulate sufficient amounts of nanoparticles to allow for in vivo detection.
Although the type and quantity of labeling agent internalized by cells plays a major role in determining the sensitivity of MR imaging–based cell tracking, acquisition pulse sequences also represent a major factor. The development of new MR pulse sequences such as off-resonance MR imaging, which generates positive contrast through suppression of background tissue, may help increase the robustness of cell detection.
Given the overall large numbers of articles on nanoparticles in preclinical molecular imaging, the actual amount of translation into clinical trials is low. To the best of our knowledge, ClinicalTrials.gov shows one completed trial in the United States (NCT01169935; process date: September 24, 2013; http://clinicaltrials.gov/show/NCT01169935) (81); four clinical trials have been reported (82).
In 2005, MR imaging tracking of ex vivo–labeled autologous dendritic cells with SPIO particles and 111In-oxiquinolon was described (79). Their trafficking after injection into lymph nodes was imaged in humans with use of MR imaging (Fig 5) and scintigraphy. MR imaging was shown to be at least as sensitive as scintigraphy. Misadministration of cells (missing the lymph nodes) was only visualized with MR imaging.
Figure 5:
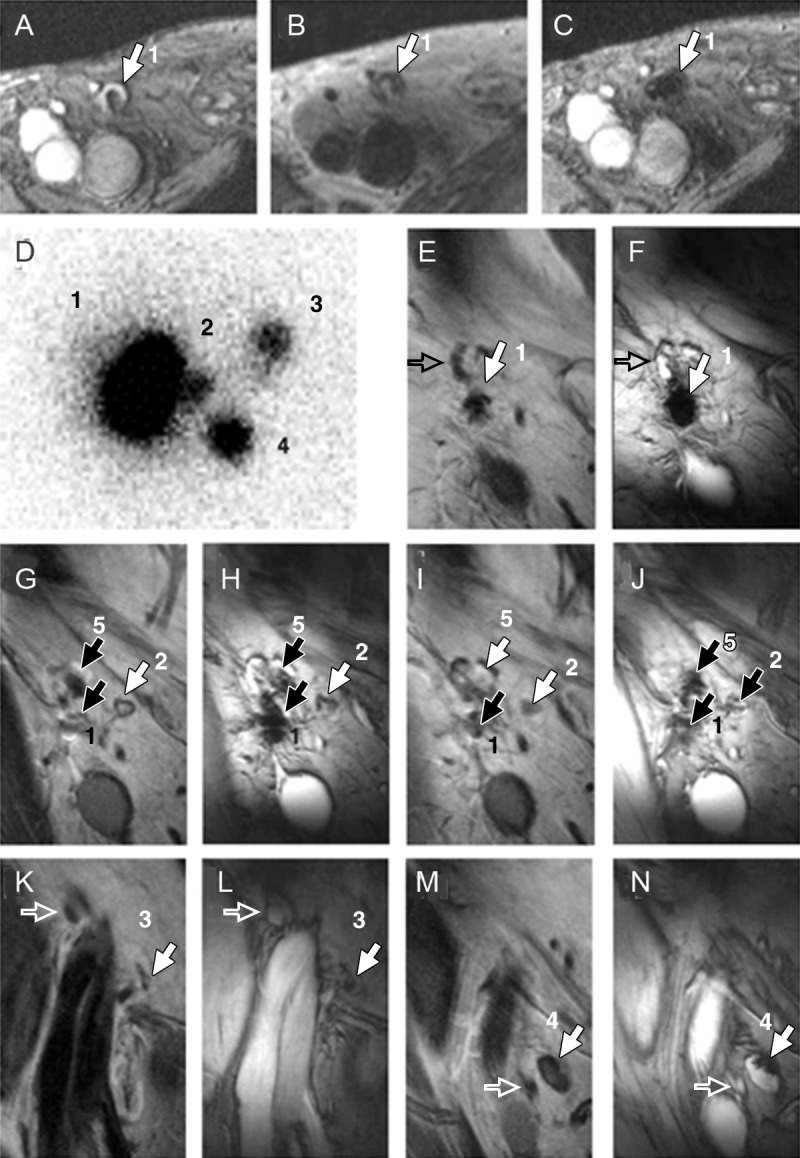
Clinical cell tracking. A–C, MR images obtained before and after injection of dendritic cells labeled with SPIO and 111In into patient’s lymph node. A, Gradient-echo transverse image obtained before vaccination shows right inguinal lymph node with high signal intensity (1). B, Spin-echo transverse image of same lymph node after vaccination. C, Gradient-echo transverse image obtained after vaccination in same position as in B shows decreased signal intensity in lymph node 1. D–N, Images help monitor in vivo migration of labeled dendritic cells 2 days after injection in another patient. D, Image from in vivo scintigraphy shows migration of dendritic cells from injection lymph node (1) to three additional lymph nodes (2–4). E–N, Five image pairs (coronal gradient-echo and spin-echo images) show migration of dendritic cells from, E, F, injection lymph node 1 to, G–N, four additional lymph nodes (2–5). Open arrows indicate lymph nodes that do not contain SPIO, solid arrows indicate lymph nodes that are positive for SPIO. (Reprinted, with permission, from reference 79.)
Tracking of autologous neuronal stem cells in two patients with traumatic brain injury was reported in 2006. Cell labeling was achieved by using an additional transfection agent, and the cells were stereotactically injected near the side of the brain injury. Dynamic changes in the observed hypointensities were attributed to assumed movement of the cells toward the lesion (83). In 2007, a group in Brazil demonstrated tracking of CD34+ bone marrow stem cells in patients with chronic spinal cord injury. Again, movement of cells was observed toward the side of injury, and no signal migration was observed in patients receiving just particles. In this study, however, Dynabeads (Life Technologies, NY), and not a clinically approved preparation, were used for magnetic cell separation (84).
Another clinical study published in 2010 reported on the localization of SPIO-labeled pancreatic islets cells. The transplanted islets were located with MR imaging in the liver over months, and no negative interference between the label and the cellular function was found (80).
However, given the overall large number of articles that describe labeling of cells with nanoparticles and, in particular, iron oxide nanoparticles, as for many other innovative contrast-enhanced imaging approaches, the actual amount of use in ongoing clinical trials is disappointingly low. To the best of our knowledge, ClinicalTrials.gov shows one completed trial (NCT01169935; process date: September 24, 2013; http://clinicaltrials.gov/show/NCT01169935) (81).
Labeling Implants, Transplants, and Grafts
Surgical interventions for many diseases require biocompatible textiles, including vascular grafts, patches, and different types of meshes. Approaches that combine tailored mechanical properties and rapid cellularization with controlled drug release to modulate environmental cells and tissues have led to a promising generation of multifunctional polymers and the design of improved implant structures (85) (Fig 6). To ensure the functionality and safety of the implanted material, it is of critical importance to accurately assess its correct placement during implantation and to longitudinally monitor its fate and function over time. Noninvasive imaging is therefore a crucial element in this field because it has the potential to longitudinally help visualize the implant in vivo, depict potential failure, and monitor tissue function and host response simultaneously.
Figure 6:
Labeling implants, transplants, and grafts. A, MR image of phantom and, B, C, in vivo MR images of surgical mesh loaded with SPIO nanoparticles. (Reprinted, with permission, from reference 86.) Nanoparticle labeling enabled accurate delineation of mesh at implantation (arrow in B ). D, MR image enables noninvasive monitoring of tissue-engineered vascular graft in vivo. Graft was colonized with iron oxide–labeled smooth muscle cells, which facilitated evaluation of graft location (arrowheads), structure, and function during a 3-week period. * = Air in large bowel, ** = air in stomach. (Reprinted, with permission, from reference 87.) E–K, Labeling and multimodal imaging of islet cell containing microcapsules with different nanoparticles. E, Schematic of immune protective microcapsule loaded with gadolinium-gold nanoparticles, which were clearly visualized on, F, T1-weighted MR image, G, T2-weighted MR image, H, CT scan, and, I, US scan. (Reprinted, with permission, from reference 88.) J, Fluorine 19 (18F) MR image hybridized with hydrogen 1 image (gray scale) for highly specific in vivo imaging of perfluorocarbon-containing microcapsules. (Reprinted, with permission, from reference 89.) K, Chemical exchange saturation transfer–magnetization transfer-weighted overlay shows in vivo visualization of cell containing microcapsules labeled with chemical exchange saturation transfer l-arginine liposomes, enabling local sensing of pH changes associated with cell death. (Reprinted, with permission, from reference 90.)
Unfortunately, polymers are difficult to visualize with conventional radiologic methods. To overcome this limitation, nanoparticles can be conjugated to or integrated into polymeric scaffolds, improving their noninvasive visualization. For instance, the integration of SPIO into the base material of surgical textile implants has been shown to be a suitable method for visualizing polyvinylidene fluoride–based mesh implants with MR imaging (91). The accurate MR assessment enabled the determination of the proper operation of the implants and revealed related complications such as shrinkage or adhesions without additional invasive surgical revision (86) (Fig 6, A–C). Beyond that, gold and silver nanoparticles have also been incorporated into a biodegradable polymeric blend of poly(l-lactide)/poly/(4-hydroxybutryrate) to improve image enhancement in x-ray and near-infrared imaging of an absorbable vascular graft (92).
Diagnostic particles have also been used to label biohybrid tissue–engineered implants and cellular transplants (Fig 6, D–J). In addition to the depiction of the implant material itself, noninvasive imaging methods provide fast and accurate assessments of cell growth, viability, differentiation, and tissue development—including matrix development.
Studies have demonstrated the possibility of using MR imaging to monitor tissue-engineered vascular grafts in vivo with use of human aortic smooth muscle cells labeled with iron oxide nanoparticles (Fig 6, D). MR imaging was used to evaluate the structure and function of the human tissue-engineered vascular grafts in addition to investigating the fate of the cells in vivo during a 3-week time course (87). Especially important is gathering information about the fate of tissue-engineered endothelium in a vascular graft, as this cell layer provides an anticoagulant and antithrombotic barrier and modulates vascular tone, growth, inflammation, and hemostasis by secreting biologically active mediators. Labeling of the endothelial cells with superparamagnetic nanoparticles provided a valuable tool for monitorable seeding procedures and offered details about the intactness of the endothelial monolayer after implantation (93).
In the field of cellular transplants, islet cell transplantation has received considerable attention as an effective therapy for type I diabetes mellitus. However, the therapeutic effect is often limited owing to immunologic reactions and graft rejection. To protect pancreatic islet cells from immune reactions leading to rejection of the graft, encapsulation into hydrogel structures has effectively prolonged islet cell viability and improved their insulin secretion. Several studies demonstrated that the incorporation of nanoparticles allowed noninvasive visualization of the encapsulated graft, enabling monitoring of distribution and determination of transplantation sites (Fig 6, E–K). Capsules that contain, for example, iron oxide nanoparticles (magnetocapsules) have been used to monitor hepatic delivery and engraftment with MR imaging (94). Capsules labeled with gadolinium-containing or gold nanoparticles could be further visualized with MR imaging, CT, and US (88,95) (Fig 6, E–I). Immunoprotective alginate microcapsule formulations with coembedded perfluorocarbons also enabled multimodality imaging with CT, US, and 19F MR imaging (Fig 6, J). In addition, perfluorocarbons increased oxygen tension in the immediate proximity of the cells owing to their high oxygen solubility (89). As a first approach to also enable monitoring of the viability of engrafted cells, a chemical exchange saturation transfer l-arginine liposome probe was embedded into the capsule formulation (90). This probe allowed the local sensing of pH changes in the microenvironment associated with cell death and provided conclusive information about the time period of cell survival and the reasons for incomplete immune protection (Fig 6, K).
In conclusion, it has been demonstrated that the labeling of implant material and cells with nanoparticles is an efficient method for visualizing the location and fate of implanted devices. Increased imaging characterization of scaffold material, engineered tissues, and cellular transplants can lead to improved design of implant-based therapies and may accelerate translation to clinical practice.
Nano- and Microparticles for Molecular Imaging
From a pharmacologic and imaging point of view, molecular imaging probes should fulfill several demands: good delivery to the target, highly specific binding (and internalization), low nonspecific accumulation, rapid elimination of the unbound probe, and highly sensitive detection (optimally enabling microdosing).
Until now, most clinically applied molecular imaging agents have been small molecules with fast renal clearance for imaging with PET or SPECT—the modalities with the highest sensitivity for probes (pico- and nanomolar concentrations can be detected). For other imaging modalities with lower contrast agent sensitivity, such as MR imaging (sensitivity up to nanomolar range) or CT (sensitivity in the millimolar range), nanoparticles can be designed as highly potent signal-altering molecules. Examples are targeted SPIO nanoparticles such as USPIO and crossed-linked iron oxide (96–99), as well as paramagnetic liposomes (100) for MR imaging and different functionalized gold nanoparticles for CT (Table) (111,121–123). Many preclinical studies successfully showed an enhanced accumulation of particles in the target tissue in comparison to control tissues or control probes. Considering these examples, the question arises as to why more molecularly targeted micro- and nanoparticles have not yet entered clinical evaluation. In addition to the limited market and the high development costs mentioned earlier, there are several reasons for this, including: (a) Many of the published studies lack proper controls such as competitive binding (blocking) experiments and/or they present insufficient data about the biodistribution of the probes; (b) substantial underlying contribution from nonspecific tissue uptake of the probes owing to varying EPR effects and MPS uptake can make it difficult to reliably draw conclusions on the expression of the target structure (especially in the absence of suitable controls); and (c) extravasation, extravascular convection, and penetration of most diagnostic nanoparticles is low and thus there ultimately is insufficient delivery to extravascular targets (7,9). Because these problems are much less relevant for small molecules, it is not surprising that targeted nanoparticles for PET and SPECT, the most widely used molecular imaging modalities in clinical applications, are rarely used. In addition, injected amounts of targeted nanoparticles in many preclinical studies are much higher than would ever be acceptable for humans.
Table.
Representative Examples for Nanoparticle-based Imaging Probes

Note.—CEST = chemical exchange saturation transfer, HYNIC-GGC = hydrazinonicotinamide-glycine-glycine-cysteine, MAOETIB = methacryloyloxyethyl triiodobenzaote, VSOP = very small superparamagnetic iron oxide.
Review article.
Some of these problems can be overcome by directing the nanoparticles against targets of angiogenesis and inflammation. On one hand, the expected key market for molecular imaging probes that target angiogenesis and inflammation is large because both processes play a pivotal role in a multitude of diseases, including cancer, atherosclerosis, and cardiac and cerebral infarction. Furthermore, because many related target structures are located at the surface of endothelial cells, extravasation of the diagnostic probes is not required and the low extravasation can even be beneficial to keep the nonspecific background signal low. Thus, nano- and microparticles are well suited for these targets. It is not surprising that substantially more publications report targeting of nano- and microparticles to intravascular rather than extravascular targets (Table). Nevertheless, to our knowledge, molecular US contrast agents are the only ligand-targeted diagnostic microparticles that are being clinically evaluated at the moment. Depending on the shell composition, soft- and hard-shell microbubbles can be distinguished, as reviewed by Kiessling et al (112). After preclinical testing in models of breast (113) and prostate (114) cancer in addition to their use for monitoring antiangiogenic tumor therapies (115), the diagnostic potential of vascular endothelial growth factor receptor 2–targeted soft-shell microbubbles with a perfluorobutane core and a PEGylated phospholipid shell (116) is now being evaluated in patients suspected of having prostate carcinoma. Advantages of this class of contrast agents are as follows: (a) highly sensitive detection with US, (b) rapid binding (short time between injection and imaging), (c) no extravasation (low nonspecific background), and (d) rapid elimination from the body (after microbubble disintegration, by means of exhalation of the core gas and metabolization of the phospholipids).
Furthermore, these particles show substantial early nonspecific retention in the liver, and initial preclinical data on their use in the differentiation between normal and dysplastic liver tissue showed promising results (117), prompting clinical evaluation of their use in the detection and characterization of liver diseases.
Nanoparticle-based Theranostics
Nanoparticles for Imaging-guided Interventions
For decades, nano- and microparticles have been used for tumor embolization in the kidney, liver, and brain. However, state-of-the-art embolization materials often are not detectable with MR imaging and radiography; thus, clinically useful low-molecular-weight contrast agents are required to demonstrate the correct placement of catheters and to target the area of embolization. During the past several years, nano- and microparticles for embolization therapies have been developed that can be imaged with CT and MR imaging. Examples of such particles include chitosan microspheres loaded with SPIO that were detectable at MR imaging (127) and multimodal particles measuring 40–200 µm that consist of a copolymerized methacryloyloxyethyl triiodobenzaote core coated with USPIO measuring approximately 150 nm, which are detectable with both radiography and MR imaging (128). Transarterial chemoembolization is another procedure that may benefit from the use of novel theranostic nano- and/or microparticle agents, which have been excellently reviewed by Lewis and Dreher (129). Here, chemotherapeutic drugs can be encapsulated in degradable and nondegradable carrier materials based, for example, on polymers of poly (lactic/glycolic) acid, polyvinyl acetate, and natural substances like plasma proteins, alginate, and chitosan. The addition of imaging markers (eg, Lipiodol, Guerbet) to these constructs will provide the opportunity to pretest correct particle delivery and monitor the success of the intervention (Fig 7a).
Figure 7:
Nanoparticle-based theranostics. (a) Images show detection of polyvinyl acetate–based contrast agent–labeled microspheres during transcatheter embolization of swine liver. Image in top left is preembolization transcatheter CT angiogram obtained with dilute contrast material. The other images were obtained after transcatheter embolization with 0.2-mL increments of Lipiodol-containing radiopaque microspheres from same catheter tip location within a lobar branch of hepatic artery. (Reprinted, with permission, from reference 136.) (b) Three-dimensional reconstruction of fused MR image and CT scan in patient with glioblastoma treated with magnetic fluid hyperthermia by using Nanotherm (Magforce, Berlin, Germany) depicts primary tumor lesion (brown; MR assessment in upper inset), intratumorally administered magnetic fluid (blue; CT scan in lower inset), and thermometry catheter (green). (Reprinted, with permission, from reference 137.) (c) Gamma camera image of 99mTc-labeled PEGylated liposomal doxorubicin (Doxil) in patient with Kaposi sarcoma of palmer area of the hand (arrow) demonstrates the potential of using noninvasive imaging techniques to visualize and quantify target site accumulation of nanomedicine formulations. (Reprinted, with permission, from reference 133.) (d) Images of drug targeting to liver with 123I-labeled sugar-modified polymer-drug conjugate show efficient liver localization (left panel: gamma camera image), but inefficient tumor accumulation (upper right panel: SPECT scan shows distribution of galactosamine-targeted poly-N-[2-hydroxypropyl]methacrylamide-doxorubicin [known as PK2] within liver; lower right panel: CT scan of central liver tumor). (Reprinted, with permission, from reference 134.)
The properties of theranostic microparticles with variable constitution and radiolabels that are used for selective internal radiation therapy treatment of liver tumors are reviewed by Van de Wiele et al (138). In this context, 90Y is the most commonly used therapeutic isotope and shows predominantly β− decay but marginally (<1%) β+ decay. Because of the local administration and the use of high doses, however, PET-based distribution measurements of 90Y microspheres are feasible during selective internal radiation therapy treatment. Preliminary data suggest that PET of 90Y microspheres may not be very useful for detailed PET imaging but reasonably useful for dose assessment with patient-specific dosimetry (130).
Nano- or Microparticles for Imaging-guided Hyperthermia Treatment
The basic principle of nano- or microparticle-based hyperthermia is to use particles that absorb energy and transform it into heat. This can be achieved by means of irradiation with acoustic (139,140) and electromagnetic (137) waves, as well as with light (141–144). The major problem and the motivation for choosing a theranostic approach is the control of the homogeneous and quantitatively sufficient accumulation of the nanoparticles in the target tissue—which is the major problem for most of these approaches and limits the therapeutic outcome. In this context, injections of particles into the target tissue, catheter-based local arterial injections, and systemic intravenous injections have been evaluated (131,145). In addition, there are efforts to locally retain magnetic particles with the application of strong magnetic fields (146).
The prospects and indications for such approaches, however, must be considered carefully, taking into account the available alternative treatments, which include high-intensity focused ultrasound, radiofrequency ablation, cryoablation and embolization, as well as radiation therapy, transarterial chemoembolization, and selective internal radiation therapy treatment.
Malignant brain tumors are suited for nanoparticle-based magnetic fluid hyperthermia because, after surgery, radiation therapy, and chemotherapy, further therapeutic options for malignant brain tumors are limited. Therefore, in a clinical phase II study, magnetic nanoparticles were installed under CT control into glioblastomas and heated up by applying magnetic forces (137). As exemplified in Figure 7b, the distribution and persistence of the particles could be controlled with CT, enabling repetitive thermal therapy. In patients with recurrent glioblastoma, overall survival was longer than that with conventional therapies (137), demonstrating the potential of this theranostic approach.
Imaging-guided Drug Delivery
A final interesting and increasingly recognized application of nanoparticles is for imaging-guided drug delivery. By developing nanomedicine formulations containing both diagnostic and therapeutic properties, their biodistribution and target site accumulation can be visualized and quantified, their local activation can be monitored (in case of triggered drug release), and their therapeutic efficacy can be assessed noninvasively and longitudinally (147–149). The latter strategy could, for example, be relevant in case of long-circulating blood pool contrast agents that contain antiangiogenic drugs or vascular disrupting agents, which would enable targeted reduction of blood vessels in tumors as well as simultaneous monitoring of therapeutic efficacy (150,151). The potential value of monitoring triggered drug release has, on several occasions, been elegantly demonstrated by using temperature-sensitive liposomes containing chemotherapeutic drugs and T1-weighted MR imaging contrast agents (152). When used in combination with local radiofrequency ablation or MR imaging–guided high-intensity focused ultrasound–mediated hyperthermia, such stimuli-sensitive nanotheranostics provide real-time feedback on the efficacy of temperature-induced drug release (153–155).
The use of nanoparticles that contain both drugs and imaging agents for visualizing their biodistribution and target site accumulation is arguably the most relevant application of imaging-guided drug delivery. As exemplified in Figure 7c and d, patients likely to respond to nanomedicine treatment can be preselected on the basis of noninvasive imaging with use of such approaches (156,157). Convincing clinical proof of principle is available showing that tumors associated with a strong EPR effect, such as Kaposi sarcomas and head and neck tumors, respond relatively well to liposome-based nanotherapeutics, whereas tumors that generally have poor EPR, such as breast carcinomas, do not seem to benefit too much from such targeted interventions—at least not when it comes to increasing response rates and prolonging progression-free and overall survival times (8,133,156). It should be noted, however, that even in case of the latter, there are clear advantages of using nanomedicine formulations, but these are largely based on changing the nature and/or reducing the incidence and intensity of side effects, rather than on improving therapeutic outcome (12,13).
On the basis of the above notion that a substantial target site accumulation of nanomedicines, as observed in Kaposi sarcomas and head and neck cancers, also leads to significant therapeutic efficacy, it seems reasonable to much more strongly incorporate imaging into nanomedicine-based chemotherapeutic interventions (156,157). By doing so, patients with a low degree of target site accumulation (and therefore unlikely to respond to such treatments) (Fig 7), as well as individuals with a high degree of off-target localization (and therefore at high risk of developing side effects), can be excluded from nanomedicine treatment at very early stages, and they can be referred to alternative established or experimental interventions (156). And, vice versa, patients with a high degree of target site accumulation can likely be treated relatively efficiently (Fig 7). Moreover, in these patients, hybrid imaging approaches that aim to noninvasively monitor therapeutic efficacy can be implemented to further refine and personalize nanomedicine treatments.
Conclusion
Diagnostic nano- and microparticles are favorably suited for labeling the MPS (eg, liver, spleen, lymph nodes), and the use of diagnostic nanoparticles is indicated for ex vivo labeling of cells, scaffolds, and other kinds of tissue-engineered implants that are to be visualized in vivo. Another important application field is their use as theranostics and drug release systems, in particular for imaging-guided interventions, where the particles are directly delivered to the tissue (eg, by means of catheter-based arterial injection or direct injection into the tissue).
However, due to several reasons the potential of nanoparticles as molecularly targeted diagnostic agents is limited and a clear advantage over small molecules is usually not apparent. High background signal is often generated by nanoparticle uptake in the MPS and by EPR effects. Furthermore, because of the relatively low penetration into tissues, molecularly targeted micro- and nanoparticles are often not sufficiently delivered to their targets. This is particularly true for all targets localized outside of the vasculature. For targets inside the vasculature, however, larger microparticles may even be preferable because these will hardly extravasate and thus generate less nonspecific background. However, besides these biologic prerequisites for a successful application of nanoparticles, other factors are of crucial importance: There is a clear need to more accurately quantify the targeting efficacy of nanoparticulates to be capable of better estimating their diagnostic and therapeutic potential. In addition, regulatory challenges related to good manufacturing practice production, uniformity, batch-to-batch reproducibility and upscaling must be resolved to facilitate clinical translation. This is especially important because batch-dependent differences in particle size, in addition to shape and charge, have a strong influence on the blood half-life, biodistribution, and elimination of the particles (10).
In summary, there are numerous publications suggesting nanoparticles as diagnostics and theranostics, and there are several applications with high clinical potential. However, when reviewing the literature, important fundamental requirements on diagnostic drugs are not considered because of fundamental conceptual shortfalls. Thus, many publications only present basic ideas that are conceptually truly exciting but remain without any foreseeable clinical impact. These limitations may be overcome and more clinically relevant nanodiagnostics and theranostics may be developed if the interaction between scientists from chemistry, biology, pharmacology, and medicine is improved.
Essentials
■ Theranostic micro- and nanoparticles have substantial potential for interventional radiology (eg, as embolization material and drug delivery systems).
■ Diagnostic micro- and nanoparticles are favorably suited for imaging of the mononuclear phagocytic system and for ex vivo labeling of implants and cells.
■ The value of micro- and nanoparticles as molecular diagnostics is overestimated because their pharmacokinetic properties are difficult to control; in addition, extravascular targets are difficult to reach and small molecules often perform better.
■ Targeted microbubbles used for US are the only target-specific nano- and microparticles that are currently being evaluated in clinical trials.
■ If micro- and nanoparticles are used for molecular imaging, intravascular targets should be preferred over extravascular targets.
Received August 12, 2013; revision requested September 20; revision received October 11; accepted October 28; final version accepted November 6.
Supported by the European Research Council (ERC starting grant 309495: NeoNaNo).
Supported by the National Institutes of Health (grants 1R01EB014944-01 and P30 CA08748).
Disclosures of Conflicts of Interest: F.K. disclosed no relevant relationships. M.E.M. disclosed no relevant relationships. J.G. disclosed no relevant relationships. T.L. disclosed no relevant relationships.
Abbreviations:
- EPR
- enhanced permeability and retention
- MPS
- mononuclear phagocytic system
- SPIO
- superparamagnetic iron oxide
- USPIO
- ultrasmall SPIO
References
- 1.Yigit MV, Moore A, Medarova Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm Res 2012;29(5):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakhmola A, Anton N, Vandamme TF. Inorganic nanoparticles based contrast agents for x-ray computed tomography. Adv Healthc Mater 2012;1(4):413–431. [DOI] [PubMed] [Google Scholar]
- 3.Wahajuddin AS, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine 2012;7:3445–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiessling F, Morgenstern B, Zhang C. Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Curr Med Chem 2007;14(1):77–91. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Kiessling F, Gaetjens J. Advanced nanomaterials in multimodal imaging: design, functionalization, and biomedical applications. J Nanomater 2010;894303. [Google Scholar]
- 6.Heneweer C, Gendy SE, Peñate-Medina O. Liposomes and inorganic nanoparticles for drug delivery and cancer imaging. Ther Deliv 2012;3(5):645–656. [DOI] [PubMed] [Google Scholar]
- 7.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm 2011;8(6):2101–2141. [DOI] [PubMed] [Google Scholar]
- 8.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res 2011;44(10):1029–1038. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol 2013;24(6):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm J, Scheinberg DA. Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol 2011;21(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 12.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7(11):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release 2012;161(2):175–187. [DOI] [PubMed] [Google Scholar]
- 14.Wagner M, Wagner S, Schnorr J, et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: initial experience in humans. J Magn Reson Imaging 2011;34(4):816–823. [DOI] [PubMed] [Google Scholar]
- 15.Wheaton AJ, Miyazaki M. Non–contrast enhanced MR angiography: physical principles. J Magn Reson Imaging 2012;36(2):286–304. [DOI] [PubMed] [Google Scholar]
- 16.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res 2009;42(7):851–862. [DOI] [PubMed] [Google Scholar]
- 17.Turetschek K, Preda A, Novikov V, et al. Tumor microvascular changes in antiangiogenic treatment: assessment by magnetic resonance contrast media of different molecular weights. J Magn Reson Imaging 2004;20(1):138–144. [DOI] [PubMed] [Google Scholar]
- 18.Persigehl T, Wall A, Kellert J, et al. Tumor blood volume determination by using susceptibility-corrected ΔR2* multiecho MR. Radiology 2010;255(3):781–789. [DOI] [PubMed] [Google Scholar]
- 19.Persigehl T, Bieker R, Matuszewski L, et al. Antiangiogenic tumor treatment: early noninvasive monitoring with USPIO-enhanced MR imaging in mice. Radiology 2007;244(2):449–456. [DOI] [PubMed] [Google Scholar]
- 20.Pannetier N, Lemasson B, Christen T, et al. Vessel size index measurements in a rat model of glioma: comparison of the dynamic (Gd) and steady-state (iron-oxide) susceptibility contrast MRI approaches. NMR Biomed 2012;25(2):218–226. [DOI] [PubMed] [Google Scholar]
- 21.Zwick S, Strecker R, Kiselev V, et al. Assessment of vascular remodeling under antiangiogenic therapy using DCE-MRI and vessel size imaging. J Magn Reson Imaging 2009;29(5):1125–1133. [DOI] [PubMed] [Google Scholar]
- 22.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 2001;11(11):2319–2331. [DOI] [PubMed] [Google Scholar]
- 23.Leike J, Sachse A, Ehritt C, Krause W. Biodistribution and CT-imaging characteristics of iopromide-carrying liposomes in rats. J Liposome Res 1996;6(4):665–680. [Google Scholar]
- 24.Desser TS, Rubin DL, Muller H, McIntire GL, Bacon ER, Toner JL. Blood pool and liver enhancement in CT with liposomal iodixanol: comparison with iohexol. Acad Radiol 1999;6(3):176–183. [DOI] [PubMed] [Google Scholar]
- 25.Krause W, Leike J, Sachse A, Schuhmann-Giampieri G. Characterization of iopromide liposomes. Invest Radiol 1993;28(11):1028–1032. [DOI] [PubMed] [Google Scholar]
- 26.Hallouard F, Anton N, Choquet P, Constantinesco A, Vandamme T. Iodinated blood pool contrast media for preclinical x-ray imaging applications: a review. Biomaterials 2010;31(24):6249–6268. [DOI] [PubMed] [Google Scholar]
- 27.Kong WH, Lee WJ, Cui ZY, et al. Nanoparticulate carrier containing water-insoluble iodinated oil as a multifunctional contrast agent for computed tomography imaging. Biomaterials 2007;28(36):5555–5561. [DOI] [PubMed] [Google Scholar]
- 28.Aviv H, Bartling S, Kieslling F, Margel S. Radiopaque iodinated copolymeric nanoparticles for x-ray imaging applications. Biomaterials 2009;30(29):5610–5616. [DOI] [PubMed] [Google Scholar]
- 29.de Vries A, Custers E, Lub J, van den Bosch S, Nicolay K, Grüll H. Block-copolymer-stabilized iodinated emulsions for use as CT contrast agents. Biomaterials 2010;31(25):6537–6544. [DOI] [PubMed] [Google Scholar]
- 30.Torchilin VP, Frank-Kamenetsky MD, Wolf GL. CT visualization of blood pool in rats by using long-circulating, iodine-containing micelles. Acad Radiol 1999;6(1):61–65. [DOI] [PubMed] [Google Scholar]
- 31.Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. An x-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater 2006;5(2):118–122. [DOI] [PubMed] [Google Scholar]
- 32.Aviv H, Bartling S, Grinberg I, Margel S. Synthesis and characterization of Bi2O3/HSA core-shell nanoparticles for x-ray imaging applications. J Biomed Mater Res B Appl Biomater 2013;101(1):131–138. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Ai K, Liu J, Yuan Q, He Y, Lu L. Hybrid BaYbF(5) nanoparticles: novel binary contrast agent for high-resolution in vivo x-ray computed tomography angiography. Adv Healthc Mater 2012;1(4):461–466. [DOI] [PubMed] [Google Scholar]
- 34.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010;257(1):24–39. [DOI] [PubMed] [Google Scholar]
- 35.Kim YK, Kwak HS, Kim CS, Chung GH, Han YM, Lee JM. Hepatocellular carcinoma in patients with chronic liver disease: comparison of SPIO-enhanced MR imaging and 16–detector row CT. Radiology 2006;238(2):531–541. [DOI] [PubMed] [Google Scholar]
- 36.Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ. Superparamagnetic iron oxide–enhanced MR imaging of head and neck lymph nodes. Radiology 2002;222(1):239–244. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. J Magn Reson Imaging 2001;14(4):355–361. [DOI] [PubMed] [Google Scholar]
- 38.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003;348(25):2491–2499. [DOI] [PubMed] [Google Scholar]
- 39.Saleh A, Wiedermann D, Schroeter M, Jonkmanns C, Jander S, Hoehn M. Central nervous system inflammatory response after cerebral infarction as detected by magnetic resonance imaging. NMR Biomed 2004;17(4):163–169. [DOI] [PubMed] [Google Scholar]
- 40.Heesakkers RA, Jager GJ, Hövels AM, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10–enhanced MR imaging. Radiology 2009;251(2):408–414. [DOI] [PubMed] [Google Scholar]
- 41.Madru R, Kjellman P, Olsson F, et al. 99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes. J Nucl Med 2012;53(3):459–463. [DOI] [PubMed] [Google Scholar]
- 42.Ocampo-García BE, Ramírez FdeM, Ferro-Flores G, et al. 99mTc-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection. Nucl Med Biol 2011;38(1):1–11. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol 2004;22(1):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai X, Li W, Kim CH, Yuan Y, Wang LV, Xia Y. In vivo quantitative evaluation of the transport kinetics of gold nanocages in a lymphatic system by noninvasive photoacoustic tomography. ACS Nano 2011;5(12):9658–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sever AR, Mills P, Weeks J, et al. Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in patients with breast cancer. AJR Am J Roentgenol 2012;199(2):465–470. [DOI] [PubMed] [Google Scholar]
- 46.Sever AR, Mills P, Jones SE, et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011;196(2):251–256. [DOI] [PubMed] [Google Scholar]
- 47.Morana G, Salviato E, Guarise A. Contrast agents for hepatic MRI. Cancer Imaging 2007;7(Spec No A):S24–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissleder R, Elizondo G, Stark DD, et al. The diagnosis of splenic lymphoma by MR imaging: value of superparamagnetic iron oxide. AJR Am J Roentgenol 1989;152(1):175–180. [DOI] [PubMed] [Google Scholar]
- 49.Weissleder R. Liver MR imaging with iron oxides: toward consensus and clinical practice. Radiology 1994;193(3):593–595. [DOI] [PubMed] [Google Scholar]
- 50.Rappeport ED, Loft A, Berthelsen AK, et al. Contrast-enhanced FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol 2007;48(4):369–378. [DOI] [PubMed] [Google Scholar]
- 51.Ward J, Guthrie JA, Wilson D, et al. Colorectal hepatic metastases: detection with SPIO-enhanced breath-hold MR imaging—comparison of optimized sequences. Radiology 2003;228(3):709–718. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto H, Yamashita Y, Yoshimatsu S, Baba Y, Takahashi M. MR enhancement of hepatoma by superparamagnetic iron oxide (SPIO) particles. J Comput Assist Tomogr 1995;19(4):665–667. [DOI] [PubMed] [Google Scholar]
- 53.Reimer P, Tombach B. Hepatic MRI with SPIO: detection and characterization of focal liver lesions. Eur Radiol 1998;8(7):1198–1204. [DOI] [PubMed] [Google Scholar]
- 54.Grandin C, Van Beers BE, Robert A, Gigot JF, Geubel A, Pringot J. Benign hepatocellular tumors: MRI after superparamagnetic iron oxide administration. J Comput Assist Tomogr 1995;19(3):412–418. [PubMed] [Google Scholar]
- 55.Wunderbaldinger P, Josephson L, Bremer C, Moore A, Weissleder R. Detection of lymph node metastases by contrast-enhanced MRI in an experimental model. Magn Reson Med 2002;47(2):292–297. [DOI] [PubMed] [Google Scholar]
- 56.Ross RW, Zietman AL, Xie W, et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging 2009;33(4):301–305. [DOI] [PubMed] [Google Scholar]
- 57.Pandharipande PV, Harisinghani MG, Ozanne EM, et al. Staging MR lymphangiography of the axilla for early breast cancer: cost-effectiveness analysis. AJR Am J Roentgenol 2008;191(5):1308–1319. [DOI] [PubMed] [Google Scholar]
- 58.Guimaraes AR, Tabatabei S, Dahl D, McDougal WS, Weissleder R, Harisinghani MG. Pilot study evaluating use of lymphotrophic nanoparticle–enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology 2008;71(4):708–712. [DOI] [PubMed] [Google Scholar]
- 59.Majmudar MD, Yoo J, Keliher EJ, et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res 2013;112(5):755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Settles M, Etzrodt M, Kosanke K, et al. Different capacity of monocyte subsets to phagocytose iron-oxide nanoparticles. PLoS ONE 2011;6(10): e25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye Q, Yang D, Williams M, et al. In vivo detection of acute rat renal allograft rejection by MRI with USPIO particles. Kidney Int 2002;61(3):1124–1135. [DOI] [PubMed] [Google Scholar]
- 62.Yilmaz A, Dengler MA, van der Kuip H, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J 2013;34(6):462–475. [DOI] [PubMed] [Google Scholar]
- 63.Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A 2004;101(34):12634–12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 2011;121(1):442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol 2011;8(11):677–688. [DOI] [PubMed] [Google Scholar]
- 66.Alam SR, Shah AS, Richards J, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging 2012;5(5):559–565. [DOI] [PubMed] [Google Scholar]
- 67.Sosnovik DE, Nahrendorf M. Cells and iron oxide nanoparticles on the move: magnetic resonance imaging of monocyte homing and myocardial inflammation in patients with ST-elevation myocardial infarction. Circ Cardiovasc Imaging 2012;5(5):551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med 2000;6(3):351–355. [DOI] [PubMed] [Google Scholar]
- 69.Tannous BA, Grimm J, Perry KF, Chen JW, Weissleder R, Breakefield XO. Metabolic biotinylation of cell surface receptors for in vivo imaging. Nat Methods 2006;3(5):391–396. [DOI] [PubMed] [Google Scholar]
- 70.Kircher MF, Allport JR, Graves EE, et al. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res 2003;63(20):6838–6846. [PubMed] [Google Scholar]
- 71.Pittet MJ, Grimm J, Berger CR, et al. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc Natl Acad Sci U S A 2007;104(30):12457–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou R, Thomas DH, Qiao H, et al. In vivo detection of stem cells grafted in infarcted rat myocardium. J Nucl Med 2005;46(5):816–822. [PMC free article] [PubMed] [Google Scholar]
- 73.Grimm J, Swirski FK, Pittet M, Josephson L, Weissleder R. A nanoparticle-based cell labeling agent for cell tracking with SPECT/CT. Mol Imaging 2006;5(Suppl 3):364. [Google Scholar]
- 74.Astolfo A, Schültke E, Menk RH, et al. In vivo visualization of gold-loaded cells in mice using x-ray computed tomography. Nanomedicine (Lond Print) 2013;9(2):284–292. [DOI] [PubMed] [Google Scholar]
- 75.Gilad AA, Walczak P, McMahon MT, et al. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn Reson Med 2008;60(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pala A, Liberatore M, D’Elia P, et al. Labelling of granulocytes by phagocytic engulfment with 64Cu-labelled chitosan-coated magnetic nanoparticles. Mol Imaging Biol 2012;14(5):593–598. [DOI] [PubMed] [Google Scholar]
- 77.Zelivyanskaya ML, Nelson JA, Poluektova L, et al. Tracking superparamagnetic iron oxide labeled monocytes in brain by high-field magnetic resonance imaging. J Neurosci Res 2003;73(3):284–295. [DOI] [PubMed] [Google Scholar]
- 78.Sipe JC, Filippi M, Martino G, et al. Method for intracellular magnetic labeling of human mononuclear cells using approved iron contrast agents. Magn Reson Imaging 1999;17(10):1521–1523. [DOI] [PubMed] [Google Scholar]
- 79.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005;23(11):1407–1413. [DOI] [PubMed] [Google Scholar]
- 80.Saudek F, Jirák D, Girman P, et al. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010;90(12):1602–1606. [DOI] [PubMed] [Google Scholar]
- 81.Tracking inflammatory cells using superparamagnetic particles of iron oxide (spio) and magnetic resonance imaging (MRI) . http://clinicaltrials.gov/show/NCT01169935. Updated February 4, 2013. Accessed July 2013.
- 82.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol 2009;193(2):314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med 2006;355(22):2376–2378. [DOI] [PubMed] [Google Scholar]
- 84.Callera F, de Melo CMTP. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev 2007;16(3):461–466. [DOI] [PubMed] [Google Scholar]
- 85.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater 2009;8(6):457–470. [DOI] [PubMed] [Google Scholar]
- 86.Kuehnert N, Kraemer NA, Otto J, et al. In vivo MRI visualization of mesh shrinkage using surgical implants loaded with superparamagnetic iron oxides. Surg Endosc 2012;26(5):1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nelson GN, Roh JD, Mirensky TL, et al. Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo. FASEB J 2008;22(11):3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arifin DR, Long CM, Gilad AA, et al. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 2011;260(3):790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnett BP, Ruiz-Cabello J, Hota P, et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 2011;258(1):182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan KW, Liu G, Song X, et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater 2013;12(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krämer NA, Donker HC, Otto J, et al. A concept for magnetic resonance visualization of surgical textile implants. Invest Radiol 2010;45(8):477–483. [DOI] [PubMed] [Google Scholar]
- 92.Luderer F, Begerow I, Schmidt W, et al. Enhanced visualization of biodegradable polymeric vascular scaffolds by incorporation of gold, silver and magnetite nanoparticles. J Biomater Appl 2013;28(2):219–231. [DOI] [PubMed] [Google Scholar]
- 93.Perea H, Aigner J, Heverhagen JT, Hopfner U, Wintermantel E. Vascular tissue engineering with magnetic nanoparticles: seeing deeper. J Tissue Eng Regen Med 2007;1(4):318–321. [DOI] [PubMed] [Google Scholar]
- 94.Barnett BP, Arepally A, Karmarkar PV, et al. Magnetic resonance–guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med 2007;13(8):986–991. [DOI] [PubMed] [Google Scholar]
- 95.Kiessling FM. Science to practice: are theranostic agents with encapsulated cells the key for diabetes therapy? Radiology 2011;260(3):613–615. [DOI] [PubMed] [Google Scholar]
- 96.Jayapaul J, Arns S, Lederle W, et al. Riboflavin carrier protein-targeted fluorescent USPIO for the assessment of vascular metabolism in tumors. Biomaterials 2012;33(34):8822–8829. [DOI] [PubMed] [Google Scholar]
- 97.Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res 2011;44(10):842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fréchou M, Beray-Berthat V, Raynaud JS, et al. Detection of vascular cell adhesion molecule-1 expression with USPIO-enhanced molecular MRI in a mouse model of cerebral ischemia. Contrast Media Mol Imaging 2013;8(2):157–164. [DOI] [PubMed] [Google Scholar]
- 99.Burtea C, Ballet S, Laurent S, et al. Development of a magnetic resonance imaging protocol for the characterization of atherosclerotic plaque by using vascular cell adhesion molecule-1 and apoptosis-targeted ultrasmall superparamagnetic iron oxide derivatives. Arterioscler Thromb Vasc Biol 2012;32(6):e36–e48. [DOI] [PubMed] [Google Scholar]
- 100.Strijkers GJ, Kluza E, Van Tilborg GA, et al. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 2010;13(2):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Preda A, van Vliet M, Krestin GP, Brasch RC, van Dijke CF. Magnetic resonance macromolecular agents for monitoring tumor microvessels and angiogenesis inhibition. Invest Radiol 2006;41(3):325–331. [DOI] [PubMed] [Google Scholar]
- 102.Arbab AS, Pandit SD, Anderson SA, et al. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells 2006;24(3):671–678. [DOI] [PubMed] [Google Scholar]
- 103.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J 2007;21(8):1647–1654. [DOI] [PubMed] [Google Scholar]
- 104.Hoehn M, Wiedermann D, Justicia C, et al. Cell tracking using magnetic resonance imaging. J Physiol 2007;584(Pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoehn M, Küstermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A 2002;99(25):16267–16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 2001;19(12):1141–1147. [DOI] [PubMed] [Google Scholar]
- 107.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 2005;23(8):983–987. [DOI] [PubMed] [Google Scholar]
- 108.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med 2004;10(9):993–998. [DOI] [PubMed] [Google Scholar]
- 109.Zhang C, Jugold M, Woenne EC, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res 2007;67(4):1555–1562. [DOI] [PubMed] [Google Scholar]
- 110.Schmieder AH, Winter PM, Williams TA, et al. Molecular MR imaging of neovascular progression in the Vx2 tumor with αvβ3-targeted paramagnetic nanoparticles. Radiology 2013;268(2):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim YH, Jeon J, Hong SH, et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small 2011;7(14):2052–2060. [DOI] [PubMed] [Google Scholar]
- 112.Kiessling F, Bzyl J, Fokong S, Siepmann M, Schmitz G, Palmowski M. Targeted ultrasound imaging of cancer: an emerging technology on its way to clinics. Curr Pharm Des 2012;18(15):2184–2199. [DOI] [PubMed] [Google Scholar]
- 113.Bzyl J, Lederle W, Rix A, et al. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur Radiol 2011;21(9):1988–1995. [DOI] [PubMed] [Google Scholar]
- 114.Tardy I, Pochon S, Theraulaz M, et al. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest Radiol 2010;45(10):573–578. [DOI] [PubMed] [Google Scholar]
- 115.Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55). Radiology 2010;256(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol 2010;45(2):89–95. [DOI] [PubMed] [Google Scholar]
- 117.Grouls C, Hatting M, Rix A, et al. Liver dysplasia: US molecular imaging with targeted contrast agent enables early assessment. Radiology 2013;267(2):487–495. [DOI] [PubMed] [Google Scholar]
- 118.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 2004;22(8):969–976. [DOI] [PubMed] [Google Scholar]
- 119.De la Zerda A, Zavaleta C, Keren S, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol 2008;3(9):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kresse M, Wagner S, Pfefferer D, Lawaczeck R, Elste V, Semmler W. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Magn Reson Med 1998;40(2):236–242. [DOI] [PubMed] [Google Scholar]
- 121.Reuveni T, Motiei M, Romman Z, Popovtzer A, Popovtzer R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine 2011;6:2859–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J, Chaudhary A, Chmura SJ, et al. A novel functional CT contrast agent for molecular imaging of cancer. Phys Med Biol 2010;55(15):4389–4397. [DOI] [PubMed] [Google Scholar]
- 123.Ghann WE, Aras O, Fleiter T, Daniel MC. Syntheses and characterization of lisinopril-coated gold nanoparticles as highly stable targeted CT contrast agents in cardiovascular diseases. Langmuir 2012;28(28):10398–10408. [DOI] [PubMed] [Google Scholar]
- 124.Lindner JR. Molecular imaging of myocardial and vascular disorders with ultrasound. JACC Cardiovasc Imaging 2010;3(2):204–211. [DOI] [PubMed] [Google Scholar]
- 125.Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J Nucl Med 2012;53(3):345–348. [DOI] [PubMed] [Google Scholar]
- 126.Oostendorp M, Douma K, Wagenaar A, et al. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation 2010;121(6):775–783. [DOI] [PubMed] [Google Scholar]
- 127.Chung EY, Kim HM, Lee GH, et al. Design of deformable chitosan microspheres loaded with superparamagnetic iron oxide nanoparticles for embolotherapy detectable by magnetic resonance imaging. Carbohydr Polym 2012;90(4):1725–1731. [DOI] [PubMed] [Google Scholar]
- 128.Bartling SH, Budjan J, Aviv H, et al. First multimodal embolization particles visible on x-ray/computed tomography and magnetic resonance imaging. Invest Radiol 2011;46(3):178–186. [DOI] [PubMed] [Google Scholar]
- 129.Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release 2012;161(2):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.D’Arienzo M, Chiaramida P, Chiacchiararelli L, et al. 90Y PET-based dosimetry after selective internal radiotherapy treatments. Nucl Med Commun 2012;33(6):633–640. [DOI] [PubMed] [Google Scholar]
- 131.Stone R, Willi T, Rosen Y, Mefford OT, Alexis F. Targeted magnetic hyperthermia. Ther Deliv 2011;2(6):815–838. [DOI] [PubMed] [Google Scholar]
- 132.Harrington KJ, Mohammadtaghi S, Uster PS, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res 2001;7(2):243–254. [PubMed] [Google Scholar]
- 133.Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas: rationale for combination with radiotherapy. Acta Oncol 2000;39(2):207–211. [DOI] [PubMed] [Google Scholar]
- 134.Seymour LW, Ferry DR, Anderson D, et al. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol 2002;20(6):1668–1676. [DOI] [PubMed] [Google Scholar]
- 135.Seymour LW, Ferry DR, Kerr DJ, et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol 2009;34(6):1629–1636. [DOI] [PubMed] [Google Scholar]
- 136.Sharma KV, Dreher MR, Tang Y, et al. Development of “imageable” beads for transcatheter embolotherapy. J Vasc Interv Radiol 2010;21(6):865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011;103(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van de Wiele C, Maes A, Brugman E, et al. SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imaging 2012;39(10):1646–1655. [DOI] [PubMed] [Google Scholar]
- 139.Wang X, Chen H, Zheng Y, et al. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials 2013;34(8):2057–2068. [DOI] [PubMed] [Google Scholar]
- 140.Niu D, Wang X, Li Y, et al. Facile synthesis of magnetite/perfluorocarbon co-loaded organic/inorganic hybrid vesicles for dual-modality ultrasound/magnetic resonance imaging and imaging-guided high-intensity focused ultrasound ablation. Adv Mater 2013;25(19):2686–2692. [DOI] [PubMed] [Google Scholar]
- 141.Xu QC, Zhang Y, Tan MJ, et al. Anti-cAngptl4 Ab-conjugated N-TiO(2)/NaYF(4):Yb,Tm nanocomposite for near infrared-triggered drug release and enhanced targeted cancer cell ablation. Adv Healthc Mater 2012;1(4):470–474. [DOI] [PubMed] [Google Scholar]
- 142.Shen H, You J, Zhang G, et al. Cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv Healthc Mater 2012;1(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Su Y, Wei X, Peng F, et al. Gold nanoparticles–decorated silicon nanowires as highly efficient near-infrared hyperthermia agents for cancer cells destruction. Nano Lett 2012;12(4):1845–1850. [DOI] [PubMed] [Google Scholar]
- 144.Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem Soc Rev 2013;42(2):530–547. [DOI] [PubMed] [Google Scholar]
- 145.Chertok B, David AE, Yang VC. Brain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topography. J Control Release 2011;155(3):393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.David AE, Cole AJ, Chertok B, Park YS, Yang VC. A combined theoretical and in vitro modeling approach for predicting the magnetic capture and retention of magnetic nanoparticles in vivo. J Control Release 2011;152(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev 2010;62(11):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lammers T, Kiessling F, Hennink WE, Storm G. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol Pharm 2010;7(6):1899–1912. [DOI] [PubMed] [Google Scholar]
- 149.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 2010;62(11):1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kiessling F, Heilmann M, Lammers T, et al. Synthesis and characterization of HE-24.8: a polymeric contrast agent for magnetic resonance angiography. Bioconjug Chem 2006;17(1):42–51. [DOI] [PubMed] [Google Scholar]
- 151.Lammers T, Subr V, Peschke P, et al. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br J Cancer 2008;99(6):900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaittanis C, Shaffer TM, Ogirala A, et al. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat Commun doi:10.1038/ncomms4384. Published online March 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J Control Release 2011;150(1):102–110. [DOI] [PubMed] [Google Scholar]
- 154.Ranjan A, Jacobs GC, Woods DL, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release 2012;158(3):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grüll H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release 2012;161(2):317–327. [DOI] [PubMed] [Google Scholar]
- 156.Lammers T, Rizzo LY, Storm G, Kiessling F. Personalized nanomedicine. Clin Cancer Res 2012;18(18):4889–4894. [DOI] [PubMed] [Google Scholar]
- 157.Lammers T. Smart drug delivery systems: back to the future vs. clinical reality. Int J Pharm 2013;454(1):527–529. [DOI] [PMC free article] [PubMed] [Google Scholar]








