Abstract
This overview covers many well-characterized mouse models of autism spectrum disorders (ASDs). As there are numerous mouse models of varying design and description in the literature, the models presented here represent examples of genetically engineered models where human genetic evidence supports a causative relationship between the targeted mutation and the behavioral phenotype of the mouse models. As the diagnosis of ASDs is primarily based on behavioral evaluations in humans, we highlight the murine behavioral analogs to human autistic behaviors in different models. However, because the characteristic behaviors of ASDs in humans are in the domains of social interaction, communication, and restricted interests, the translational value of various behavioral assays used in rodents remains a subject of debate. Using genetically engineered mouse models with good construct and face validity will provide a platform for investigating underlying pathophysiological mechanisms and discovering potential therapeutic interventions which will hopefully translate into effective treatments for ASDs. We also illustrate several significant challenges in the field of modeling ASDs in rodents due to the considerable clinical and molecular heterogeneity associated with ASDs in humans.
Keywords: autism spectrum disorders, ASDs, mouse models, genetically engineered, behavior, therapeutic interventions
Introduction
Autism spectrum disorders (ASDs) are a range of neurodevelopmental disorders defined in The Diagnostic and Statistical Manual of Mental Disorders (5th ed; DSM-5; American Psychiatric (American Psychiatric Association, 2013) as persistent difficulties in both social communication/interaction and repetitive/restrictive patterns of behavior presenting early in childhood and causing significant functional impairment. The clinical presentations and severity of symptoms are quite heterogeneous. Comorbidities including intellectual disability (ID), movement disorders, and seizures are common. As the incidence of ASDs has climbed to 1-in-68 children and the costs of providing support to affected individuals is estimated at $35–85 billion per year in the United States alone, development of effective therapies has become an essential public health priority(Autism Developmental Disabilities Monitoring Network, 2014; Ganz, 2007). However, the diverse and often unknown etiologies of ASDs combined with limited experimental techniques available in human research have emphasized the need for valid and well-characterized animal models in order to further elucidate disease mechanisms and identify opportunities for intervention.
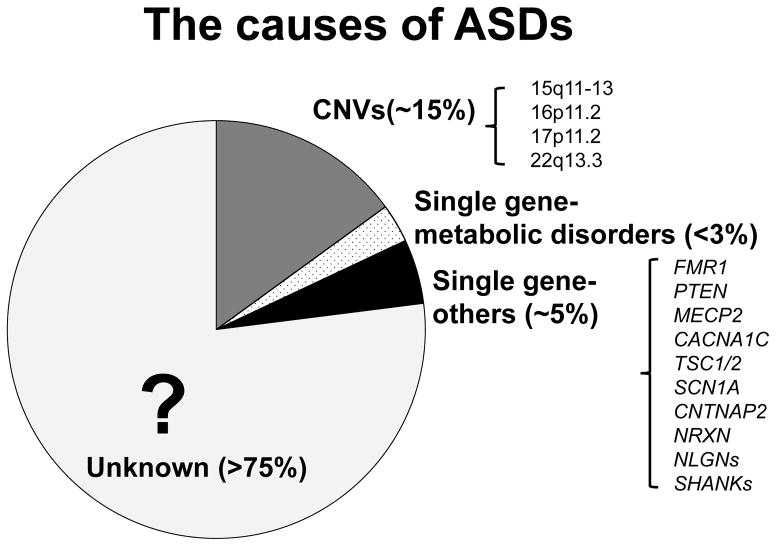
Twin and family studies indicate that genetic heritability contributes as much as 80–90% to the occurrence of ASDs, although the roles of environment and development are not well understood in the manifestation of symptoms (Miles, 2011). While the cause of ASDs has not been identified for the majority of patients, 20–30% of them have known genetic causes (see Figure 1) including ~5% with cytogenetically visible chromosomal abnormalities by traditional karyotyping technique, ~10–20% with sub-microscopic copy number variations (CNVs) detected by array based comparative genomic hybridization, and ~5% due to single-gene mutations (Miles, 2011). Caglayan (2010) provides a review of the known genetics of ASDs, but with the application of new genome technology in clinical and research settings, many more ASD genes have been discovered with more to come in the near future.
Figure 1. The causes of ASDs.
The cause for the majority (>75%) of ASD cases is unknown. Human genetics research and clinical studies have implicated chromosomal copy number variations (CNVs) in ~15% and single gene mutations in ~8% of ASD cases. Recessive mutations in single gene resulting in metabolic disorders account for a small number (<3%) of ASD patients. Mutations in non-metabolic disease related genes, frequently de novo mutations in synaptic genes, account ~5% of ASD cases. Both CNVs and single gene mutations have been associated with syndromic and non-syndromic forms of ASDs.
The considerable clinical and molecular heterogeneity of ASDs have posed a great challenge to studying autism in animal models. Ideally, a valid model for autism is required to meet both “construct validity” (the molecular defect mimics that seen in human ASD) and “face validity” (the phenotypes are analogous to human clinical presentation). The finding of causative genes or chromosomal defects in a small subset of ASD patients provides the opportunity to make genetically engineered mouse models with good construct validity. There is no reliable neuropathological hallmark found in ASD brains that reflect the underlying pathophysiology. Given that the current diagnostic criteria are purely behavioral in ASDs, valid mouse models should recapitulate analogous behavioral abnormalities relevant to the ASD diagnosis.
Great progress has been made in developing murine behavioral assays of social interaction, vocalizations, and repetitive/restrictive behaviors which are thoroughly reviewed elsewhere (Silverman et al., 2010b). Table 1 describes examples of commonly used behavioral tests for ASD-related and frequent comorbid behaviors. Many mouse models of known pathogenic mutations for human ASDs (i.e. with good construct validity) demonstrate abnormal behaviors which correlate to the pathological human behaviors in ASDs. However, caution is still warranted when evaluating ASD model mice, as there are few tests which have direct analogs between humans and mice. Future efforts should focus on refining existing behavioral paradigms and developing new ones with attention to reproducibility across cohorts of mice and between laboratories, sensitivity to quantifying subtle behavioral differences, and translational utility from pre-clinical models to human research. Likewise, the heterogeneity and spectrum of behavioral impairments seen in human patients likely have origins outside of a given pathogenic mutation and thus are difficult to capture in a single-gene mouse model. Future efforts to study multiple genes as well as gene and environment interactions are warranted.
Table 1.
Summary of ASD-Related Behavioral Tests
| Test | Description |
|---|---|
| Social Behavior | |
| 3-Chamber Social Test | Test mouse explores partitioned arena with neutral center chamber and side chambers containing stimuli; sociability phase measures time spent in chamber containing caged mouse versus chamber containing empty cage; social novelty phase measures time spent in chamber containing novel mouse versus chamber containing familiar mouse |
| Partition Test | Test mouse explores one half of partitioned arena with novel mouse on the other side of the barrier; time spent near the partition is a measure of social approach |
| Habituation-Dishabituation | Test mouse is repeatedly introduced to stimulus mouse; decreasing time spent in social interaction represents social recognition of stimulus mouse; then novel mouse can be introduced as stimulus mouse and increased time spent in social interaction represents social recognition |
| Direct Interaction Tests | Test mouse freely interacts with stimulus mouse and number, duration, and types of social interactions measured; variations include neutral pairing, i.e. stimulus is ovariectomized female or juvenile male, or agonistic pairing, i.e. stimulus is adult males in novel arena social (social dyadic) or test mouse’s home cage (resident intruder) |
| Juvenile Play | Pairs of juvenile mice allowed to freely interact; number, duration, and types of play behaviors can be measured |
| Nest Building | Test mouse is given pressed cotton material; amount of material shredded and arranged into nest is a measure of nest construction ability |
| Communication Behavior | |
| Pup Ultrasonic Vocalizations | Test mouse pup is briefly separated from dam and littermates; number, duration, and types of ultrasonic isolation calls recorded |
| Adult Ultrasonic Vocalizations | Test male mouse introduced to reproductive setting, i.e. estrus female or female urine, or agonistic setting, i.e. another male mouse (such as in resident-intruder paradigm); number, duration, and types of ultrasonic calls recorded |
| Scent-Marking | Test male mouse exposed to urine from estrus females; amount and proximity of male urine scent-marks to female urine stimuli is a measure of scent-marking communication behavior |
| Repetitive/Restricted Behavior | |
| Repetitive Grooming | Test mouse is observed for self-grooming in home cage or novel environments; duration and bouts of grooming are measures of repetitive behavior; skin lesions are measures of self-injurious behavior |
| Marble Burying | Test mouse introduced to cage containing marbles atop bedding; number of marbles buried is a measure of compulsive digging and bedding sifting behavior |
| Holeboard Exploration | Test mouse explores an arena with grid pattern of circular holes in floor allowing for head-dips into the holes; number and pattern of holes explored is a measure of perseverative exploration |
| Maze Reversal Learning | Test mouse must learn new location of hidden platform or escape hole in spatial mazes (see below); ability to learn new location is a measure of cognitive flexibility |
| Frequent Comorbidities | |
| Elevated Zero or Plus Mazes | Test mouse explores annular or cross-shaped arena with closed and open arms; time spent in open arms is a measure of anxiety-like behavior |
There have been many mouse models of ASDs involving environmental and pharmacological manipulations, possible naturally occurring mutations in inbred strains, and genetically engineered models targeting candidate human ASD genes. Efforts have been made to generate a comprehensive database of animal models of ASDs, with details about all levels of molecular, electrophysiological, and behavioral phenotypes (Kumar et al., 2011). While all of these categories have models which exemplify the analog murine behavioral abnormalities across the ASD domains, inclusion criteria for the scope of this overview were defined as models with good construct validity, i.e. multiple lines of human genetic evidence for the causality of the candidate mutation, as well as good face validity across murine ASD-like behavioral tests and associated comorbidities. Particular attention is drawn to models which have been used for testing therapeutic interventions, including genetic and pharmacological manipulations, as these hypotheses warrant further pre-clinical testing such that progress towards targeted treatments for patients may be made. These represent ideal opportunities for targeted animal models which can result in better understanding of disease mechanisms and directed therapeutic research without the confounds of genetic, developmental, and environmental diversity found in humans.
This overview covers a range of mouse models for genetic defects including (i) autism associated with defined genetic syndromes due to mutations in a single gene such as fragile X or Rett syndromes, (ii) non-syndromic autism associated with pathological mutations in single genes, such as the Neuroligin or SHANK family genes, and (iii) CNVs associated with autism such as 15q11-q13 or 16p11.2. Table 2 summarizes the key features of the ASD-like phenotype, comorbidities, therapeutic interventions and source for the models discussed in this review. One non-genetic model of an inbred strain with face validity across the three ASD-related behavioral domains is included due to its extensive characterization and use in pre-clinical testing, although many other examples of inbred, environmental, or exposure based models exist and are reviewed elsewhere (Moy and Nadler, 2008). When considering which mouse model to select, there are additional criteria to consider beyond how well the genetic construct recapitulates the human mutations and how many ASD-related behavioral domains are affected and to what degree. These include reproducibility of phenotypes across genetic backgrounds, laboratories, and testing conditions, as well as sensitivity to therapeutic interventions. While this information is not available for many of the more recently created models, we highlight those mutant mice which have withstood such rigorous evaluation and may hold great utility for mechanistic and interventional research. For all genetically engineered models, analyses from molecular, electrophysiological, and behavioral aspects were usually conducted by variable methods. Due to the limited scope of this overview, and considering the diagnosis of ASDs in humans is currently behavioral, we will primarily focus on reviewing and comparing the evidence from behavioral analyses. For readers interested in other aspects of these models, select reviews are available for some models (Chung et al., 2012; Zoghbi and Bear, 2012).
Table 2.
Summary of ASD Mouse Models
| Model | Social Interaction | Communication | Repetitive | Comorbidities | Interventions | Source |
|---|---|---|---|---|---|---|
| Single-Gene Syndromic Models | ||||||
| Fmr1 (−/Y) | ± sociability − social novelty ↑social anxiety |
↑pup calls | ↑stereotypies ↑perseveration |
±anxiety ↑activity ↓learning/memory |
enrichment ↓ mGluR |
JAX 004634 (FVB) JAX 003025 (B6) |
| Pten (cKO) | ↓sociability ↓social novelty ↓social recognition |
not tested | ↑self-grooming | ↑anxiety ↑activity ↓learning/memory ↑seizures |
rapamycin | JAX 006440 (B6) |
| Mecp2tm1.1Jae (exon 3) | ↑sociability ↑social novelty |
↑pup calls | not tested | ↓anxiety ↓activity ↓motor skills ↓learning/memory |
enrichment Ampakine CX546 choline diet IGF-1 |
MMRRC 000415 (B6) UC Davis |
| Mecp2tm1Hzo (308X) | −sociability −social novelty ↓pair interaction |
↓pup calls | ↑self-grooming | ↑anxiety ↓activity ↓motor skills ↓learning/memory |
none tested | JAX 005439 (B6) |
| Mecp2tm1.1Joez (T158A) | not tested | not tested | not tested | ↓anxiety ↓activity ↓motor skills ↓learning/memory |
none tested | JAX 017741 (B6) |
| Cacna1c (Ts2-Neo KI) | ↓sociability −recognition |
↓pup calls | ↑self-grooming ↑inflexibility ↑perseveration |
−anxiety ↓activity ↑learning/memory |
none tested | JAX 019547 (B6) |
| Tsc1 (+/−) | ↓pair interaction | not tested | not tested | ↓anxiety (♂) −activity −motor skills ↓learning/memory |
rapamycin | Dr. Cheadle of Cardiff University Dr. Kobayashi of Juntendo University |
| Tsc1 (cKO) | ↓sociability ↓social novelty |
↑pup calls | ↑self-grooming ↑inflexibility |
−activity ↓motor skills |
rapamycin | JAX 005680 (B6) |
| Tsc2 (+/−) | ↓sociability ↓social novelty (♂) ↓pair interaction (♀) |
↑pup calls | −inflexibility −perseveration |
−anxiety −activity ↓motor skills ↓learning/memory |
rapamycin lovastatin |
Dr. Kobayashi of Juntendo University JAX 004686 (B6) |
| Tsc2 (cKO) | ↓sociability ↓social novelty |
not tested | ↑perseveration −inflexibility |
±anxiety ↓motor skills |
rapamycin | Dr. Gambello of University of Texas |
| Scn1a (+/−) | ↓sociability ↓social novelty |
not tested | ↑self-grooming ↑circling |
↑anxiety ↑activity ↓learning/memory |
clonazepam | Dr. Catterall of University of Washington |
| Single-Gene Non-syndromic Models | ||||||
| Cntnap2 (−/−) | ↓sociability ↓juvenile play |
↓pup calls | ↑self-grooming ↑inflexibility |
↑activity ↑seizures |
risperidone | JAX 017482 (B6) |
| Nrxn1α (−/−) | ± sociability ± social novelty −recognition |
not tested | ±self-grooming | ± anxiety ± activity ↑motor skills −learning/memory |
none tested | JAX 006377 (B6-129) |
| Nlgn1 (−/−) | ↓sociability −social novelty |
not tested | ↑self-grooming | −anxiety −activity −motor skills ↓learning/memory |
D-cycloserine | JAX 008236 (B6-129) |
| Nlgn2 (−/−) | −sociability | ↓pup calls | −self-grooming | ↑anxiety ↓development ↓activity ↓motor skills |
none tested | JAX 008139 (B6) |
| Nlgn3 (−/−) | −sociability ↓social novelty |
↓adult calls | −perseveration −inflexibility |
−anxiety ↑activity −motor skills −learning/memory |
none tested | JAX 008394 (B6-129) |
| Nlgn3 (R451C KI) | ±sociability | ↓pup calls | −perseveration | −anxiety ↓development −activity ↓motor skills ±learning/memory |
none tested | JAX 008475 (B6) |
| Nlgn4 (−/−) | ±sociability ±social novelty −juvenile play |
−pup calls ±adult calls |
±self-grooming ↑perseveration ↑stereotypies −inflexibility |
−anxiety −development −activity −motor skills −learning/memory |
none tested | Dr. Ehrenreich of the Max Planck Institute |
| Shank1 (−/−) | −sociability −juvenile play |
↓pup calls ↓adult calls |
−self grooming −inflexibility |
↑anxiety ↓development ↓activity ↓motor skills ±learning/memory |
none tested | JAX 008108 (B6) |
| Shank2 (ex6–7) | ↓sociability −social novelty |
↓pup calls | ↑self-grooming ↑jumping |
↑anxiety ↑activity ↓learning/memory |
D-cycloserine CPDDB |
Dr. Kim of KAIST, South Korea |
| Shank2 (ex7) | ↓pair interaction | ↓pup calls ↓adult calls |
↑self-grooming | ↑anxiety ↑activity −learning/memory |
none tested | Dr. Boeckers of Ulm University |
| Shank3 (ex4–9B) | −sociability −social novelty ↓pair interaction |
−pup calls ↓adult calls |
↑self-grooming ↑inflexibility |
−anxiety ↓activity ↓motor skills ±learning/memory |
IGF-1 | JAX 017890 (B6) |
| Shank3 (ex4–9J) | ↓sociability ↓social novelty ↓pair interaction |
↓adult calls (♀) ↑adult calls (♂) |
↑self-grooming ↑inflexibility ↑stereotypy ↑perseveration |
−anxiety −activity ↓motor skills ↓learning/memory |
none tested | JAX 017442 (B6) |
| Shank3 (ex4–7) | −sociability ↓social novelty |
not tested | −self-grooming | −anxiety | none tested | Dr. Feng of Massachusetts Institute of Technology |
| Shank3 (ex13–16) | ↓sociability ↓social novelty ↓pair interaction |
not tested | ↑self-grooming −inflexibility |
↑anxiety −activity −motor skills −learning/memory |
none tested | JAX 017688 (B6) |
| Shank3 (ex11) | not tested | not tested | ↑self-grooming | not tested | none tested | Dr. Boeckers of Ulm University |
| Shank3 (ex21) | −sociability ↓social novelty −pair interaction −social recognition |
−adult calls | ↑self-grooming ↑inflexibility |
±anxiety ↓activity ↓motor skills ↓learning/memory |
none tested | JAX 018398 (B6) |
| Copy Number Variation Models | ||||||
| 15q11-13dup (Dp(7)15) | ↓sociability | ↑pup calls ↓adult calls |
↑inflexibility | ↑anxiety ↓activity |
none tested | Dr. Takumi of Hiroshima University |
| Ube3a BAC (triple copy) | ↓sociability | −pup calls ↓adult calls |
↑self-grooming | −anxiety −activity −motor skills |
none tested | Dr. Anderson of Harvard University |
| 16p11.2del (Df(7)16) | −sociability −social novelty |
not tested | ↑stereotypies | ↑activity ↓survival |
none tested | JAX 013128 (B6-129) |
| 16p11.2dup (Dp(7)16) | −sociability −social novelty |
not tested | −stereotypies | ↓activity −survival |
none tested | JAX 013129 (B6-129) |
| 17p11.2dup (Dp(11)17) | ↓sociability ↓social novelty |
↓pup calls | ↑perseveration | ↑anxiety ↑activity ↓motor skills ↓learning/memory |
enrichment | Dr. Lupski of Baylor College |
| Non-genetic Models | ||||||
| BTBR | ↓sociability (♂) ↓social novelty ↓pair interaction |
↑pup calls ↓adult calls |
↑self-grooming ↑inflexibility ↑stereotypy ↑perseveration |
↑anxiety −activity ↓motor skills −learning/memory |
D-cycloserine MPEP rapamycin risperidone |
JAX 002282 |
Key:
↑ is increased and ↓ is decreased; − is no change; ± is conflicting reports; ♂ is male; ♀ is female; −/− is homozygous for null allele; +/− is heterozygous for null allele; cKO is conditional knockout; KI is knock-in; JAX is Jackson Laboratory Mouse Supplier; MMRRC is Mutant Mouse Regional Resource Center; for strains of mice: B6 is C57BL/6J; FVB is FVB/NJ; 129 is 129SV
Single-Gene Syndromic Models
Fragile X Syndrome
Genetics
Fragile X Syndrome (FXS), the most common monogenic intellectual disability (ID) syndrome in males, is caused by CGG triplet repeat expansion in the 5′ untranslated (UTR) region of the FMR1 gene on the X chromosome. Clinically, FXS is characterized by ID, epilepsy, hyperactivity, dysmorphologies, and macroorchidism (Bhakar et al., 2012). In addition, 25–50% of FXS patients meet full ASD diagnostic criteria with many more demonstrating some degree of autistic behavior, and FXS patients account for up to 5% of the known causes of ASD cases. FMR1 mutations are the most frequent single-gene cause of ASD, primarily in males, but females can also be affected due to skewed patterns of X chromosome inactivation (Caglayan, 2010).
Fmr1 mutant mice
The Fmr1 mutant mouse was produced in 1994 by targeting coding exon 5 using gene targeting method in embryonic stem (ES) cells (The Dutch-Belgian Fragile X Consortium, 1994). The most commonly used mice are male mutants (Fmr1−/Y) in the FVB background (JAX 004624), although Fmr1 mice backcrossed to C57BL/6J, the most commonly used inbred strain for neurobehavioral research, are also available (JAX 003025). Ever since the Fmr1 knockout (KO) mice were reported, they have been extensively studied to dissect the molecular pathogenesis of ID and ASD. It should be noted that the Fmr1 mutant mouse has the same molecular consequence for the expression of the Fmr1 gene, i.e. loss of its encoded fragile X mental retardation protein (Fmrp), as that caused by triplet repeat expansion in humans. However, the molecular nature of the mutation mechanism is different between human and mouse. Fmr1 KO mice were produced by deleting coding exon 5, while in humans, the triplet repeat mutation causes the loss of expression of FMRP due to methylation of the promoter region of FMR1. Male Fmr1 mutant mice have demonstrated ASD-related and comorbid behaviors including abnormal sociability and social recognition as well as hyperactivity and impaired learning and memory, although there have been many conflicting reports (Moy and Nadler, 2008; Silverman et al., 2010b). Recently, Spencer et al. (2011) demonstrated that genetic background (such as congenic C57BL/6J versus individual hybrids of C57BL/6J with each of A/J, DBA/2J, FVB/NJ, 129S1/SvImJ, and CD-1) impacts the expression of all three ASD-related domains. No two genetic backgrounds had the same phenotypes, and only one out of six tested backgrounds (C57BL/6J x DBA/2J hybrid) showed abnormalities in all three of the ASD domains: sociability, USVs, and repetitive behaviors. Of note, some groups have identified specific ASD-related phenotypes which can be ameliorated by pharmacological and genetic interventions targeting excessive metabotropic glutamate receptor (mGluR)-mediated protein synthesis, hypothesized to be an important pathophysiological mechanism (Bhakar et al., 2012). These include abnormal social interaction, compulsive marble burying, and comorbidities such as increased seizures and abnormal motor learning on the rotarod. In addition, environmental enrichment has been shown to ameliorate anxiety-like behavior and aberrant habituation responses in Fmr1 mutant mice, and it remains to be seen whether this intervention can impact more ASD-relevant behaviors (Restivo et al., 2005).
Assessment
Despite the presence of inconsistent findings in some reports, Fmr1 mice, which mimic both the genetic causes as well as many of the neurobehavioral features of FXS, have been a valid and popular mouse model to dissect a monogenic form of human ASD. Perhaps more so than any other known genetic cause of ASD, the underlying pathophysiologic mechanism of dysregulated protein synthesis downstream of aberrant mGluR-mediated signaling in synapses has been characterized and ameliorated in the Fmr1 mouse. This body of research has translated into multiple drug candidates currently under testing in human clinical trials, with the promise of targeted therapeutic development on the horizon. Given the wide use of this model, reproducibility of key phenotypes, and sensitivity to a range of mGluR-modulating therapeutic interventions, we recommend continued research into understanding the correlation between the synaptic, cellular, and circuit defects and the behavioral phenotypes in these mutant mice in order to discover new therapies and refine existing drug targets. In addition, the mGluR hypothesis should be examined in other mouse models of ASDs in order to identify disparate genetic causes with shared underlying pathophysiology which may prove similarly sensitive to pharmacological treatments.
PTEN Hamartoma Tumor Syndromes
Genetics
Mutations in the tumor-suppressor gene phosphatase and tensin homolog (PTEN) were initially associated with Cowden Syndrome and other related hamartomatous disorders such as Bannayan-Riley-Ruvalcaba and Proteus syndromes. These syndromes are characterized by hamartomas and macrocephaly, with variably penetrant neurobehavioral features such as ID and ASD. In addition, mutations in PTEN have also been reported in cases with primary presentation of ASD and significant macrocephaly (Butler et al., 2005; Goffin et al., 2001). Recently, mutations in PTEN have been associated with sporadic or non-syndromic cases of ASD by whole exome sequencing methods (O’Roak et al., 2012a; O’Roak et al., 2012b). It is estimated that anywhere from 1–17% of patients with ASD and macrocephaly harbor PTEN mutations from several studies with small sample sizes, although the total number of ASD patients with PTEN mutations is unclear, but likely quite small (Butler et al., 2005; Buxbaum et al., 2007).
Pten conditional knockout mice
As conventional Pten homozygous mice are lethal, and heterozygotes develop tumors in a variety of tissues which impacts survival and performance on behavioral tests, conditional knockout (Pten-cKO) mice with loxP sites flanking exon 5 (JAX 006440) represent the best option to examine the ASD-related behaviors produced by Pten deficiency (Stiles et al., 2004). Kwon et al. (2006) conditionally deleted both copies of Pten in a subset of post-mitotic neurons and found that these mice recapitulated the macrocephaly seen in patients as well as ASD-related behavior including reduced sociability and preference for social novelty in the 3-chamber test, reduced interaction and impaired social recognition when investigating a juvenile conspecific, and impaired nest-building. Neither communication nor repetitive behavioral domains were tested. While motor learning using the rotarod and contextual and cued fear conditioning were normal, Pten-cKO demonstrated many other comorbid behaviors including spontaneous seizures, anxiety-like behavior in the open field and light/dark test, hyperactivity in novel environments, impaired learning and memory in the Morris water maze (MWM), and enhanced auditory startle, impaired prepulse inhibition (PPI), and reactivity during handling all suggestive of altered sensitivity to sensory stimuli. A subsequent study found that treatment with rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR), could rescue both the macrocephalic/hypertrophic and the behavioral phenotypes of Pten-cKO mice, including sociability and anxiety-like behavior (Zhou et al., 2009). A different group used the same Cre line to generate haploinsufficiency of Pten in post-mitotic neurons and again found that these mice also demonstrated altered sociability and social novelty preference in the 3-chamber test, as well as enhanced self-grooming, again validating the ASD-relevant phenotype of Pten mutants (Napoli et al., 2012).
Assessment
While PTEN mutations are probably rare, the human genetic evidence supports pathological mutations in this gene as causative for a subset of ASD cases. The Pten cKO mouse model exhibits several strengths, including robust and reproducible ASD-related and comorbid behaviors, and sensitivity to pharmacological interventions. Caveats include a lack of testing of communication-related and higher order repetitive behaviors and some weakness with regards to construct validity, as the cKO mice have deficiency of Pten in only a subset of post-mitotic neurons whereas humans with PTEN mutations are deficient in every cell throughout development. However, in addition to future research designed to address these areas, further work investigating the mechanisms responsible for ASD-related behavior in this model could lead to fruitful discoveries of targeted therapeutics for not only PTEN-related patients but perhaps a subset of ASDs that are known to have mild macrocephaly.
Rett Syndrome
Genetics
Rett syndrome (RTT) is a neurodevelopmental disorder predominantly affecting girls caused by mutations in the gene encoding methyl-CpG binding protein 2 (MECP2) on the X chromosome. The key clinical features of Rett syndrome include ID, progressive motor impairment, seizures, breathing abnormalities, and shortened lifespan. RTT was considered a prototypical single-gene cause of ASD in the DSM IV, and remains the most common cause of ASDs in females. RTT patients demonstrate autistic behaviors including stereotypies, loss of language abilities, and social withdrawal (Moretti and Zoghbi, 2006; Neul, 2012).
Mecp2 mutant mice
More than 10 different lines of mouse models targeting Mecp2 have been made (Calfa et al., 2011; Katz et al., 2012). Similar to MeCP2 mutations in humans, germline absence of Mecp2 is embryonically lethal in mice, so many models have been made with disruption of Mecp2 in various tissues, at various time points, or with various mutation strategies to produce a hypomorphic Mecp2 mutant allele (Calfa et al., 2011). Several lines of Mecp2 mice have been used more frequently and tested more rigorously for ASD-related phenotypes. Examples of Mecp2 mutant mice which are publically available and demonstrate ASD-relevant behavioral phenotypes include: targeted deletion of exon 3 (Mecp2tm1.1Jae available from the Mutant Mouse Regional Resource Center, UC Davis #000415 )(Chen et al., 2001), and a nonsense mutation generating a premature stop codon after amino acid 308 (Mecp2tm1Hzo, JAX 005439)(Shahbazian et al., 2002). Others have made point mutations with good construct validity to human mutations, such as a missense mutation knock-in of T158A (Mecp2tm1.1Joez, JAX 017741), but limited testing of ASD-relevant phenotypes have been conducted (Goffin et al., 2012). These mutant mice were tested both in heterozygous females and hemizygous males, the former representing better construct validity but often having milder and delayed onset of phenotypes, and the latter with more severe and earlier onset of phenotypes due to the lack of a compensatory wild-type allele provided by the mosaicism that arises from X chromosome inactivation. Each of these lines recapitulates the gross motor dysfunctions and shortened lifespan that characterize the disease. However, for behaviors relevant to ASD, as reviewed in Katz et al. (2012), different lines of Mecp2 mutant mice demonstrate variable phenotypes. We will highlight a few ASD-relevant but inconsistent features between two of the widely used lines. Social phenotypes range from decreased social interaction in Mecp2tm1Hzo mice to enhanced sociability and preference for social novelty in Mecp2tm1.1Jae mice. These two models also have altered isolation USVs as pups, although Mecp2tm1Hzo pups produce fewer isolation calls whereas the Mecp2tm1.1Jae pups produce more calls. Excessive and injurious self-grooming has been reported as an example of enhanced repetitive behaviors or motor stereotypies in the Mecp2tm1Hzo mice. Like social and communication behavior, anxiety-like behavior has also been inconsistent across models with some lines, such as Mecp2tm1.1Jae or Mecp2tm1.1Joez mice, demonstrating less anxiety-like behavior, whereas others, such as the Mecp2tm1Hzo mice, exhibit more anxious behavior. The reason for the significant variability related to ASD-like behavior is not immediately clear. While the molecular nature for the mutations in different lines of Mecp2 mice could be a factor, different experimental conditions may also be considered as a cause for the phenotypic variability. In contrast, poor motor performance and cognitive impairment have been more reliable phenotypes across lines, with all three of the mutants highlighted in this overview demonstrating impaired motor skills on the rotarod or in tests of gait abnormalities, as well as impaired learning and memory in a variety of paradigms including novel object recognition and contextual fear conditioning. Numerous pharmacological interventions have been tried and are reviewed in Katz et al. (2012), although few have made it into large pre-clinical or clinical trials.
Assessment
Like FXS and the Fmr1 mice, the multiple Mecp2 lines and extensive and often inconsistent behavioral phenotypes make this particular animal model challenging to cover in this overview. The comprehensive and critical studies of these mice represent the level of investigation that all ASD mouse models should be submitted to prior to validating their use for identifying therapeutic targets and pre-clinical studies. The robust and reproducible behavioral impairments in motor coordination and learning and memory, as well as the sensitivity of these phenotypes to various pharmacological manipulations, strongly support the use of Mecp2 mutant mice for continued pre-clinical research targeting improvement in these comorbid behaviors which greatly impair the lives of RTT patients. Based on the construct validity of the T158A knock-in model, it will be of great interest to determine whether these mice exhibit an ASD-like behavioral phenotype, and whether ASD-related phenotypes prove as reproducible and sensitive to intervention as the motor and learning phenotypes.
Timothy Syndrome
Genetics
Timothy Syndrome (TS) is caused by a common de novo mutation of pGly406Arg in exon 8 of the CACNA1C gene encoding the L-type voltage gated calcium channel, CaV1.2. The pGly406Arg mutation is believed to be a gain-of-function mutation affecting the calcium channel’s function. The mutation results in congenital heart disease, cardiac arrhythmias, dysmorphologies, and ID, with ASD affecting ~30–50% of TS patients (Splawski et al., 2011). The number of ASD patients with CACNA1C mutations is unknown, but likely quite rare. However, the finding of a mutation in a calcium channel in autism makes this direction particularly interesting regarding the neurobiology of ASDs.
Cacna1c mutant mice Ts2-neo mice
Due to the strong evidence supporting a gain-of-function mutation in human TS, mutant mice with the common point mutation of pGly406Arg are most appropriate to model human TS. Unexpectedly, mutant mice carrying homozygous and heterozygous pGly406Arg are not viable. Interestingly, mice heterozygous for this point mutation but retaining the neomycin-selection cassette (Ts2-neo, JAX 019547) are viable, which presumably is due to reduced levels of the mutant protein via transcriptional interference. Ts2-neo heterozygous mice demonstrated ASD-like behavior including reduced sociability in an extended version (measured across 4 hours) of the 3-chamber test although performance in the standard 10-minute version of this test and the habituation-dishabituation test of social recognition were normal. Reduced duration of USVs but normal number, amplitude, and frequency were observed in Ts2-neo pups. In addition, enhanced self-injurious grooming, repetitive marble burying and inflexibility in reversal learning of the MWM were observed (Bader et al., 2011; Bett et al., 2012). As far as comorbidities, the Ts2-neo mice demonstrated normal spatial learning in the initial acquisition of the MWM, and enhanced associative learning and memory in cued and contextual fear conditioning. These mice also demonstrated reduced locomotion and approach to novelty, although they had normal activity in familiar environments (i.e. home cage) and normal anxiety levels in light/dark test and elevated zero maze (Bader et al., 2011; Bett et al., 2012).
Assessment
Although TS is quite rare, mutations in other ion channel proteins account for additional cases of ASDs (Schmunk and Gargus, 2013). The Ts2-neo mice recapitulate the point mutation responsible for human TS, as well as abnormal behaviors across all three ASD-relevant domains and several comorbid behaviors. Given this degree of construct and face validity and the direct impact of channel mutations on neuronal excitability, further research into how this putative gain-of-function mutation results in aberrant circuit and behavioral function is warranted. Targeted therapies to restore normal calcium-mediated signaling and thus regulate excitability represent an exciting possibility for this syndrome and other ASDs resulting from mutations in ion channels and associated proteins.
Tuberous Sclerosis Complex
Genetics
Tuberous sclerosis complex (TSC) is an autosomal dominant neurodevelopmental syndrome caused by mutations in either TSC1 or TSC2, encoding the respective proteins hamartin and tuberin, which function as inhibitors of the mammalian target of rapamycin (mTOR) signaling cascade. TSC is characterized by the development of astrocytomas and cortical tubers with variable penetrance of epilepsy, ID, and autism. Fifty percent of TSC patients carry an ASD diagnosis, and TSC mutations account for ~1–4% of ASD cases (Caglayan, 2010).
Tsc1 heterozygous and conditional knockout mice
Mouse mutants for tuberous sclerosis complex have been characterized extensively. At least two lines of Tsc1 mutant mice have been made, one targeting exons 6–8 (available from Dr. Kobayashi, Juntendo University, Japan) and another targeting exons 5–7 (available from Dr. Cheadle, Cardiff University, United Kingdom) with similar results: homozygous Tsc1 mutant mice are lethal while heterozygotes are viable. Although, they have not shown the pathognomonic tuber formation in the brain or seizures, these mice have demonstrated ASD-like behavior. Tsc1 heterozygous mice exhibit reduced social interaction in pairs, which could be improved by treatment with rapamycin, and impaired nest building (Goorden et al., 2007; Sato et al., 2012). Communication and repetitive behaviors have not been formally evaluated. As for comorbidities, male mutants demonstrated reduced anxiety-like behavior in the light/dark test, and both sexes show impaired learning and memory in the MWM and contextual fear conditioning, with the learning and memory deficits rescued by rapamycin. Locomotor activity in the open field and motor performance on the rotarod were normal (Goorden et al., 2007; Sato et al., 2012). Interestingly, cKO mice targeting exons 17–18 of Tsc1 (JAX 005680) solely in cerebellar Purkinje cells demonstrate ASD-like behaviors including reduced sociability and social novelty in the 3-chamber test, increased pup USVs, increased self-grooming, and impaired reversal learning. They also showed comorbid behaviors including ataxic gait and impaired motor learning, but normal levels of locomotor activity. Treatment with rapamycin rescued social behavior and reversal learning in the MWM task (Tsai et al., 2012). These findings suggest an important role of Tsc1 in cerebellum in ASD-like behaviors but how the cerebellar circuit contributes to ASD-like behaviors, particularly social behaviors, remains unknown.
Tsc2 heterozygous mice and conditional knockout mice
Several lines of Tsc2 mutant mice have also been made (targeting exon 2 available as JAX 004686 or exons 2–5 available from Dr. Kobayashi), and again the homozygous KO mice are not viable. Tsc2 heterozygous mice also show some ASD-related phenotypes including reduced sociability (females) and reduced preference for social novelty (males) in the 3-chamber test, and reduced interaction in pairs (Reith et al., 2013; Sato et al., 2012). Abnormal pup USVs were observed in heterozygous or wild-type pups born to Tsc2 heterozygous dams, although adult USVs have not been tested (Young et al., 2010). Conventional heterozygous Tsc2 mice did not show increased repetitive behavior in marble burying or reversal learning of the MWM (Reith et al., 2013). Results from comorbid behavioral tests included normal levels of anxiety in the open field, impaired motor performance on the rotarod, and impaired learning and memory in the MWM and contextual fear conditioning, with the impaired memory phenotype rescued by treatment with rapamycin (Ehninger et al., 2008; Sato et al., 2012). Similar to Tsc1, mice with a conditional allele of Tsc2 were also made by floxing exons 2–4 of the Tsc2 gene (Tsc2flox) (mice available from Dr. Gambello, University of Texas Health Science Center). By crossing the Tsc2flox allele to a Cre line expressed in cerebellar Purkinje cells and to the conventional Tsc2 heterozygotes, mice with Tsc2 haploinsufficiency in all tissues but complete deficiency in Purkinje cells were produced. This manipulation is capable of recapitulating more severe ASD-related behaviors than those found in the conventional heterozygote, including reduced sociability and social novelty in the 3-chamber test, increased marble burying and poor motor learning, although tests for reversal learning in the MWM did not reveal a deficit (Reith et al., 2013).
Assessment
TSC represents one of the more common causes of syndromic ASD. The conventional heterozygous Tsc1/Tsc2 mice have high construct validity for recapitulating haploinsufficiency of TSC1 or TSC2 in human patients, and they demonstrate ASD-relevant social phenotypes and sensitivity to pharmacological intervention with rapamycin. However, these mice need further validation in the domains of communication and repetitive behavior, which make them weaker mouse models than some of the others presented in this overview. On the other hand, both Tsc1/Tsc2 cKO mice demonstrate both abnormal social and repetitive behavior and sensitivity to treatment with rapamycin, although how cerebellar circuitry gives rise to these ASD-relevant behaviors remains less understood. However, the reproducibility of several key behaviors across labs and the success of rapamycin for reversing both cellular and behavioral phenotypes underscore this potential therapeutic option for TSC and other ASDs, should they also be shown to be related to dysregulation of the mTOR pathway.
Dravet Syndrome
Genetics
Dravet syndrome is a rare developmental epilepsy syndrome caused by autosomal dominant mutations in the SCN1A gene encoding the voltage-gated sodium channel NaV1.1, and is characterized by intractable epilepsy, ID, and autism. About 25% of Dravet patients meet the full diagnostic criteria of ASD, although a greater proportion had autistic behavior (Li et al., 2011).
Scn1a heterozygous mice
While initial efforts developing and characterizing a mouse model of Scn1a haploinsufficiency focused on the epilepsy-related phenotypes, a recent paper from Han et al (2012) demonstrated that Scn1a heterozygous mice (targeting the last coding exon; from Dr. Caterall at the University of Washington) demonstrate ASD-like behavior including reduced sociability and preference for social novelty in the 3-chamber test, reduced sociability in pair interactions, avoidance of novel and social odors, increased circling in the open field which may represent a motor stereotypy, and enhanced self-grooming. These mice also exhibit anxiety-like behavior in the open field and elevated plus maze, hyperactivity in the open field, and impaired learning and memory in the MWM, Barnes maze, and contextual fear conditioning. Treatment with the benzodiazepine, clonazepam, at low doses which did not induce sedation or anxiolysis, rescued social and learning/memory phenotypes in these mice. Also, conditional deletion of Scn1a in forebrain inhibitory neurons recapitulated many of the ASD-related behaviors. Taken together, this evidence supports a mechanism whereby reduced GABA-ergic tone in Scn1a mutants results in disrupted excitatory/inhibitory balance and thus mediates the ASD behaviors.
Assessment
Scn1a mutants demonstrate both social and lower order repetitive behaviors, as well as comorbid epilepsy and impaired learning and memory consistent with Dravet syndrome patients. Also intriguing is the sensitivity of these animals to both pharmacological and genetic manipulations targeting the GABA neurotransmitter. Additional molecular studies are warranted in this direction. Further efforts to characterize communication and higher order repetitive behaviors as well as confirm the reproducibility of these findings across backgrounds, laboratories, and testing conditions may be very important. Given the frequent comorbidity of autism in epilepsy syndromes and vice versa, along with the face validity for both ASD-related behaviors and spontaneous seizures in the Scn1a mice, this model provides an opportunity for investigating how hyperexcitability or loss of inhibitory/excitatory balance can result in these often overlapping neuropsychiatric conditions.
Single-Gene Non-syndromic Models
Contactin-associated protein-like 2 (CNTNAP2)
Genetics
Homozygous or compound heterozygous mutations in contactin-associated protein-like 2 (CNTNAP2), a member of the neurexin super-family of neuronal cell adhesion molecules, were originally associated with a rare syndrome characterized by epilepsy, cortical dysplasia, and ID. Further genetic evidence implicates heterozygous variants in this gene with ASD or autism-related endophenotypes such as impaired language, although the contribution of these mutations to the prevalence of ASDs is likely quite small (Alarcon et al., 2008; Arking et al., 2008).
Cntnap2 mutant mice
Cntnap2 homozygous mice (targeting exon 1, JAX 017482) demonstrate reduced sociability in the 3-chamber test as well as during a juvenile play paradigm, reduced pup isolation USVs, enhanced self-grooming, and poor reversal learning in the MWM, in addition to seizures and hyperactivity (Penagarikano et al., 2011). This mouse model also demonstrates abnormal neuronal migration, reduced numbers of interneurons, and abnormal neuronal synchrony which may underlie the observed behavioral deficits. Treatment with risperidone, one of two FDA approved drugs for treatment of ASD-related behavior, reduced the hyperactivity and repetitive behavior phenotypes in Cntnap2 homozygous mice, but did not improve their sociability defect. Heterozygous mice did not show any abnormal behaviors or neuropathological findings.
Assessment
While human genetic evidence supports heterozygous CNTNAP2 mutations as associated with ASD, only mice homozygous for Cntnap2 mutations recapitulate ASD-related behavioral deficits in all three domains, with heterozygotes appearing normal. In addition to efforts to reproduce behavioral findings and identify the pathophysiological mechanisms underlying CNTNAP2 mutations, further work is warranted to identify why mice with haploinsufficiency of Cntnap2 did not have significant ASD-related phenotypes, unlike in humans with heterozygous CNTNAP2 mutations, and to determine whether this gene falls within a pathway shared with other causes of ASDs.
Neurexin1
Genetics
A number of studies have associated single gene mutations in and copy-number variations of the gene NRXN1, encoding neurexin1, with idiopathic autism, as well as other neuropsychiatric disorders including ID and schizophrenia (Bena et al., 2013). Neurexins are a family of pre-synaptic cell-surface proteins which serve as synaptic cell-adhesion molecules through binding to post-synaptic neuroligins, which have also been implicated in ASDs and will be covered below.
Nrxn1α mutant mice
Nrxn1contains two promoters, which produce two major classes of isoforms, Nrxn1α and Nrxn1β. Targeting the first coding exon produced mice lacking Nrxn1α isoforms (JAX 006377). These mice demonstrated a relatively mild behavioral phenotype with normal social behavior including normal social interaction with both juvenile and adult partners, normal social preference in the 3-chamber test, and intact social recognition; however, the mice did demonstrate impaired nest building and enhanced self-grooming. Anxiety-like behaviors in the elevated plus maze, locomotor activity in the open field, and memory/learning in the MWM were reported to be normal. Interestingly, they demonstrated improved motor learning in a rotarod task. Nrxn1α mutant mice also demonstrated impaired PPI, a phenotype seen in schizophrenia patients and variably reported to be found in ASD patients as well (Etherton et al., 2009). It is critical to note that this study was conducted using 129/SvJ x C57BL/6J hybrid mice. A recent study of social behaviors of Nrxn1α KO mice on a backcrossed C57BL/6J background revealed enhanced sociability (both sexes) and preference for social novelty (females only) in the 3-chamber test, increased aggression in free interaction with adult and juvenile conspecifics (males only), as well as hypoactivity (females only) and anxiety-like behavior (males only) (Grayton et al., 2013). The reason for sex specific difference in various behaviors is interesting because a similar phenomenon, but not exactly the same direction, is well documented in human ASDs.
Assessment
To date, the ASD-relevant social and repetitive phenotypes of Nrxn1α have not been shown to be particularly robust or reproducible across laboratories or genetic backgrounds. These conflicting data emphasize the importance of these factors in altering possible both the expression of behavioral phenotypes as well as the sensitivity of behavioral testing paradigms. However, given the strong genetic evidence implicating NRXN1 as an ASD causative gene, further experiments, such as examination of their ultrasonic vocalizations or other higher-order repetitive behaviors, are warranted in order to further validate this model before promoting its use for pre-clinical research into therapeutic interventions.
Neuroligin Family
Genetics
The neuroligin gene family (NLGN1-4) encodes post-synaptic cell-adhesion molecules, and like their pre-synaptic binding partners, the neurexins, are critical for synaptic function (Sudhof, 2008). A few studies have identified an enrichment of NLGN1 variants in ASD patients relative to controls or rare de novo mutations in NLGN1 identified only in single individuals with ASD (Glessner et al., 2009; O’Roak et al., 2012b; Ylisaukko-oja et al., 2005). Several studies have implicated mutations in NLGN3 and 4 with ASDs, although incidence of these mutations is unknown but is thought to be quite low (Jamain et al., 2003; Laumonnier et al., 2004; Ylisaukko-oja et al., 2005; Yu et al., 2011).
Nlgn1 mutant mice
Nlgn1 homozygous mice, targeting the first two coding exons (JAX 008136), demonstrated a mild ASD-related phenotype with reduced social approach to a caged conspecific, although their 3-chamber sociability and social novelty was normal, as was free interaction with a juvenile. They also demonstrated impaired nest-building and increased self-grooming, the latter being rescued by treatment with D-cycloserine, an NMDA receptor agonist. These mice also demonstrated altered sensitivity to pain (increased threshold) and heat (decreased threshold) stimuli although auditory stimuli were perceived normally, which may be related to the sensory processing abnormalities seen in ASD patients. As far as comorbidities go, these mice also demonstrated impaired spatial learning and memory in the MWM task, although their fear memory and learning was normal, with no increases in anxiety-like behavior in the open field, elevated plus maze, or light/dark test, normal activity levels in the open field, and normal motor performance on the rotarod (Blundell et al., 2010).
Nlgn2 mutant mice
Nlgn2 mutant mice, targeting the first coding exon (JAX 008139), have been tested for ASD-related behaviors and demonstrate abnormal communication with reduced number of pup USVs. However, tests of social behavior were normal as were tests of repetitive behavior. These mice did demonstrate several comorbid behaviors including delayed development (including growth parameters, eye opening, teeth eruption, and the acquisition of several reflexes), increased anxiety-like behavior, reduced activity and exploration, altered sensory sensitivity, and motor incoordination. However, it was noted that these phenotypes were not consistent across three different groups analyzing the mice emphasizing the importance of experimental conditions and genetic background once again (Blundell et al., 2009; Wohr et al., 2013).
Nlgn3 mutant mice
Nlgn3 homozygous mice, targeting exons 2–3 (JAX 008394), demonstrate a mild ASD-related phenotype with normal social interaction in pairs, normal sociability but reduced preference for social novelty in the 3-chamber test, reduced adult USVs, and no perseveration on the holeboard test or inflexibility on the MWM. They did demonstrate locomotor hyperactivity in the open field, but they had normal levels of anxiety-like behavior in the elevated plus maze, normal motor performance on the rotarod, and normal learning and memory in the MWM or (Radyushkin et al., 2009).
Nlgn3 R451C mice
In addition to the Nlgn3 knockout (KO) mouse, a knock-in (KI) was designed to recapitulate one of the mutations, R451C (JAX 008475), found in human ASD patients. Interestingly, the KI but not the KO mice demonstrated impaired sociability and enhanced spatial learning reproduced across two studies (Jaramillo et al., 2014; Tabuchi et al., 2007), whereas sociability was normal but USVs were reduced, and developmental delays and impaired motor learning were observed in another study of an independently generated KI line (Chadman et al., 2008). The explanation for these discrepancies is not immediately clear and may be due to genetic background or experimental conditions.
Nlgn4 mutant mice
Mice lacking Nlgn4 (from Dr. Ehrenreich at the Max Plank Institute, Germany) also demonstrate ASD-related behaviors including reduced social interaction in pairs, a very mild and gender-dependent difference in sociability and preference for social novelty in the 3-chamber test, and impaired nest-building. They also exhibited reduced adult USVs, increased self-grooming, compulsive marble burying and repetitive circling, although they did not show inflexibility in the reversal phase of the MWM. These mice demonstrated no increases in anxiety-like behavior, activity, learning and memory, or motor coordination (El-Kordi et al., 2013; Jamain et al., 2008). However, when Ey et al. (2012) examined later generations of this same line of mice in the same genetic background, they failed to recapitulate the social or communication deficits, again highlighting the possible impacts of genetic drift and variable experimental or environmental conditions on behavioral phenotypes.
Assessment
While the incidence of individual NLGN mutations is probably rare, the occurrence of pathogenic mutations in multiple gene family members and ASD-related behavior observed when disrupting each gene in mice highlights the importance of this gene family in normal synaptic function, and warrants future study of shared pathophysiological mechanisms between NLGN mutations as well as other ASD-causative genes which interact with members of this gene family including the NRXNs and SHANKs. However, the reduced robustness and reproducibility of the ASD-related behaviors across each of the Nlgn mutant lines may weaken their utility for testing pharmacological interventions at this time. Further investigation to understand the discrepancies among different studies may be helpful.
Shank Family
Genetics
The SHANK gene family includes 3 members encoding similar master scaffold proteins Src homology 3 and multiple ankyrin repeat domains (SHANK1-3), all of which, in particular SHANK3, have been strongly implicated from human genetic studies to contribute to ASD occurrence, accounting for 1–2% of cases. Most notably, deletions of human chromosome 22q13.3 including the SHANK3 locus results in a neurodevelopmental disorder known as Phelan-McDermid syndrome which includes severe autistic behavior in the majority of patients. Idiopathic cases of ASDs have also been associated with point mutations in or microdeletions of all SHANK family members (Jiang and Ehlers, 2013).
Shank1 mutant mice
Shank1 mutant mice, targeting exons 14–15 (JAX 008108), were the first to be reported, and several groups have published behavioral characterizations revealing mild or absent ASD-related phenotypes including normal sociability in the 3-chamber test as well as a juvenile play paradigm, reduced pup and adult USVs and abnormal scent-marking which may represent an important murine mode of communication, and normal levels of grooming; although, paradoxically, they demonstrated enhanced reversal learning in a radial maze. Behavioral tests for comorbidities revealed anxiety-like behavior in the open field and light/dark test, developmental delays, hypoactivity in the open field, impaired motor performance on the rotarod, impaired contextual fear memory, but intriguingly, enhanced spatial learning and memory in the radial maze (Hung et al., 2008; Silverman et al., 2011; Wohr et al., 2011).
Shank2 mutant mice
Two different lines of Shank2 mice were generated, with one line targeting exons 6–7 of Shank2 (Dr. Kim, KAIST, Korea). These mice demonstrated ASD-related behaviors including impaired sociability but normal social novelty in the 3-chamber test, reduced adult USVs, context-specific increases in self-grooming and repetitive jumping behavior, in addition to comorbidities including anxiety-like behavior in the elevated plus maze, profound hyperactivity in the open field test, and impaired learning and memory in the MWM. Treatment with the NMDAR agonist, D-cycloserine, and with the mGluR positive allosteric modulator, CPDDB, which can also enhance NMDAR function, improved the sociability phenotype as well as other biochemical and electrophysiological abnormalities (Won et al., 2012). The other line also had a similar mutation targeting exon 7 (Dr. Boeckers, Ulm University, Germany), and remarkably given the difficulty in replicating behavioral phenotypes in the same line of mice let alone in a different line of mice in different experimental conditions, these mice also demonstrated ASD-related behaviors. They exhibited reduced social contact in free interaction paradigms, increased pup USVs and more simplistic adult USVs, and increased self-grooming, as well as anxiety-like behavior in the light/dark test and profound hyperactivity in the open field test, but normal learning in memory in novel object recognition (Schmeisser et al., 2012).
Shank3 isoform-specific knockout mice
Five groups have made six different lines of Shank3 mutant mice by targeting different portion of exons. Due to the complex structure of Shank3’s transcripts, all of these lines result in an isoform-specific but not complete KO of Shank3. As the behavioral profiles differ from line to line, they will be described separately. First, exons 4–9 of Shank3 (JAX 017890) were targeted by Bozdagi et al. (2010) and further characterized by Yang et al (2012), finding an overall mild ASD-related phenotype including normal sociability and social novelty in the 3-chamber test, mild impairment in juvenile or male-female pair interactions, normal pup USVs but reduced adult USVs, increased self-grooming and inflexibility in the MWM. Tests of comorbid behavior revealed no anxiety-like behavior in the elevated plus maze or light/dark test, slight hypoactivity in the open field, impaired motor performance on the rotarod, and impaired learning and memory in novel object recognition, although acquisition of the MWM and fear conditioning were normal. It was also reported that IGF-1 can rescue the impaired motor performance in these animals, but its effects on ASD-related behaviors remain to be discovered (Bozdagi et al., 2013). Wang et al. (2011) also targeted exons 4–9 (JAX 017442) using a slightly different design, and found many ASD-related behaviors including impaired sociability and social novelty in the three chamber, reduced social interaction in freely interacting pairs, abnormal adult USVs, and increased self-grooming, perseveration in the holeboard test, stereotypic investigation of novel objects, and inflexibility in the reversal phase of the MWM. They also showed comorbid behaviors such as impaired motor performance on the rotarod and impaired learning and memory in social transmission of food preference, the MWM, and novel object recognition, although the mice displayed normal levels of anxiety and locomotor activity. Peca et al. (2011) generated one line targeting exons 4–7 (Dr. Feng, Massachusetts Institute of Technology) and another targeting exon 13–16 (JAX 017688), and due to the more profound phenotypes in the latter mutant, they focused their studies on exon 13–16 mutants. They found impaired sociability and preference for social novelty, as well as reduced pair interaction, and profound self-grooming leading to skin lesions although normal flexibility in MWM reversal learning. As well, they observed increased anxiety in the elevated zero maze and light/dark test, but normal activity in the open field, normal motor performance on the rotarod, and normal acquisition of the MWM. Communication behaviors were not assessed. Schmeisser et al. (2012) targeted exon 11 (Dr. Boeckers), but reported very minimal behavioral characterization of this line, although prominent self-grooming and skin lesions did occur. Lastly, the Worley lab targeted exon 21 of Shank3 (JAX 018398), and a recent paper by Kouser et al. (2013) tested ASD-related behaviors and found normal sociability but impaired social novelty in the 3-chamber test, normal social interaction and recognition when paired with a juvenile, impaired nest building, normal adult USVs, increased self-grooming and impaired reversal learning of the MWM. In addition, they observed increased anxiety-like behavior in the light/dark test but not in elevated plus maze or open field, reduced activity when initially presented to the open field, impaired motor performance on the rotarod, and impaired learning and memory in the MWM. In addition to experimental conditions and background which have been demonstrated to contribute to conflicting behavioral data in many of the other mutant models previously described, the question of targeting mutation design could explain some of the phenotypic variance seen in these mice. Shank3 has multiple intragenic promotors and alternatively spliced exons giving rise to great transcriptional complexity leading to many protein isoforms. As each mutant targeted only a subset of Shank3’s coding exons, it is possible that each mouse only lacks a subset of Shank3 protein isoforms which may have differential functions across brain regions, developmental time periods, or cell types.
Assessment
The strong genetic evidence implicating SHANKs in ASDs, plus the SHANK family proteins’ interaction with multiple other putative ASD-causative genes, provide support for the value of Shank mutant mice to model human ASDs. While the number of Shank mutant mice makes summarizing these models concisely a challenge, it is remarkable that the myriad Shank2 and Shank3 mice with different exonic deletions, genetic backgrounds, and testing paradigms reveal robust and relatively consistent findings of aberrant social, communicative, repetitive, and comorbid behavioral domains. This underscores these models as important for future investigation of pathophysiological mechanisms underlying mutations in synaptic genes and corresponding behavioral manifestations. These models may provide tools for the identification of therapeutic targets which may extend to other cases of ASD involving synaptic protein dysfunction. These mice also exemplify the importance of the specific targeting mutation in genes with such transcriptional complexity like the Shank family, which may result in different isoform-specific knockouts with varying consequences on synaptic and behavioral phenotypes. This principle may well apply to many other neuronal proteins with similar or even increased levels of isoform complexity, such as the neurexins and neuroligins. However, in order to better understand the human condition, complete knockout, rather than isoform-specific knockout mice are warranted, as microdeletions of SHANK genes are the genetic defect responsible for greater than 95% of SHANK-causing ASDs. A comprehensive study to compare different lines of isoform-specific mutant mice with complete Shank3 KO mice may provide insight for correlating genotype and phenotype in humans.
Models of Human Copy Number Variations (CNVs)
Copy Number Variations at human chromosome 15q11-q13
Genetics
Human chromosome 15q11-q13 is a genetic locus with multiple variants associated with distinct disease entities including maternal deletions causing Angelman Syndrome, paternal deletions causing Prader-Willi Syndrome, and increased copy numbers associated with sporadic autism. While Angelman and Prader-Willi syndromes have prominent neurobehavioral features including autistic behaviors, this overview will focus on the third category, as duplication or even triplication of 15q11-q13 of maternal origin is one of the most commonly (estimated prevalence of 1–4%) occurring cytogenetic abnormalities associated with ASDs (Depienne et al., 2009; Moreno-De-Luca et al., 2013). Patients carrying triplications of 15q11-q13 with maternal origin have nearly 100% penetrance of autistic phenotypes. In contrast, increased gene copy number of paternal origin is usually associated with much milder developmental delay (Depienne et al., 2009). There are more than 15 genes, including maternally, paternally, and biallelically expressed genes in the 15q11-q13 region. The brain-specific and maternally expressed UBE3A gene is the causative gene for Angelman syndrome. Because of the strong association of maternal origin of this duplication with ASD, it has been hypothesized that the Angelman UBE3A gene is a strong candidate gene in the region responsible for the molecular pathogenesis of ASD phenotypes.
Dp(7)15 duplication mice
As mouse chromosome 7 has a syntenic region with conservation of the genes, gene order, and imprinting pattern of the homologous human 15q11-q13 linkage group, mutant mice with a duplication of a 6 Mb region homologous to human 15q11-q13 chromosomal region (mice available from Dr. Takumi, Hiroshima University, Japan) by chromosomal engineering technique were made (Nakatani et al., 2009). Unexpectedly, mice carrying a paternal duplication, but not maternal duplication, demonstrated face validity in many of the ASD behavioral domains including reduced sociability in the 3-chamber test, aberrant number, quality, and developmental profile of pup and adult USVs, and reduced behavioral flexibility in reversal phases of the MWM and Barnes maze. In addition, they demonstrated increased anxiety-like behavior in both generalized fear following fear conditioning and reduced exploration of the open arms of an elevated plus maze (Nakatani et al., 2009).
Ube3a BAC transgenic mice
Another group examined mice carrying duplications or triplications of the gene Ube3a by BAC (bacterial artificial chromosome) mediated transgenic mice (mice available from Dr. Anderson, Harvard University) to examine the sufficiency of increasing this gene’s copy number to cause ASD-related behaviors (Smith et al., 2011). Indeed, mice carrying two additional transgenic copies of Ube3a demonstrated reduced sociability in the 3-chamber test, reduced adult USVs but normal pup USVs, and increased self-grooming without any abnormalities in activity or anxiety levels in the open field, motor performance on the rotarod, or cognition in a novel object recognition task. Mice with only one additional copy of Ube3a showed milder or normal behavior on ASD-related tests.
Assessment
As the 15q11-q13 locus demonstrates both relatively frequent prevalence and high penetrance for causing ASDs, these two lines of model mice represent excellent opportunities for exploring ASD mechanisms and pre-clinical tests of novel therapeutics. While the Dp(7)15 mice capture the genetic construct more precisely, by disrupting a similar number of genes as those found in most CNVs of human locus 15q11-q13, the opposite pattern of parental origin effect on this duplication between humans and rodents remains unexplained because other molecular features including parental origin expression of many genes in the region are highly conserved between humans and mice. The Ube3a triple copy mice offer the unique opportunity to examine whether dosage of the UBE3A gene alone is responsible for neurobehavioral phenotypes of this disorder, and similar work targeting other possible causative genes within the 15q11-q13 locus should be conducted. For both of these lines of mice, efforts to reproduce these behavioral findings across laboratories or genetic backgrounds, determine the pathophysiological mechanisms underlying the behavioral phenotypes, and test sensitivity to pharmacological, genetic, or environmental interventions are necessary.
Copy Number Variations at human chromosome 16p11.2
Genetics
Deletions and duplications at the 16p11.2 locus were first identified in ~1% of idiopathic ASD from genome-wide CNV analysis. Subsequently, these same CNVs have also been associated with a wide range of clinical presentation including ID, seizures, developmental delay, and obesity (Miller et al., 2011; Moreno-De-Luca et al., 2013). Similar to other CNVs associated with disorders, there are more than 20 genes mapped within the deleted or duplicated interval at 16p11.2 region. It is unclear which gene or genes are responsible for the major ASD-related clinical features.
Df(7)16 and Dp(7)16 mice
Taking advantage of the syntenic region on mouse chromosome 7, Horev et al. (2011) created mice carrying either a deletion (JAX 013128, Df(7)16) or the reciprocal duplication (JAX 013129, Dp(7)16) homologous to the human 16p11.2 region, and found some reciprocal behavioral phenotypes for these respective CNVs. Despite the strong genetic association of this locus in human ASD, both deletion and duplication mice showed no or relatively mild ASD-like behaviors, although behavioral phenotyping was limited to the 3-chamber test and observation in a novel, automated home cage environment. Df(7)16 mice demonstrated normal sociability and preference for social novelty in the 3-chamber test, but they did demonstrate prominent stereotypies in climbing behaviors in a modified home cage environment. While the deletion mice had impaired post-natal survival, those animals surviving weaning appeared grossly normal but demonstrated hyperactivity when introduced to a novel cage. Dp(7)16 mice demonstrated normal sociability and preference for social novelty in the 3-chamber test and no prominent stereotypies. The duplication mice did not have any viability problems, but they demonstrated hypoactivity in the modified home cage environment suggesting that the deletion and reciprocal duplication have opposite effects on some behavioral phenotypes.
Assessment
Given the prevalence of 16p11.2 CNVs and their frequent association with ASD in genetic studies, the 16p11.2 deletion and duplication mice clearly have strong molecular construct validity. However, these model mice are as of yet not validated across the ASD-relevant behavioral domains, and their utility as pre-clinical models hinges on whether and how robustly they recapitulate social, communicative, and repetitive behaviors. In addition, identification of the dose-sensitive genes within this locus, and why both duplications and deletions can manifest in ASD diagnoses, remain critical areas of future study.
Potocki-Lupski Syndrome or human chromosome 17p11.2 duplication
Genetic Evidence
Like the genetic locus at human chromosome 15q11-13, reciprocal CNVs at human chromosome 17p11.2 have been associated with neurobehavioral syndromes, in particular, with deletions and duplications of 17p11.2 causing Smith-Magenis (SMS) and Potocki-Lupski syndromes (PLS), respectively, with the latter characterized by ASD in 80% of patients, developmental delay, ID, hypotonia, failure-to-thrive, and congenital cardiovascular abnormalities (Potocki et al., 2007). This region includes the dosage-sensitive gene, Retinoic Acid Inducible 1 (RAI1) which likely is responsible for many aspects of the phenotype, because point mutations in RAI1 gene display major features of SMS. However, duplications of isolated RAI1 have not been identified in PLS (Potocki et al., 2007).
Dp(11)17 Mice
Mice share a syntenic region to human chromosome 17 on murine chromosome 11 allowing for the construction of mouse models (available from Dr. Lupski, Baylor College of Medicine) by chromosomal engineering for this disorder with good molecular construct validity (Walz et al., 2003). Duplication of 17p11.2 (Dp(11)17) mice have demonstrated ASD relevant behaviors including reduced sociability and preference for social novelty in the 3-chamber test, enhanced social dominance in a tube test, enhanced aggression and reduced habituation in social cognition tests, altered developmental time course of pup USVs, and perseverative exploration of the holeboard (Lacaria et al., 2012; Molina et al., 2008; Ricard et al., 2010). Comorbid behaviors include anxiety-like behavior in the open field and elevated plus maze, hyperactivity in the open field, motor impairments on the rotarod, and impaired contextual fear memory (Lacaria et al., 2012; Molina et al., 2008; Walz et al., 2006). Rearing Dp(11)17 mice in an enriched environment rescued a subset of behaviors including aberrant social interaction, anxiety-like behavior, and impaired contextual fear memory, but exacerbated others such as perseverative holeboard exploration (Lacaria et al., 2012).
Assessment
The high penetrance of ASD behavior in Potocki-Lupski Syndrome patients together with the many ASD-related behavioral abnormalities in this mouse model support its use to investigate the pathophysiology of this CNV. As a series of studies of these mice have produced ASD-relevant findings, the reproducibility and robustness of this model’s behavioral phenotype, as well as its amenability to an environmental intervention, makes it one of the stronger models of CNV-causing ASDs. Further work should be focused to explore which genes within the region represent dosage-sensitive targets for mediating neurobehavioral phenotypes, and whether pharmacological interventions can be identified to target these genes.
Non-genetic models: the example of BTBR T+tff J mice
In addition to the genetic engineering strategy for constructing mouse mutants recapitulating human mutations, many other mouse models for ASDs have been developed by examining behavioral variability between inbred mouse strains or by manipulating the environmental or toxicological exposures of non-genetic models. While this overview does not aim to cover the full spectrum of non-genetic models, attention is drawn to one of the best studied models of ASD-related behavior in an inbred strain of mice, the BTBR T+tff J mice (JAX 002282), as they compare to C57BL/6J mice, the most commonly used strain of mice for neurobehavioral studies. Extensive behavioral testing on this strain of mice has been conducted, and Meyza et al. (2013) reviews it in greater detail. As for selected ASD-related phenotypes, BTBR male mice regularly show a lack of sociability and preference for social novelty in the 3-chamber task (although females show more variable phenotypes), and both adult and juvenile BTBR mice also demonstrate reduced social interaction in pairs. USVs represent a more complicated picture, as isolated BTBR pups call more loudly and more frequently than pups of other inbred strains. However, adult male BTBR mice emit fewer USVs, with reduced complexity of the call structure, and they display reduced scent-marking behavior. BTBR mice also demonstrate increased self-grooming, perseverative marble burying, stereotyped object exploration, and impaired reversal learning on the MWM. When tested for comorbid behaviors, they demonstrate inconsistent performance in tests of anxiety-like behavior, hyperactivity in the open field, impaired motor performance on the rotarod, and normal learning and memory in the MWM acquisition phase. Many pharmacological and non-pharmacological interventions have been tested, and highlighted results include reduced self-grooming but unchanged sociability with MPEP (mGluR5 antagonist), GRN-529 (selective negative modulator of mGluR5) or risperidone (D2 antagonist) treatment; improved sociability and reduced self-grooming with D-cycloserine (NMDA receptor agonist) treatment; and improved sociability with rapamycin (mTOR pathway inhibitor) treatment (Burket et al., 2013; Burket et al., 2014; Silverman et al., 2010a). Interestingly, these drugs have had positive effects in other genetic mouse models of ASDs.
Assessment
While not originally bred with ASD-phenotypes in mind, many strains of inbred mice demonstrate variable behavioral phenotypes, including prominent ASD-related phenotypes in the BTBR strain. While these models do not recapitulate known genetic causes of ASDs in humans, and thus caution is warranted about how well findings from these mice will translate from pre-clinical studies to effective therapies before further genetic evidence emerges. Studies underway will possibly identify novel genetic variants in these mice which contribute to the development of ASD-related behaviors, with the hope of using this knowledge to better understand the causes of human autism. They also offer a unique opportunity to test pharmacological interventions discovered in genetically engineered ASD models to determine whether novel therapeutics can ameliorate ASD-related behaviors across models, and thus support shared pathophysiological mechanisms uniting these diverse etiologies.
Conclusions
This overview has highlighted the common genetic models of ASDs with the strongest human genetic and murine behavioral evidence to support their use for developing hypotheses about underlying pathogenic mechanisms and testing therapeutic interventions. The considerable molecular and clinical heterogeneity of ASDs in humans make it harder to generalize the knowledge learned from individual animal models. The challenging but urgent question is whether some shared molecular and circuit mechanisms can be defined in near future. Before that, selection of the most appropriate mouse model of ASDs depends on both the strength of the construct and face validity, but also the specific research question being asked and specific patient population to be targeted. In those models with the longest history of use or largest number of genetically engineered mice targeting specific gene families, there is more known about the robustness and reproducibility of behavioral findings, the underlying cellular and synaptic pathophysiology, as well as the sensitivity of these models to amelioration with pharmacological and environmental rescues. This may also emphasize the need for replication of preliminary findings and continued refinement of hypotheses about how a given mutation leads to the observed behaviors for newly developed models.
There also exist many opportunities for the creation and validation of other mouse models, as recent genetic studies have provided strong evidence for other ASD-causative genes such as CHD8 or MET, although whether mice harboring these mutations recapitulate autistic phenotypes and prove to have usefulness and predictive validity remains to be seen. Likewise, there are existing mouse models with good face validity across the three domains of ASD-related behavior, such as Reelin or Cadps2 mutant mice. However, pathological mutations of these genes have not been found in ASD patient populations in recent whole genome sequencing projects.
Modeling human ASDs in mice remains a significant challenge. The lack of reliable biomarkers or neuropathological hallmarks in human ASD is the major obstacle to dissecting the pathophysiology using these genetically engineered ASD mouse models. The current analytic paradigm for ASD mouse models is primarily focused on the analysis of behavioral impairments and defects in synaptic function. As illustrated by many models in this review, significant variability of behaviors occurs between different models and the sensitivity of behavioral assays poses many challenging questions for the translational value of these models in pre-clinical trials. Similarly, but not reviewed here, synaptic function studies in these models have also uncovered a wide range of synapse-specific or model-specific defects associated with a given genotype. These observations demand new analytic paradigms to better model human ASDs using genetically engineered mice.
Acknowledgments
We would like to thank Jiang lab members for their contribution to this review, in particular Andrea L. Pappas for her critical reading of the manuscript. The research in the Jiang lab is supported by NIMH grant 5R01MH098114-02, Ruth K. Broad Foundation, and Duke Institute for Brain Sciences. ALB is a pre-doctoral fellow in Duke University’s Medical Scientist Training Program supported by the Ruth K. Broad Foundation and Autism Science Foundation.
Footnotes
Due to the limited space for references, we apologize for many papers that are not included in this overview.
References
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American journal of human genetics. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. American journal of human genetics. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism Developmental Disabilities Monitoring Network. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and mortality weekly report. Surveillance summaries. 2014;63(Suppl 2):1–21. [PubMed] [Google Scholar]
- Bader PL, Faizi M, Kim LH, Owen SF, Tadross MR, Alfa RW, Bett GC, Tsien RW, Rasmusson RL, Shamloo M. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bena F, Bruno DL, Eriksson M, van Ravenswaaij-Arts C, Stark Z, Dijkhuizen T, Gerkes E, Gimelli S, Ganesamoorthy D, Thuresson AC, Labalme A, Till M, Bilan F, Pasquier L, Kitzis A, Dubourgm C, Rossi M, Bottani A, Gagnebin M, Sanlaville D, Gilbert-Dussardier B, Guipponi M, van Haeringen A, Kriek M, Ruivenkamp C, Antonarakis SE, Anderlid BM, Slater HR, Schoumans J. Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2013;162B:388–403. doi: 10.1002/ajmg.b.32148. [DOI] [PubMed] [Google Scholar]
- Bett GC, Lis A, Wersinger SR, Baizer JS, Duffey ME, Rasmusson RL. A Mouse Model of Timothy Syndrome: a Complex Autistic Disorder Resulting from a Point Mutation in Cav1.2. North American journal of medicine & science. 2012;5:135–140. doi: 10.7156/najms.2012.053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annual review of neuroscience. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes, brain, and behavior. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Molecular autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Molecular autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. D-Cycloserine improves sociability in the BTBR T+ Itpr3tf/J mouse model of autism spectrum disorders with altered Ras/Raf/ERK1/2 signaling. Brain research bulletin. 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Benson AD, Tang AH, Deutsch SI. Rapamycin improves sociability in the BTBR T(+)Itpr3(tf)/J mouse model of autism spectrum disorders. Brain research bulletin. 2014;100:70–75. doi: 10.1016/j.brainresbull.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. Journal of medical genetics. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsater H, Rastam M, Smith CJ, Silverman JM, Hollander E, Leboyer M, Gillberg C, Verloes A, Betancur C. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2007;144B:484–491. doi: 10.1002/ajmg.b.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan AO. Genetic causes of syndromic and non-syndromic autism. Developmental medicine and child neurology. 2010;52:130–138. doi: 10.1111/j.1469-8749.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- Calfa G, Percy AK, Pozzo-Miller L. Experimental models of Rett syndrome based on Mecp2 dysfunction. Experimental biology and medicine. 2011;236:3–19. doi: 10.1258/ebm.2010.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism research: official journal of the International Society for Autism Research. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature genetics. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chung L, Bey AL, Jiang YH. Synaptic plasticity in mouse models of autism spectrum disorders. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2012;16:369–378. doi: 10.4196/kjpp.2012.16.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, Delorme R, Chaste P, Siffroi JP, Chantot-Bastaraud S, Benyahia B, Trouillard O, Nygren G, Kopp S, Johansson M, Rastam M, Burglen L, Leguern E, Verloes A, Leboyer M, Brice A, Gillberg C, Betancur C. Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biological psychiatry. 2009;66:349–359. doi: 10.1016/j.biopsych.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature medicine. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kordi A, Winkler D, Hammerschmidt K, Kastner A, Krueger D, Ronnenberg A, Ritter C, Jatho J, Radyushkin K, Bourgeron T, Fischer J, Brose N, Ehrenreich H. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behavioural brain research. 2013;251:41–49. doi: 10.1016/j.bbr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey E, Yang M, Katz AM, Woldeyohannes L, Silverman JL, Leblond CS, Faure P, Torquet N, Le Sourd AM, Bourgeron T, Crawley JN. Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin4. Genes, brain, and behavior. 2012 doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Archives of pediatrics & adolescent medicine. 2007;161:343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, Glaberson W, Flory J, Otieno F, Garris M, Soorya L, Klei L, Piven J, Meyer KJ, Anagnostou E, Sakurai T, Game RM, Rudd DS, Zurawiecki D, McDougle CJ, Davis LK, Miller J, Posey DJ, Michaels S, Kolevzon A, Silverman JM, Bernier R, Levy SE, Schultz RT, Dawson G, Owley T, McMahon WM, Wassink TH, Sweeney JA, Nurnberger JI, Coon H, Sutcliffe JS, Minshew NJ, Grant SF, Bucan M, Cook EH, Buxbaum JD, Devlin B, Schellenberg GD, Hakonarson H. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. American journal of medical genetics. 2001;105:521–524. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- Goffin D, Allen M, Zhang L, Amorim M, Wang IT, Reyes AR, Mercado-Berton A, Ong C, Cohen S, Hu L, Blendy JA, Carlson GC, Siegel SJ, Greenberg ME, Zhou Z. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nature neuroscience. 2012;15:274–283. doi: 10.1038/nn.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Annals of neurology. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Grayton HM, Missler M, Collier DA, Fernandes C. Altered social behaviours in neurexin 1alpha knockout mice resemble core symptoms in neurodevelopmental disorders. PloS one. 2013;8:e67114. doi: 10.1371/journal.pone.0067114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, Krieger AM, Buja A, Henkelman RM, Wigler M, Mills AA. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T Paris Autism Research International Sibpair S. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature genetics. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo TC, Liu S, Pettersen A, Birnbaum SG, Powell CM. Autism-related neuroligin-3 mutation alters social behavior and spatial learning. Autism research: official journal of the International Society for Autism Research. 2014;7:264–272. doi: 10.1002/aur.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, Guy J, Howell CJ, Kron M, Nelson SB, Samaco RC, Schaevitz LR, St Hillaire-Clarke C, Young JL, Zoghbi HY, Mamounas LA. Preclinical research in Rett syndrome: setting the foundation for translational success. Disease models & mechanisms. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, Liu S, Jaramillo TC, Bangash M, Xiao B, Worley PF, Powell CM. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:18448–18468. doi: 10.1523/JNEUROSCI.3017-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Wadhawan R, Swanwick CC, Kollu R, Basu SN, Banerjee-Basu S. Animal model integration to AutDB, a genetic database for autism. BMC medical genomics. 2011;4:15. doi: 10.1186/1755-8794-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaria M, Spencer C, Gu W, Paylor R, Lupski JR. Enriched rearing improves behavioral responses of an animal model for CNV-based autistic-like traits. Human molecular genetics. 2012;21:3083–3096. doi: 10.1093/hmg/dds124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. American journal of human genetics. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BM, Liu XR, Yi YH, Deng YH, Su T, Zou X, Liao WP. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy & behavior: E&B. 2011;21:291–295. doi: 10.1016/j.yebeh.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Defensor EB, Jensen AL, Corley MJ, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. The BTBR T+ tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behavioural brain research. 2013;251:25–34. doi: 10.1016/j.bbr.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JH. Autism spectrum disorders--a genetics review. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- Miller DT, Nasir R, Sobeih MM, Shen Y, Wu BL, Hanson E. 16p11.2 Microdeletion. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 2011. [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Human molecular genetics. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca D, Sanders SJ, Willsey AJ, Mulle JG, Lowe JK, Geschwind DH, State MW, Martin CL, Ledbetter DH. Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Molecular psychiatry. 2013;18:1090–1095. doi: 10.1038/mp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Current opinion in genetics & development. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Molecular psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld R, Giulivi C. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PloS one. 2012;7:e42504. doi: 10.1371/journal.pone.0042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues in clinical neuroscience. 2012;14:253–262. doi: 10.31887/DCNS.2012.14.3/jneul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, Munson J, Hiatt JB, Turner EH, Levy R, O’Day DR, Krumm N, Coe BP, Martin BK, Borenstein E, Nickerson DA, Mefford HC, Doherty D, Akey JM, Bernier R, Eichler EE, Shendure J. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012a;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012b;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, Glaze D, Krull K, Lee JA, Lewis RA, Mendoza-Londono R, Robbins-Furman P, Shaw C, Shi X, Weissenberger G, Withers M, Yatsenko SA, Zackai EH, Stankiewicz P, Lupski JR. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. American journal of human genetics. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes, brain, and behavior. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK, Gambello MJ. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiology of disease. 2013;51:93–103. doi: 10.1016/j.nbd.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard G, Molina J, Chrast J, Gu W, Gheldof N, Pradervand S, Schutz F, Young JI, Lupski JR, Reymond A, Walz K. Phenotypic consequences of copy number variation: insights from Smith-Magenis and Potocki-Lupski syndrome mouse models. PLoS biology. 2010;8:e1000543. doi: 10.1371/journal.pbio.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nature communications. 2012;3:1292. doi: 10.1038/ncomms2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck S, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Schmunk G, Gargus JJ. Channelopathy pathogenesis in autism spectrum disorders. Frontiers in genetics. 2013;4:222. doi: 10.3389/fgene.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain research. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nature reviews Neuroscience. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Science translational medicine. 2011;3:103ra197. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism research: official journal of the International Society for Autism Research. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R. Timothy Syndrome. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 2011. [Google Scholar]
- Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Developmental biology. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz K, Caratini-Rivera S, Bi W, Fonseca P, Mansouri DL, Lynch J, Vogel H, Noebels JL, Bradley A, Lupski JR. Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes by chromosome engineering in mice: phenotypic consequences of gene dosage imbalance. Molecular and cellular biology. 2003;23:3646–3655. doi: 10.1128/MCB.23.10.3646-3655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz K, Paylor R, Yan J, Bi W, Lupski JR. Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2) The Journal of clinical investigation. 2006;116:3035–3041. doi: 10.1172/JCI28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Human molecular genetics. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PloS one. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Silverman JL, Scattoni ML, Turner SM, Harris MJ, Saxena R, Crawley JN. Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behavioural brain research. 2013;251:50–64. doi: 10.1016/j.bbr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Rehnstrom K, Auranen M, Vanhala R, Alen R, Kempas E, Ellonen P, Turunen JA, Makkonen I, Riikonen R, Nieminen-von Wendt T, von Wendt L, Peltonen L, Jarvela I. Analysis of four neuroligin genes as candidates for autism. European journal of human genetics: EJHG. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, He X, Yao D, Li Z, Li H, Zhao Z. A sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with autism spectrum disorders in a Chinese Han cohort. Behavioral and brain functions: BBF. 2011;7:13. doi: 10.1186/1744-9081-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]