Summary
Gastrointestinal stromal tumors (GISTs) are resistant to traditional chemotherapy but are responsive to the tyrosine kinase inhibitors imatinib and sunitinib.1 The use of these agents has improved the outcome for patients but is associated with adverse effects, including hypothyroidism.2 Multiple mechanisms of this effect have been proposed, including decreased iodine organification3 and glandular capillary regression.4 Here we report the finding of consumptive hypothyroidism caused by marked overexpression of the thyroid hormone–inactivating enzyme type 3 iodothyronine deiodinase (D3) within the tumor. Affected patients warrant increased monitoring and may require supernormal thyroid hormone supplementation.
A 51-year-old man was found to have a large abdominal tumor during elective inguinal hernia repair. Given the absence of disseminated disease, a partial gastrectomy and transverse colectomy with en bloc resection of the primary tumor were performed with curative intent. Pathological examination led to the diagnosis of a GIST. Two years later, computed tomographic surveillance revealed liver lesions that were confirmed on biopsy as metastatic GIST. This finding prompted the initiation of treatment with imatinib mesylate (which was discontinued after 10 months owing to disease progression) and then sorafenib (which was discontinued after 5 months owing to disease progression). Six months after discontinuing sorafenib, the patient was enrolled in a research trial of sunitinib therapy, which at that time had not yet been approved as second-line therapy.
Baseline testing that was performed immediately before the initiation of sunitinib therapy revealed hypothyroidism, with a thyrotropin level of 149 μU per milliliter (normal range, 0.5 to 5.0 μU per milliliter), a total thyroxine level of 6.1 μg per deciliter (normal range, 5.0 to 11.0 μg per deciliter [79 nmol per liter; normal range, 64 to 142 nmol per liter]), and a triiodothyronine uptake of 28% (normal range, 25 to 35%). Testing for serum thyroperoxidase antibodies was negative. Despite the administration of levothyroxine doses as high as 300 μg (3.2 μg per kilogram of body weight) daily and excellent adherence, the patient’s subsequent serum thyrotropin levels remained elevated (range, 70 to 181 μU per milliliter), with subnormal levels of serum thyroxine (range, 1.4 to 4.2 μg per deciliter [18 to 54 nmol per liter]) and triiodothyronine (17 to 40 ng per deciliter; normal range, 70 to 170 ng per deciliter [0.3 to 0.6 nmol per liter; normal range, 1.1 to 2.6 nmol per liter]). During hypothyroxinemia, the level of serum reverse triiodothyronine was elevated, at 1545 pg per milliliter (normal range, 30 to 250 pg per milliliter [2.37 nmol per liter; normal range, 0.05 to 0.38 nmol per liter]). Sunitinib was continued until the patient’s death from tumor progression 23 months later.
Methods
Deiodination Assays
We used high-performance liquid chromatography to assay the activity of D3, as described previously,5 in 150-mm3 reactions containing 0 to 150 μg of cellular protein, 10 mM dithiothreitol, and 0.5 to 500 nM iodine-125–labeled triiodothyronine (PerkinElmer). Assays for type 1 deiodinase (D1) were in 150-mm3 reactions containing 3 μg of protein and 100 nM iodine-125–labeled reverse triiodothyronine. Assays for type 2 deiodinase (D2) were in 75-mm3 reactions containing 10 μg of protein, 0.2 versus 100 nM iodine-125–labeled thyroxine, and 100 nM triiodothyronine. Studies were approved by the institutional review board at each study center.
Other Cellular Analyses
Immunohistochemical analyses were performed with the use of 1:1000 polyclonal rabbit anti-D3 antibody (Novus Biologicals), as described previously.6 Cells were propagated in Iscove’s Modified Dulbecco’s medium with 15% fetal-calf serum (GIST-T1 cells),7 in RPMI medium with 10% fetal-calf serum (SK-N-AS neuroblastoma cells), or in Dulbecco’s Modified Eagle’s medium with 10% fetal-calf serum (MCF-7 breast-cancer cells) (Clontech). Unstripped fetal-calf serum was used to supply physiologic concentrations of thyroxine and triiodothyronine.8,9 For all cell lines, the medium contained 100 nM sodium selenite. All reagents were purchased from Sigma-Aldrich, except imatinib (LC laboratories) and iopanoic acid (MP Biochemicals).
GIST-T1 cells were plated at 5000 cells per well, and after 72-hour exposure to drugs, viability was analyzed by means of CellTiter-Glo Luminescent Cell Viability Assay (Promega). Proliferation was measured with the use of a Coulter counter.
Immunoblotting of cellular protein (20 μg per specimen) was performed with the use of rabbit polyclonal antibody against poly(adenosine diphosphate [ADP]-ribose) polymerase (Roche).
Gene Expression
We extracted total RNA using TRIzol (Ambion) and then performed reverse transcription using the iScript complementary DNA synthesis kit (Bio-Rad). All samples were quantified with the use of the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad).
Statistical Analysis
We used analysis of variance to perform all comparisons in cell-culture experiments. P values of less than 0.05 were considered to indicate statistical significance. We applied the principle of closed testing to hold the familywise type I error rate below 5% for each of the three-group comparisons of D3 activity and messenger RNA expression.
Results
Deiodinase Expression in GIST
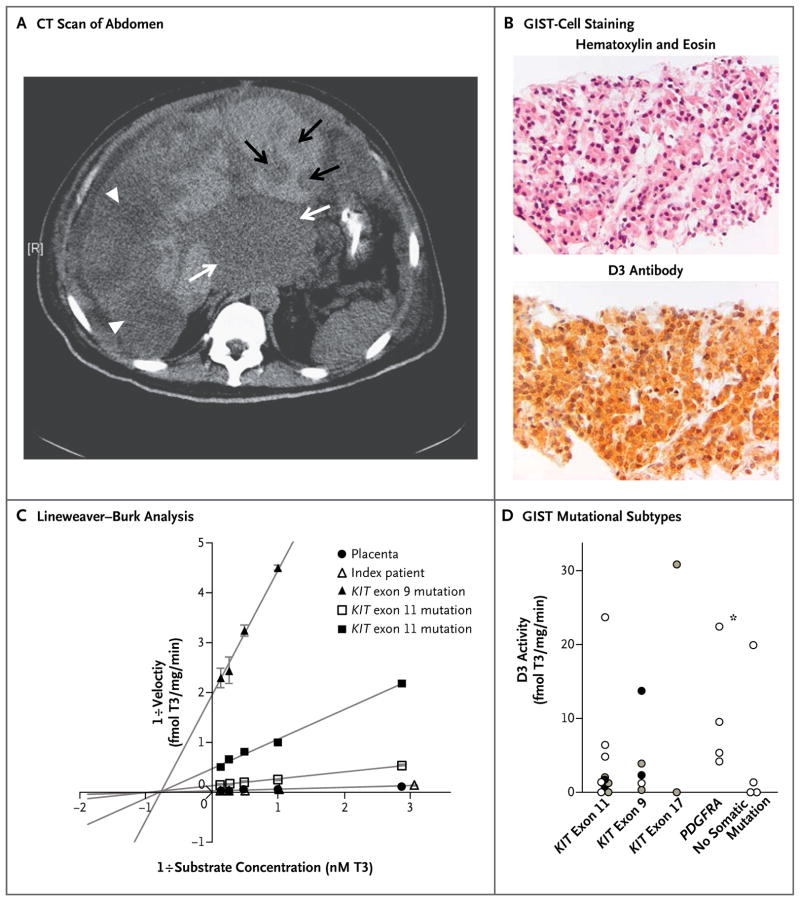
In the index patient, the massive tumor burden (Fig. 1A) and supernormal requirements for exogenous thyroid hormone raised the possibility of consumptive hypothyroidism, a rare endocrinopathy caused by the inactivation of circulating thyroid hormones by tumoral D3.10 To test this hypothesis, we immunostained biopsy samples obtained before the initiation of sunitinib therapy, which showed strong D3 expression in the GIST cells (Fig. 1B). To confirm that the D3 protein was enzymatically active, we assayed deiodinase activity in frozen GIST tissue that was stored at the time of the patient’s first surgery. This assay showed robust D3 activity approximating that of term placenta, the normal human tissue with the highest D3 activity (Fig. 1C). No D1 or D2 activity was detected.
Figure 1. Features of the Index Patient’s Tumor and D3 Expression across Genetic Subtypes of Gastrointestinal Stromal Tumor (GIST).
Computed tomography (CT) of the abdomen (Panel A) shows a massive tumor burden (low-density lesions) in the patient with GIST. A large mass at the gastrointestinal junction is evident in the center of the image (white arrows), and the majority of the right lobe of the liver is replaced with tumor (white arrowheads). Smaller metastases are present in the left liver lobe (black arrows). A tumor-biopsy sample obtained from the patient shows specific staining of GIST cells (Panel B) (top, hematoxylin and eosin; bottom, immunohistochemical staining for type 3 iodothyronine deiodinase [D3]). D3 activity was determined by quantification of the iodine-125–labeled triiodothyronine (T3) converted to iodine-125–labeled diiodothyronine (T2) and was expressed as femtomoles of T3 deiodinated per milligram of protein per minute (fmol T3/mg/min). Lineweaver–Burk analysis of enzyme kinetics (Panel C) showed strong D3 activity in the patient’s tumor (maximum velocity [Vmax], 38.5 fmol of T3 deiodinated per milligram per minute, as compared with 37.0 fmol of T3 deiodinated per milligram per minute in placental tissue), as well as in GIST samples from three other patients with diverse KIT mutations (Vmax, 7.6, 2.1, and 0.5 fmol of T3 deiodinated per milligram per minute). As a confirmation of authentic D3 activity, all tissue samples had low nanomolar Michaelis constants for T3 (1.0 to 1.4 nM T3), indicated by the shared point of intersection through the x axis. The I bars indicate standard errors. Extended screening of additional GIST specimens (Panel D) showed D3 expression in 23 of 28 specimens tested (82%), including specimens from patients who were taking tyrosine kinase inhibitors (black circles), patients who were not receiving GIST therapy (white circles), and patients who had an unknown medication status (gray circles). The index patient is identified by an asterisk.
Genotyping revealed a somatic PDGFRA exon 18 mutation in the tumor sample. Such PDGFRA mutations are present in approximately 10% of samples obtained from patients with sporadic GISTs. To determine whether more common GIST subtypes also express D3, we performed Lineweaver–Burk analysis of tumor samples obtained from two patients with KIT exon 11 mutations (present in 60 to 80% of GISTs) and from one patient with a KIT exon 9 mutation (present in 10 to 15% of GISTs), which showed strong D3 activity (Fig. 1C).
To assess the overall prevalence of D3 expression, we next assayed a large collection of surgical GIST specimens and found D3 activity in 23 of 28 samples (82%) (Fig. 1D). D3 activity was present across all genetic subtypes of GIST and in samples obtained from 11 patients who were not receiving tyrosine kinase inhibitor therapy at the time of surgery. An assessment of thyroid status was available in the medical records of 15 patients, and retrospective review revealed 4 additional patients with hypothyroidism (all positive for tumoral D3 activity), including 2 patients with KIT-mutated GISTs who required unusually high doses of levothyroxine (300 or 400 μg per day).
Endogenous D3 Activity in GIST Cells
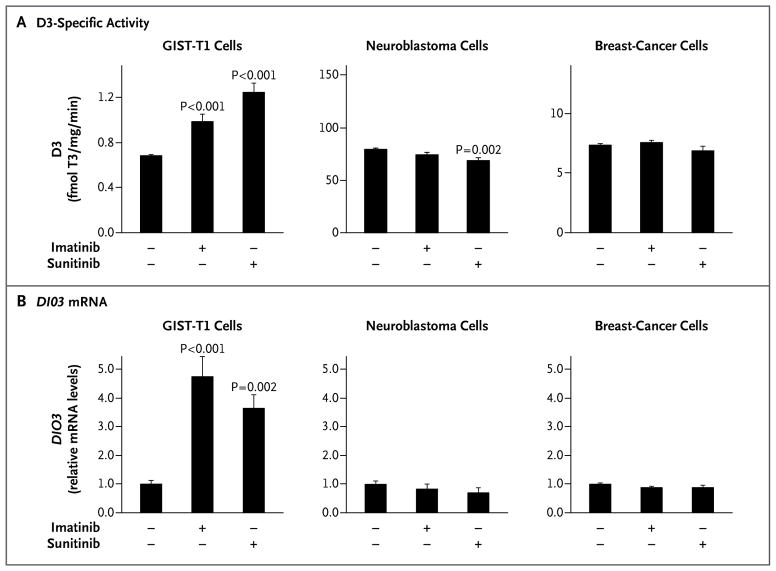
To study the regulation of D3 expression in GIST and its downstream effects, we used the well-characterized, imatinib-sensitive GIST-T1 cell line.7 In the basal state, endogenous D3 activity in GIST-T1 cells was similar to that observed in tumor tissues. Exposure to imatinib or sunitinib at doses corresponding to their clinically therapeutic ranges increased D3-specific activity by a factor of as much as 1.8 (Fig. 2A) and increased the level of D3 messenger RNA by a factor of as much as 4.8 (Fig. 2B). This effect was specific to GIST-T1 cells, with no D3 induction in either neuroblastoma or breast-cancer cells.
Figure 2. Effect of Tyrosine Kinase Inhibitors on D3 Activity in GIST-T1, Neuroblastoma, and Breast-Cancer Cells.
Treatment with tyrosine kinase inhibitors (0.2 μM) for 24 hours increased the activity of endogenous D3, expressed as femtomoles of T3 deiodinated per minute per milligram of protein (fmol T3/mg/min) (Panel A), and the relative level of messenger RNA (mRNA) encoded by the D3 gene (DIO3) (Panel B) in isolated GIST-T1 tumor cells (graph at left in each panel) but not in SK-N-AS neuroblastoma cells (middle graphs) or in MCF-7 breast-cancer cells (graphs at right). P values are for comparisons with vehicle-treated cells. The T bars indicate standard errors. Of note, the specific D3 activity is higher in neuroblastoma cells and breast-cancer cells than in GIST-T1 cells. The housekeeping gene GAPDH was used as an internal control for quantitative reverse-transcriptase–polymerase-chain-reaction assay.
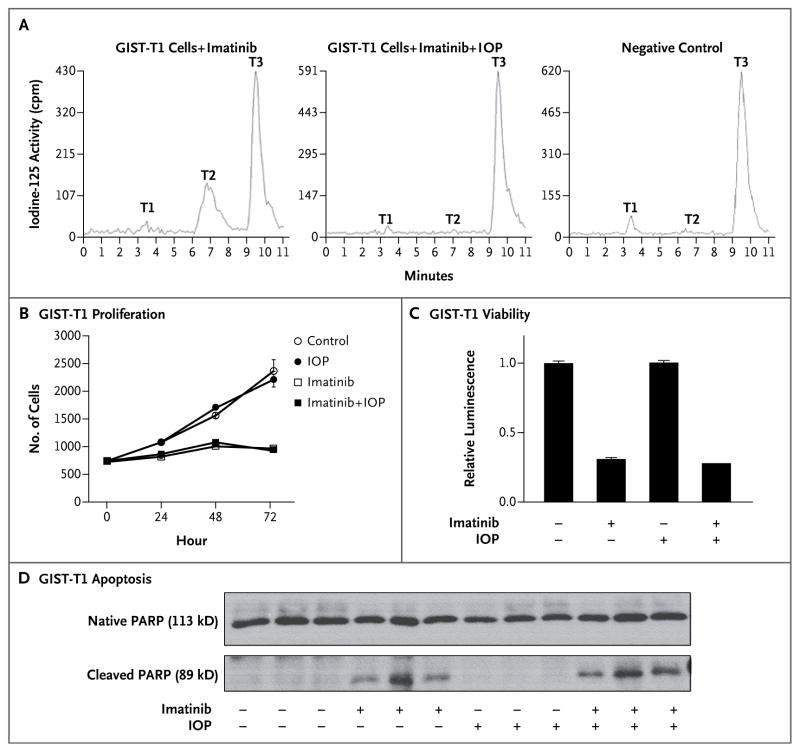
A study of basal-cell carcinoma has shown that tumoral D3 expression promotes the proliferation of malignant keratinocytes.11 To study the potential effect of D3 on GIST tumorigenesis, we developed methods to block the D3 activity of GIST-T1 cells in culture with iopanoic acid (a competitive inhibitor of deiodinase activity), using tissue-culture medium supplemented with tracer iodine-125–labeled triiodothyronine to measure deiodination in living cells. Full D3 inhibition was confirmed by the blockade of triiodothyronine inactivation in living cells (Fig. 3A). We next measured the effect of iopanoic acid on the behavior of GIST-T1 cells, using imatinib as a positive control. As expected, imatinib decreased GIST-T1–cell proliferation (Fig. 3B) and viability (Fig. 3C) and induced apoptosis (Fig. 3D) (P<0.001 for all three comparisons). No significant effect on these measurements was observed with D3 inhibition by iopanoic acid, either alone or in combination with imatinib.
Figure 3. Effects of Imatinib and Iopanoic Acid on the Proliferation, Viability, and Apoptosis of GIST-T1 Cells.
The inhibition of endogenous D3 activity with the use of 60 μM iopanoic acid (IOP) was confirmed by documenting the blockade of T3 conversion to diiodothyronine (T2) in living cells (Panel A). In GIST-T1 cells that were exposed to 0.2 μM imatinib (graph at left), 34% of the T3 in the medium was inactivated through conversion to T2. In contrast, GIST-T1 cells that were exposed to both 0.2 μm imatinib and 60 μM IOP (middle graph) showed no T2 production and resembled the negative control (graph at right). Negligible production of monoiodothyronine (T1) was observed under all conditions tested. GIST-T1 cells that were exposed to 0.2 μM imatinib, 60 μM IOP, or both were assayed for proliferation (Panel B), viability (Panel C), and apoptosis (Panel D). The I bars and T bars represent standard errors. Apoptosis was measured by means of Western blotting for poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP), which is specifically cleaved from its 113-kD native form to an 89-kD protein by apoptosis-specific proteases. Thus, a reduction in the ratio of native PARP to cleaved PARP indicates increased apoptosis. Medium contained 15% fetal-calf serum to provide physiologic concentrations of thyroxine and T3. Cell numbers rose by 3.6 cells per hour in the presence of imatinib (P = 0.0003) and by 21.8 cells per hour in the absence of imatinib (P<0.0001), a difference that was significant (P<0.001). IOP had no effect on the rate of increase, either in the presence of imatinib (P = 0.95) or in the absence of imatinib (P = 0.48). Viability, expressed as relative luminescence, decreased significantly, from 1.00 to 0.29, in the presence of imatinib (P<0.001). The amount of the reduction was not affected by the presence of IOP (P = 0.16 for interaction). After averaging of the results of two Western blot experiments, the presence of imatinib reduced the ratio of native PARP to cleaved PARP by a factor of 3.4 (95% confidence interval, 2.6 to 4.4; P<0.001). The reduction was slightly more pronounced in the presence of IOP (4.3) than in the absence of IOP (2.7) (P = 0.07).
Discussion
The patient described in this case report had severe hypothyroidism in the context of a massive GIST burden. His clinical features suggested consumptive hypothyroidism, which was confirmed by expression of the thyroid hormone–inactivating enzyme D3 in his resected tumor. Since D3 expression is common across the major GIST subtypes, this pathophysiological mechanism is applicable to the majority of patients with this tumor type. When assayed in physiologic concentrations of thyroid hormone, this D3 activity has negligible direct (cell-autonomous) effects on GIST-cell proliferation, viability, and apoptosis. However, it potently inactivates extracellular thyroid hormones, and this effect is sufficient to cause systemic hypothyroidism.
Consumptive hypothyroidism was first identified in children with infantile hemangiomas.10 Unlike all other forms of hypothyroidism, which are caused by impaired secretion, consumptive hypothyroidism results from the accelerated degradation of circulating thyroid hormone at rates that exceed the synthetic capacity of the normal stimulated thyroid gland. This pathophysiological mechanism has been well defined in infants, and its tumor dependence has been shown by the complete resolution of hypothyroidism after involution or resection of the hemangioma.12 The diagnosis of consumptive hypothyroidism requires evidence of increased thyroid hormone inactivation — either elevated levels of serum reverse triiodothyronine (the product of thyroxine inactivation) or supernormal requirements for exogenous thyroid hormone.13 Serum thyroglobulin levels and thyroid radioactive iodine uptake are elevated, reflecting stimulation of the normal thyroid gland.
To our knowledge, there have been only three previous reports of adults with consumptive hypothyroidism caused by D3-expressing vascular14,15 or fibroblastic16 tumors. Here we show that this pathophysiological mechanism extends to adults with GISTs, the most common mesenchymal tumor of the gastrointestinal tract.1 The well-characterized genetics of GISTs have made them a model for targeted therapy with tyrosine kinase inhibitors, and patient-derived cell lines such as GIST-T1 are well established in vitro models. Approximately 85% of GISTs carry pathogenic gain-of-function mutations in KIT 17 or PDGFRA18 that cause ligand-independent oncogenic signaling. These mutations activate shared downstream pathways, including PI3K–AKT and MEK–MAPK, which alter cell metabolism, apoptosis, and proliferation. Although the high level of D3 activity that is observed in all GIST subtypes that we tested suggests its stimulation by a downstream signaling pathway shared by KIT and PDGFRA, the paradoxical increase in D3 observed in GIST-T1 cells after exposure to imatinib or sunitinib (both of which inhibit KIT signaling) suggests that the regulation of D3 expression in these tumors is probably multifactorial.
Since the gene encoding D3 is a member of the DLK1-DIO3 imprinted cluster on chromosome 14q3219 and about two thirds of GISTs are characterized by either monosomy 14 or partial loss of 14q,1 we speculate that loss of an epigenetic silencing element may contribute to D3 overexpression in some of these tumors. DLK1 overexpression has been reported in GISTs with PDGFRA or KIT exon 9 mutations.20 From a clinical standpoint, a therapy-induced increase in tumoral D3 expression (as we observed in cultured GIST-T1 cells exposed to tyrosine kinase inhibitors) could contribute to the increased incidence of hypothyroidism associated with these antineoplastic agents.2 Further studies of D3 expression in tumor-biopsy specimens obtained from patients both before and during therapy with tyrosine kinase inhibitors may be warranted to test this hypothesis.
The cause of thyroid dysfunction that is associated with the use of tyrosine kinase inhibitors is multifactorial and probably varies among individual patients. Previously discovered mechanisms include thyroidal toxicity owing to reduced vascularity of the gland,4,21 inhibition of iodine uptake or organification,3,22 and the induction of hepatic D3.4,23 Thus, in most patients, tumoral D3 is only one of several mechanisms that combine to produce systemic hypothyroidism. However, it is noteworthy that our patient was not receiving tyrosine kinase inhibitor therapy when hypothyroidism developed and that robust D3 activity was present in GIST tissue from his tumor when it was resected, 2 years before he started taking imatinib. This indicates that consumptive hypothyroidism alone is sufficient to cause systemic hypothyroidism in patients with a large GIST burden. Although the incidence of this condition is unknown, it is notable that the first published prospective study of sunitinib-induced hypothyroidism described the exclusion of 15 of 69 patients with GIST (22%) because they had either levothyroxine requirements or baseline hyperthyrotropinemia that preceded the initiation of chemotherapy.21 This rate far exceeds the 4.6% prevalence of hypothyroidism in the general adult population24 and suggests that some of these patients may have had consumptive hypothyroidism.
Recognizing the presence of consumptive hypothyroidism provides useful insight into management. On the basis of experience in treating infants with massive hemangiomas, who sometimes require 10 times the typical replacement dose to restore euthyroidism,25 thyroid function should be monitored frequently in adults with rapidly growing GISTs, and when necessary, clinicians should be prepared to treat such patients with supernormal doses of levothyroxine. Since the rate of thyroid hormone inactivation depends on both the specific D3 activity of the tumor and its mass, cancer growth alone may increase the levothyroxine requirements.
On the basis of our studies of isolated GIST-T1 cells, physiologic levels of thyroid hormone have negligible direct effects on GIST proliferation or viability, so exogenous thyroid hormone should be administered as needed to restore euthyroidism. If we had diagnosed our patient’s consumptive hypothyroidism earlier, his levothyroxine dose could have been aggressively increased to rapidly correct his severe hypothyroidism and reverse its potentially deleterious effects on cardiac function and fluid balance. Monthly measurement of serum thyrotropin in all patients with a large GIST burden and symptoms of hypothyroidism, even if tyrosine kinase inhibitors have never been used, could avoid the complications of occult hypothyroidism and the risk of incorrectly attributing hypothyroid symptoms to the cancer or chemotherapy.
Acknowledgments
Supported by a donation from the Murray Family, grants from the National Institutes of Health (DK076099, DK044128, and 1P50CA12703), the William F. Milton Foundation Award, and a grant from the Harvard Clinical and Translational Science Center (NIH UL1 RR-025758 and NIH UL1 TR-001102, to Dr. Feldman).
We thank Dr. Jonathan Nowak for his review of the patient’s clinical laboratory studies and his helpful insights and comments regarding a previous draft of the manuscript, and Grant Eilers for his technical support in the analysis of GIST tissues.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111–27. doi: 10.1007/s00428-010-0891-y. [DOI] [PubMed] [Google Scholar]
- 2.Hamnvik OP, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. 2011;103:1572–87. doi: 10.1093/jnci/djr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–5. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 4.Kappers MH, van Esch JH, Smedts FM, et al. Sunitinib-induced hypothyroidism is due to induction of type 3 deiodinase activity and thyroidal capillary regression. J Clin Endocrinol Metab. 2011;96:3087–94. doi: 10.1210/jc.2011-1172. [DOI] [PubMed] [Google Scholar]
- 5.Huang SA, Mulcahey MA, Crescenzi A, et al. Transforming growth factor-beta promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol. 2005;19:3126–36. doi: 10.1210/me.2005-0173. [DOI] [PubMed] [Google Scholar]
- 6.Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–8. doi: 10.1210/jc.2002-021291. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi T, Sonobe H, Toyonaga S, et al. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab Invest. 2002;82:663–5. doi: 10.1038/labinvest.3780461. [DOI] [PubMed] [Google Scholar]
- 8.Dang ZC, Lowik CW. Removal of serum factors by charcoal treatment promotes adipogenesis via a MAPK-dependent pathway. Mol Cell Biochem. 2005;268:159–67. doi: 10.1007/s11010-005-3857-7. [DOI] [PubMed] [Google Scholar]
- 9.Refetoff S, Robin NI, Fang VS. Parameters of thyroid function in serum of 16 selected vertebrate species: a study of PBI, serum T4, free T4, and the pattern of T4 and T3 binding to serum proteins. Endocrinology. 1970;86:793–805. doi: 10.1210/endo-86-4-793. [DOI] [PubMed] [Google Scholar]
- 10.Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 11.Dentice M, Luongo C, Huang S, et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci U S A. 2007;104:14466–71. doi: 10.1073/pnas.0706754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balazs AE, Athanassaki I, Gunn SK, et al. Rapid resolution of consumptive hypothyroidism in a child with hepatic hemangioendothelioma following liver transplantation. Ann Clin Lab Sci. 2007;37:280–4. [PubMed] [Google Scholar]
- 13.Huang SA. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid. 2005;15:875–81. doi: 10.1089/thy.2005.15.875. [DOI] [PubMed] [Google Scholar]
- 14.Howard D, La Rosa FG, Huang S, et al. Consumptive hypothyroidism resulting from hepatic vascular tumors in an athyreotic adult. J Clin Endocrinol Metab. 2011;96:1966–70. doi: 10.1210/jc.2010-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SA, Fish SA, Dorfman DM, et al. A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab. 2002;87:4457–61. doi: 10.1210/jc.2002-020627. [DOI] [PubMed] [Google Scholar]
- 16.Ruppe MD, Huang SA, Jan de Beur SM. Consumptive hypothyroidism caused by paraneoplastic production of type 3 iodothyronine deiodinase. Thyroid. 2005;15:1369–72. doi: 10.1089/thy.2005.15.1369. [DOI] [PubMed] [Google Scholar]
- 17.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 19.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–16. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian S, West RB, Corless CL, et al. Gastrointestinal stromal tumors (GISTs) with KIT and PDGFRA mutations have distinct gene expression profiles. Oncogene. 2004;23:7780–90. doi: 10.1038/sj.onc.1208056. [DOI] [PubMed] [Google Scholar]
- 21.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–4. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mannavola D, Coco P, Vannucchi G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–4. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 23.Abdulrahman RM, Verloop H, Hoftijzer H, et al. Sorafenib-induced hypothyroidism is associated with increased type 3 deiodination. J Clin Endocrinol Metab. 2010;95:3758–62. doi: 10.1210/jc.2009-2507. [DOI] [PubMed] [Google Scholar]
- 24.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 25.Huang SA, Bianco AC. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 2008;4:148–55. doi: 10.1038/ncpendmet0727. [DOI] [PMC free article] [PubMed] [Google Scholar]