Abstract
Most Nuclear Receptors (NRs) are ligand-dependent transcription factors crucial in homeostatic physiological responses or environmental responses. We annotated the D. magna NRs and compared them to D. pulex and other species, primarily through phylogenetic analysis. Daphnia species contain 26 NRs spanning all seven gene subfamilies. Thirteen of the 26 receptors found in Daphnia species phylogenetically segregate into the NR1 subfamily, primarily involved in energy metabolism and resource allocation. Some of the Daphnia NRs, such as RXR, HR96, and E75 show strong conservation between D. magna and D. pulex. Other receptors, such as EcRb, THRL-11 and RARL-10 have diverged considerably and therefore may show different functions in the two species. Curiously, there is an inverse association between the number of NR splice variants and conservation of the LBD. Overall, D. pulex and D. magna possess the same NRs; however not all of the NRs demonstrate high conservation indicating the potential for a divergence of function.
Keywords: phylogenetics, crustacea, arthropod, evolution
INTRODUCTION
Daphnia species are an important aquatic bioindicator species and invertebrate toxicology model (Heckmann et al., 2008). Daphnids are commonly studied zooplankton because of their importance to aquatic ecosystems, ability to contend with environmental challenges, amenability to culture, short life-cycle, and parthenogenic reproduction (Baldwin and LeBlanc, 1994; Thomson et al., 2009). They occupy a wide array of environments with habitats ranging drastically in size, permanence, salinity, nutrient levels, and UV exposure (Colbourne et al., 1997). Daphnia magna are one of the most commonly used test species for aquatic toxicity tests. The Daphnia pulex genome has already been fully sequenced (Colbourne et al., 2011), and a concerted effort is in progress to sequence the highly studied related cladoceran, Daphnia magna (Lehman et al., 1995). These genomic models will offer a way of interpreting molecular modifications as well as convergence of adaptive traits associated with specific habitats that vary between the different species of daphnids (Shaw et al., 2008).
The regulation of physiological pathways that maintain proper metabolism and homeostasis within higher organisms continues to be a key concept in biological research. In order to maintain homeostasis cells must be able to acclimate to external and internal cues such as xenobiotics, nutrients, hormones, and other environmental cues. Nuclear receptors (NRs) are a key set of transcription factors that induce acclimation and maintain homeostasis by responding to chemical cues. Because NRs are considered so important in physiology they have been called “the Rosetta stone of physiology” (Evans, 2005). Once activated, NRs translocate to the nucleus (if not already found in the nucleus), bind DNA at specific response elements and initiate transcription. This provides for transcriptional regulation of specific proteins involved in a vast array of diverse physiological functions such as reproduction, embryonic development, cell differentiation, resource allocation, and the maintenance of homeostasis (Chawla et al., 2001; King-Jones and Thummel, 2005; Kretschmer and Baldwin, 2005).
Most NRs consist of five modules: A/B, C, D, E and F. The C module serves as the DNA-binding domain (DBD) and is responsible for binding to the response element on a target gene and is highly conserved among orthologs of different species (Hernandez et al., 2009). The A/B module binds to coactivators. The D subunit constitutes the hinge region and often controls nuclear translocation once the receptor is activated by a ligand. The E module, which is moderately conserved among orthologs of different species, is the ligand-binding domain (LBD) that controls ligand-mediated activation of the nuclear receptor (Hernandez et al., 2009). The exact function of the last subunit, F, is currently unknown. The NR superfamily is categorized into seven main subfamilies based on their structural similarities (Committee, 1999).
Some NRs are highly specific and LBD will only bind to a select molecule or group of molecules; others are much more promiscuous. Promiscuous NRs bind to a wide range of different molecules and, depending on the molecule, activate the transcription of a wide range of proteins. Some of these promiscuous NRs are involved in inducing phase I-III responses following exposure to toxicants (Kliewer et al., 1998; Kawamoto et al., 1999; Wei et al., 2000; King-Jones et al., 2006; Karimullina et al., 2012). It has been hypothesized that specificity/promiscuity comes into play when examining the evolution of nuclear receptors due to natural selection. In the most primitive species,the genomes contain significantly fewer NRs.
Either there are less NR-mediated pathways to regulate or NRs regulate more pathways by responding to more ligands in primitive species. Recently it has been postulated that with less nuclear receptors, the responsibilities of each individual receptor increases and must be able to activate a larger array of pathways (Bridgham et al., 2010; Eick et al., 2012). As evolution of species has progressed, the quantity of nuclear receptors has grown and the receptors have become increasingly specific. This observation lends itself to the idea of specificity through selection in order to make the most optimal form of the receptor (Eick et al., 2012). Through selection, new specialized nuclear receptors seem to have evolved based on the greater efficiency to carry out pathways as well as increase the regulation of these pathways in response to specific cues. Interestingly, the D. pulex genome has nearly half the receptors of humans, but more genes to regulate (Colbourne et al., 2011).
Many NRs are a conduit between internal and external environmental conditions. For example, chronic stress that may be physical or emotional increases adrenocoticotropic hormone and glucocorticoid release, and in turn glucocorticoid receptor (GR) activity. The GR responds by regulating behavior, the immune system, metabolism, growth, and reproduction (O'Connor et al., 2014) Overall, NRs induce the proper physiological responses by responding to chemical cues and transcriptionally regulating pathways that help individuals respond to current conditions.
The purpose of this study was to annotate the D. magna NRs and compare them to D. pulex and other species, primarily through phylogenetic analysis. Because daphnid species are globally distributed zooplankton, we chose to annotate and compare the NRs of two common model species, D. magna and D. pulex with the hope of providing insight into how their respective NRs evolved while under different habitats and external pressures.
MATERIALS and METHODS
Identification and genomic characterization of D. magna nuclear receptors
Identification of D. magna NRs was performed using a Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990) with each of the previously annotated 25 NRs from D. pulex (Thomson et al., 2009) against the assembled D. magna genome (http://server7.wfleabase.org:8091/gbrowse/cgi-bin/gbrowse/daphnia_magna2/). Each of the BLAST search hits was compared to the NCBI database using BLASTp to confirm its status as an NR. The DBD and the LBD of each receptor were identified at this time using the conserved domain database (CDD) (Marchler-Bauer et al., 2007). Protein family (pfam) Zf-C4 (pfam00105) was used to identify the confines of the DBD, and Hormone recep (pfam00104) was used to identify the confines of the LBD to maintain consistency in domain identification. The DBD and LBD’s were compared between D. magna and D. pulex using clustalw (http://www.ebi.ac.uk/Tools/clustalw2/) and percent identity reported. In addition, the DBD and LBD sequences were used during phylogenetic analysis (see below).
Gene Structure
D. magna homologs identified were mapped to the D. magna genome project’s browser (http://server7.wfleabase.org/genome/Daphnia_magna_prerelease/) in order to identify the gene structure (position, length, exons, introns, and intron phase). After protein translation, translation start and stop sites were determined, and protein-coding exons estimated from the gene models and number of nucleotides determined in each intron and exon.
Phylogenetics
Phylogenetic analysis was performed using analysis methods described previously (Thomson et al., 2009; Hannas et al., 2010). All non-daphnid sequences used for phylogenetic analysis were derived from the NCBI database. The D. pulex sequences are predicted protein sequences from the fleabase dataset, and the D. magna sequences are predicted protein sequences from the D. magna genome browser. D. magna was compared to D. pulex and to nuclear receptors from other species available in GenBank such as Drosophila melanogaster, Homo sapiens, Ciona intestinalis, Ixodes scapularis, Nasonia vitripennis, Bombus terrestris, Apis mellifera, Aedes aegypti, Metaseiulus occidentalis and Caenorhabditis elegans (Additional File 1).
Phylogenetic analysis was performed using only the highly conserved DBD and moderately conserved LBD of each receptor. These domains were identified using the conserved domain database CDD (Marchler-Bauer et al., 2007). Zf-C4 (pfam00105) was used to identify the boundaries of the DBD, and Hormone recep (pfam00104) was used to identify the boundaries of the LBD of each receptor. ClustalX default parameters were used to align the domains (Thompson et al., 1997). Trees were constructed using Bayesian Inference (BI) with MrBayes software version 3.1.2 (Ronquist and Huelsenbeck, 2003) on Bioportal (Kumar et al., 2009). Phylogenetic trees were constructed using the “mixed-model” approach in which the Markov chain Monte Carlo sampler explores nine different fixed-rate amino acid substitution models implemented in MrBayes. We used 4 chains with runs of 5 million generations, chains sampled every 100 generations, a burnin of 10,000 trees with the WAG model (Whelan and Goldman, 2001). The C. elegans NHR-1 receptor was used as the outgroup.
Maximum parsimony and distance parameters were used to provide additional support for the phylogenetic relationships observed. Distance parameters were measured using PAUP 4.0b10 with default characteristics (mean character difference and among site rate variation), and full heuristic searches. Branch support was measured by bootstrap analysis with 1000 replicates. Parsimony was constructed using PAUP version 4.0b10 with heuristic searches, tree-bisection-reconnection, topological constraints not enforced, and multiple tree option in effect with an initial maximum tree setting at 100,000. Branch support was measured by bootstrapping with 10,000 replicates. Trees were visualized with FigTree (http://tree.bio.ed.ac.uk/software).
Phylogenetic analysis was also confirmed with Maximum Likelihood (ML) using MEGA 6.0 (Tamura et al., 2013). “Find Best Model” was used to determine the parameters for Maximum Likelihood. In turn, the analysis was performed using the Bootstrap method with 500 replications, and the LG model was used with Gamma distributed rates among sites (2). Tree inference options included SPR level 3, BIONJ with a very strong branch filter. For consistency with BI, a WAG model with gamma distributed rates among sites was also attempted. This model showed very little difference when compared to the LG model (data not shown).
RESULTS and DISCUSSION
Nuclear Receptor Groups in Daphnia
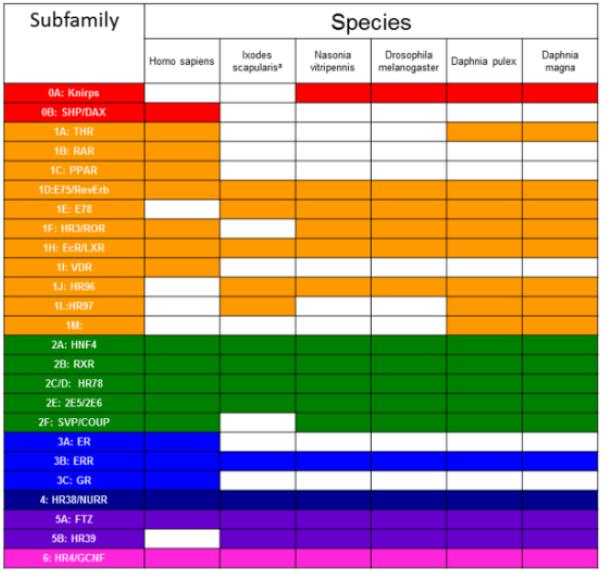
Analysis of the D. magna genome found 26 NRs. Previously, 25 NRs were found in D. pulex. The D. magna BLAST searches found an additional NR, NR2E6 (PNR-like), not previously found in D. pulex (Thomson et al., 2009). A renewed search of the D. pulex genome revealed NR2E6 in both cladoceran species (Fig. 1), thus, both D. magna and D. pulex have 26 identified NRs.
Fig 1. Comparison of nuclear receptor subfamily presence in different species.
Highlighted squares indicate the presence of a subfamily in that species.
Each of the seven subfamilies are represented within the D. magna genome, including two knirps (NR0A) that are phylogenetically related to the NR1 members. Of the 26 D. magna NRs, 13 (including the two knirps) phylogenetically segregate as NR1 subfamily members (50%) and eight are in the NR2 subfamily (31%). In comparison, of the 21 D. melanogaster NRs, eight (including three knirps) are NR1 subfamily members (38%) and eight are NR2 subfamily members (38%) (King-Jones and Thummel, 2005). Humans have 48 NRs with 19 NR1 subfamily members (40%) and 14 NR2 subfamily members, including SHP and DAX (29%). The NR1 and NR2 subfamilies are dominant in all three species. The relative drop in NR1 members in humans is in part due to the expansion of the NR3 subfamily of steroid receptors. Daphnia have a greater percentage of NR1 members than either human or Drosophila (Fig. 2).
Fig 2. Comparison of nuclear receptor percentage in each subfamily for fruitly, Daphnia, and Humans.
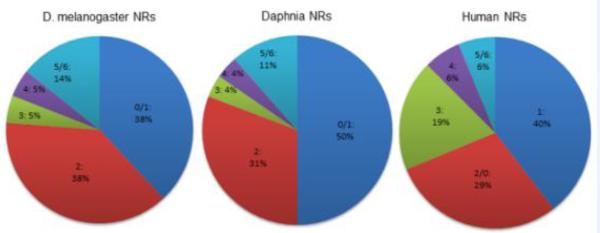
The number before the colon represents the group number. The percentage after the colon represents the percent of total NRs is in that group for each species. For Daphnia and D. melanogaster, the knirps (0A) are grouped with Group 1, and in humans, the knirps (0B) are grouped with Group 2 because of phylogenetic determinations. For all species, Groups 5 and 6 are grouped together.
The expansion of the NR1 family in Daphnia relative to Drosophila is the result of several receptors including a THR, an additional ecdysone receptor (EcRβ), three members of the newly found HR97 (NR1L) subfamily first discovered in D. pulex (Thomson et al., 2009), and a receptor putatively related to RAR (RARL-10). Two NR1L members, magnaHR97a/HR97b are found in tandem repeat, and two NR0A members, magna-KNR-R1/KNR-R2, are found in tandem repeat. The duplication of the ecdysone receptor appears to be unique to Daphnia species.
Phylogenetics
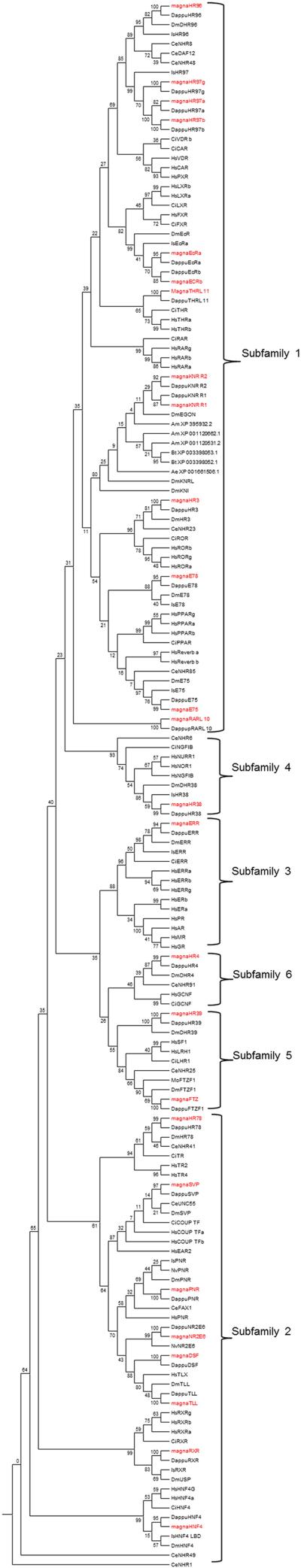
Phylogenetic analysis performed using the LBD and DBD amino acid sequences derived from the D. magna genome indicate that there are three major NR clades in Daphnia; subfamilies NR2, NR3/5/6, and NR1/4. Both Maximum Likelihood (Fig. 3) and BI (Additional File 2) indicate that there are three major clades. ML bootstrap values are low and range from 23-61 at these nodes (Fig. 3), but BI poster probabilities are much stronger at these nodes and range from 0.99 – 1.0 (Additional File 2). The ML tree is also available as an expandable pdf (Additional File 3), and the BI tree has been subsectioned by clade into individual figures (see Results below)The NR 3/5/6 clade further subdivides into NR3 and NR5/6 clades, and the NR1/4 clade further subdivides into NR1ABHIJ, NR1DEF, and NR4 clades similar to previous reports (Bridgham et al., 2010). The four different phylogenetic models used typically agreed at the group and sometimes the subfamily level, but ML and BI showed much greater resolution at the base of the phylogram. However, several of the ancient posterior probabilities are not significant using ML and BI. In contrast, NJ and maximum parsimony were unable to discern the distinct clades on the left hand side of the tree.
Fig 3. Phylogenetic relationship of nuclear receptors as determined by Maximum Likelihood.
The phylogenetic tree is shown with bootstrap support values from ML at each node. Species included are D. pulex (Dappu), D. magna (magna), D. melanogaster (Dm), H. sapiens (Hs), I. scapularis (Is), C. elegans (Ce), C. intestinalis (Ci), B. terrestris (Bt), A. mellifera (Am), A. Aegypti (Ae), N. vitripennis (Nv), an M. occidentalis (Mo).
NR0 Subfamily
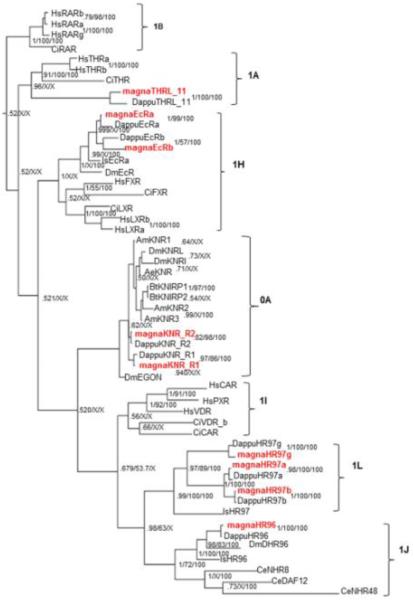
The NR0 group is not named for its phylogenetic positioning, but instead for its lack of either a LBD (NR0A) or a DBD (NR0B) (Germain et al., 2006). Group 0A NRs consist of eagle and the knirps-related receptors, two of which, KNR-R1 and KNR-R2 are present in D. magna and D. pulex. There are no group 0B members (SHP, DAX) in Daphnia. The knirps, unlike their closest phylogenetic relatives, Group 1 NRs, encode orphan nuclear receptors with a C4 zinc finger motif that lacks a corresponding LBD (Perl et al., 2013). BI (Fig. 4) indicates that the knirps are most closely related to the NR1I/J/L groups that contain CAR, PXR, HR96, and HR97. However, ML indicates that the knirps most closely resemble the NR1CDEF clade of NR1 receptors (Fig. 3). Neither method has particularly strong support with 0.53 and 11 as the posterior probability and bootstrap values for BI and ML, respectively. The knirps are found in many arthropod species, including insects, chelicerates, myriapods and crustaceans, and are involved in embryogenesis and cell fate (Peel et al., 2005; Thomson et al., 2009; Xu et al., 2010; Perl et al., 2013).
Fig 4. Phylogenetic relationship of nuclear receptors in groups NR1HIJLNAB and 0A.
NRs from different species were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Neighbor-Joining and Maximum Parsimony trees, respectively. Probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Species included are D. pulex (Dappu), D. magna (magna), D. melanogaster (Dm), H. sapiens (Hs), I. scapularis (Is), C. elegans (Ce), C. intestinalis (Ci), B. terrestris (Bt), A. mellifera (Am), A. Aegypti (Ae), N. vitripennis (Nv), an M. occidentalis (Mo). All D. magna NRs are in red.
Phylogenetic evidence indicates that a single duplication event produced the two distinct Daphnia knirps prior to separation of the crustacean and hexapod lineages (Fig. 4). Early arthropods such as the chelicerates and myriapods only have one knirp gene present (Perl et al., 2013), further supporting this phylogenetic interpretation. In addition, phylogenetic analysis suggests that similar lineage specific duplication events have occurred independently in several other species including the amphipod crustacean Parhyale hawaiensis, as well as multiple insect lineages, such as Bombus terrestris and D. melanogaster (Perl et al., 2013)(Fig. 3-4).
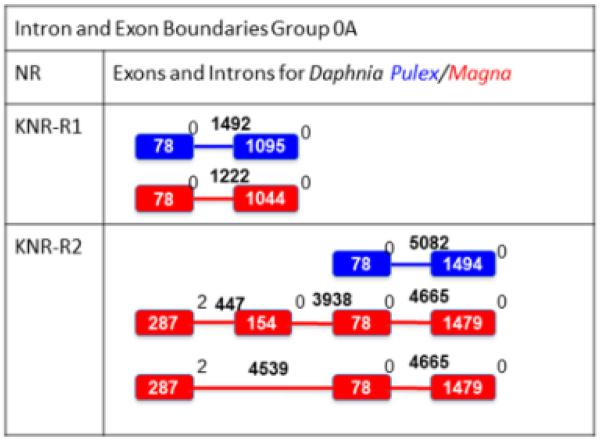
D. magna and D. pulex knirps are also located in tandem repeat on the same scaffold (Additional File 4 contains the DNA sequence, protein sequence and scaffold location of each NR), providing further evidence of a lineage specific duplication event in Daphnia (Thomson et al., 2009; Perl et al., 2013). In comparison, Drosophila melanogaster KNRL and KNI are also located in tandem repeat on the third chromosome and appear to show redundancy in their embryonic responsibilities (Gonzalez-Gaitan et al., 1994; Chen et al., 1998; Fuss et al., 2001). The Daphnia KNR-R1 intron-exon boundaries show highly similar gene structure between D. magna and D. pulex. However, D. magna KNR-R2 has at least 3 and maybe 4 exons, while we (Thomson et al., 2009) and others (Perl et al., 2013) only observed two exons in D. pulex (Fig. 5).
Fig 5. Intron-Exon structure and boundaries for Group 0A NRs for D. pulex and D. magna.
The blue/red boxes indicate coding D. pulex/D. magna exons while the blue/red lines represent D. pulex/D. magna introns. The boxes and lines are not drawn to scale. The bolded white number inside the boxes represent the number of base pairs in the exon while the bolded black number over the intron lines represent the number of base pairs in the intron. The non-bolded numbers to the right of each exon represent the intron phase. The 154 bp exon appears almost 50% of the time based on RNAseq results in D. magna, so both variations are shown.
Both D. pulex and D. magna KNR-R2 contain a significantly longer intron than the corresponding KNR-R1 homolog (Fig. 5). The intron lengths of the D. melanogaster KNRL and KNI also vary significantly. KNRL includes a transcription unit with a large intron that makes it 7.5 times the length of the transcription unit in KNI. Therefore, while the genes might code for similar proteins, they are active at different stages in development since the length of KNRL increases the time for full transcription during the quick cell divisions of early blastodermal stages (Rothe et al., 1992). It is interesting to hypothesize that the differing intron lengths between KNR-R1 and KNR-R2 in Daphnia may also play a role in temporal expression.
NR1 Subfamily
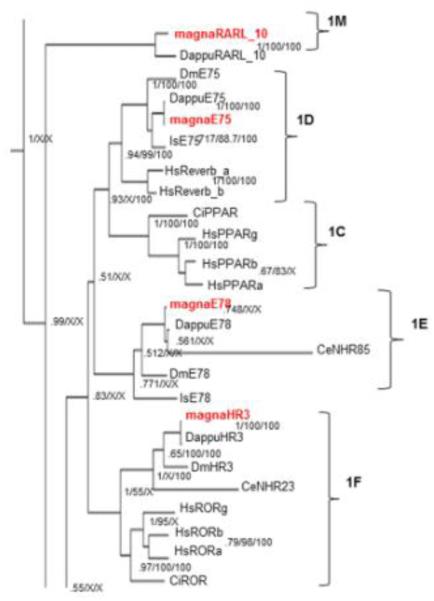
The NR1 subfamily is the largest subfamily in D. magna and separates into two distinct clades; one clade includes the NR1D (E75), NR1E (E78), and NR1F (HR3/ROR) groups (Fig. 6). This clade shows significant uncertainty and low posterior probability values between the E75, E78, and mammalian PPAR (1C) groups. Previous studies have shown stronger relationships between E75 and E78 (Thomson et al., 2009; Bridgham et al., 2010) than E75 and PPAR produced in our phylogenetic tree (Fig. 6). Alternatively, other studies have indicated stronger relationships between E75 and HR3 (Cheung et al., 2008). Further analysis by ML agrees with our BI analysis albeit with low bootstrap values and indicates that HR3 and ROR groups are closely related, HR3 is not as closely related to E75 as ROR. In addition, E75 is closely related to the rev-erbs, and E75 is more closely related to the mammalian PPARs than E78 (Fig. 3).
Fig 6. Phylogenetic relationship of nuclear receptors in subfamilies 1C-F and 1M.
The subfamily 1A-F and 1M nuclear receptors from different species were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Neighbor-Joining and Maximum Parsimony trees provided in order from left to right, respectively. Probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Species included are D. pulex (Dappu), D. magna (magna), D. melanogaster (Dm), H. sapiens (Hs), I. scapularis (Is), C. elegans (Ce), and C. intestinalis (Ci). Numbers at nodes are posterior probabilities. All D. magna sequences are in red.
The other NR1 clade includes NR1IJLAB members. NRs in this group include those activated by ecdysteroids and responsible for molting and development (EcR) (Fahrbach et al., 2012), the newly discovered HR97 group, RAR- and THR-like receptors (RARL_10, THRL_11), and HR96, a receptor involved in cholesterol and triacylglycerol homeostasis that is also promiscuous and involved in xenobiotic stress responses (King-Jones et al., 2006; Horner et al., 2009; Karimullina et al., 2012; Sieber and Thummel, 2012; Li et al., 2014). Many NR1 subfamily members are involved in resource allocation or energy metabolism, including LXR/FXR (NR1H) (Schultz et al., 2000; Zhang et al., 2012), CAR/PXR/VDR/HR96 (NR1I/J) (Dong et al., 2009; Gao and Xie, 2010; Karimullina et al., 2012; Sieber and Thummel, 2012), PPARs (NR1C) (Semple et al., 2006; Jiang et al., 2012; LeBlanc et al., 2012), and THR (NR1A) (Duntas and Brenta, 2012; Cordeiro et al., 2013). This suggests an important role for the coordination of resource allocation with other processes; several probably related to coordination with reproduction and development in this R species.
Phylogenetic analyses between the three methods used are generally in agreement at the group level when investigating the daphnid NR1 members in this clade (Fig. 4). The placement of the RARL_10 and THRL_11 receptors is of interest as these NRs previously showed significant similarity to RAR and THR NRs respectively via Blast searches, but did not phylogenetically segregate with the RAR and THR receptors (Thomson et al., 2009). Therefore, these receptors were named HR10 and HR11 and defined as putative NR1M and NR1N members (Thomson et al., 2009). Refined phylogenetic methods and the addition of the D. magna homologs improve the analysis and provide evidence that the THRL_11 receptors are THR receptors. Thus, ML and BI analysis indicates that the THRL_11 receptors belong to the NR1A group; however, there is a lack of resolution from maximum parsimony and NJ (Fig. 3-4). In contrast, the RARL_10 receptors from D. magna and D. pulex did not segregate within the NR1B clade (Fig. 3-4). Instead the RARL_10 receptors appear within the NR1 subfamily, but as early progenitors to the subfamily in both ML and BI.
Other phylogenetic disagreements within the NR1 group between methods did not include the Daphnia NRs. These included the placement of Ciona VDR-like receptors with the invertebrate HR96/NR1J group instead of the vertebrate NR1I group, the placement of CeNHR48, the relationship of the knirps to the HR96/HR97 groups or primarily to the HR97 group, and the placement of the Ixodes EcR relative to the Drosophila EcR.
There have been several duplication events within the large Daphnia NR1 clade as the 1H group (EcRα/β) has two paralogs and the new 1L group (HR97a/b/g), first discovered in D. pulex, has three paralogs (Thomson et al., 2009; Li et al., 2014). HR97a and b show high conservation between D. pulex and D. magna, but HR97g, the most ancient of the three receptors is weakly to moderately conserved relative to most NRs (Table 1). Interestingly, new data indicates that the HR97 receptors are not as promiscuous as the HR96 receptors (Li et al., 2014), and therefore may represent an example of the evolution of specificity from promiscuity (Eick et al., 2012).
Table 1.
Similarity of nuclear receptor DNA- and ligand-binding domains in D. pulex and D. magna. Domains were aligned using ClustalX and percent identity reported.
| Receptors | Domains | |
|---|---|---|
| C (DBD) |
E (LBD) |
|
| KNR-R1 | 97 | X |
| KNR-R2 | 100 | X |
| THRL_11 | 71 | 81 |
| E75 | 100 | 99 |
| E78 | 100 | 93 |
| HR3 | 100 | 98 |
| EcRa | 100 | 95 |
| EcRb | 75 | 59 |
| HR96 | 98 | 96 |
| HR97a | 100 | 92 |
| HR97b | 100 | 97 |
| HR97g | 87 | 90 |
| RARL_10 | 84 | 69 |
| HNF4 | 100 | 97 |
| RXR | 100 | 100 |
| HR78 | 100 | 99 |
| DSF | 100 | 96 |
| PNR | 98 | 91 |
| NR2E6 | 97 | 78 |
| TLL | 100 | 71 |
| SVP | 100 | 99 |
| ERR | 100 | 95 |
| HR38 | 99 | 98 |
| FTZF1 | 100 | 100 |
| HR39 | 100 | 100 |
| HR4 | 78 | 86 |
HR97a/b and the two knirps are found in tandem repeat, but the two ecdysone receptors are not in succession. To our knowledge, D. magna and D. pulex are the only invertebrate species known to contain two ecdysone receptor genes. Interestingly, the DBDs and LBDs of Daphnia EcRβ are not highly conserved, while EcRα has retained its conservation between D. pulex and D. magna (Table 1), indicating that EcRβ may respond to different cues and induce different genes in D. magna than D. pulex (Hollenberg et al., 1987; Green and Chambon, 1989).
Most of the DBDs and LBDs from the D. magna NRs show near 100% amino acid sequence identity or certainly high conservation when directly compared to their D. pulex homologs (Table 1). There are five LBDs in subfamily 1 and 2 with percent identities below 85%, and an NR4 member with 86% identity. EcRβ (59%), RARL_10 (69%), and TLL (71%) all have percent identities below 75%. There are four DBDs in subfamilies 1 and 4 with percent identities below 85%, and THRL_11 and EcRβ have DBD percent identities below 75%. Three out of the thirteen NR1 members (RARL_10, THRL_11, and EcRb) showed poor DBD conservation (< 85%) and HR97g shows 87%. EcRβ and RARL_10 are the only receptors in which both the LBD and DBD are not well conserved. Some of the DBDs and LBDs that are poorly conserved may respond to different cues or bind some different response elements in D. magna than their homologs in D. pulex.
NR2 Subfamily
The NR2 subfamily is the second largest group of NRs in Daphnia with 8 NRs (shown in green in Table 1). NR2 is further divided into 5 groups A, B, C/D, E and F. Each of these groups contain 1 receptor except the NR2E group, which contains four paralogs in D. magna and D. pulex. Group A is involved in the development of the digestive system (Zhong et al., 1993). Group B receptors bind to retinoids, are involved in growth, and are key heterodimeric partners with many other receptors (Kliewer et al., 1992; Amoutzias et al., 2007; Wang et al., 2011). There are four NR2E members and several are involved in neural development (Weber et al., 2012). Groups 2C is induced by ecdysone and both 2C and 2F members have been shown to interact with other nuclear receptors and repress transcription (Tran et al., 1992; Fisk and Thummel, 1995; Palanker et al., 2006).
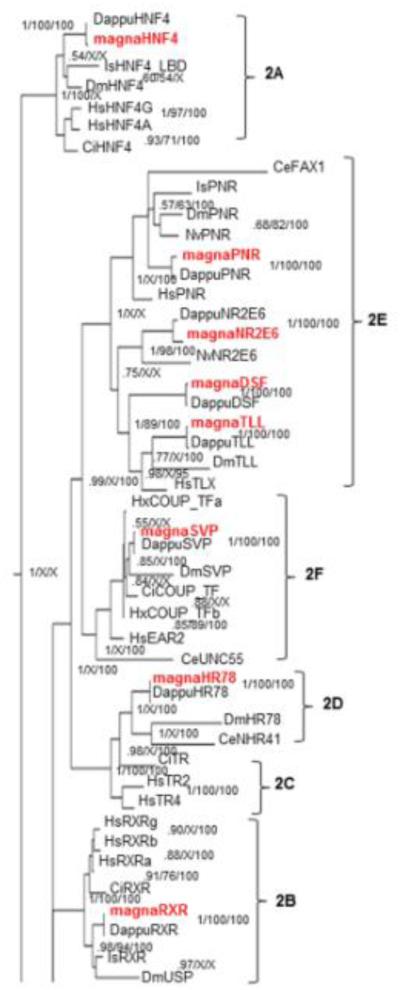
Phylogenetic analysis of NR2 receptors showed few disagreements on the right hand side (group level) of the tree with the exception of the HNF4 group of receptors where the placement of the Daphnia genes with the invertebrate clade was not well resolved (Fig. 3,7). The placement of the NR2E6 group was also poorly resolved in both BI and ML (0.75 posterior probability BI, 43 bootstrap ML) although the methods are in agreement. Previous research has not conclusively resolved the phylogenetic placement of the NR2E6 group (Bonneton et al., 2008). However, its placement in our tree is identical to research that indicates NR2E6 is related to the PNR/HR51 group (NR2E3) and with higher resolution (Bonneton et al., 2008). Other disagreements were at or near the subfamily level and were primarily due to the inability of maximum parsimony or NJ to resolve the tree at this level. The PNR clade was also poorly resolved with probabilities between 0.57 and 1.00; however, there was little disagreement between the phylogenetic methods.
Fig 7. Phylogenetic relationship of nuclear receptors in subfamily 2.
The subfamily 2 nuclear receptors from different species were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Neighbor-Joining and Maximum Parsimony trees provided in order from left to right, respectively. Probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Species included are D. pulex (Dappu), D. magna (magna), D. melanogaster (Dm), H. sapiens (Hs), I. scapularis (Is), C. elegans (Ce), C. intestinalis (Ci), and , N. vitripennis (Nv). Numbers at nodes are posterior probabilities. All D. magna sequences are in red.
The NR2 subfamily of receptors are well conserved between the two Daphnia species with the exception of the LBDs of NR2E6 and TLL (Table 1). LBDs are often less conserved than DBDs. For example, the LBDs from the 2E group in C. elegans are poorly conserved in relation to the DBDs when compared to Drosophila or vertebrate 2E members (Weber et al., 2012). It is interesting to speculate as to the purpose and selective advantage of relatively large changes in the amino acid composition of the LBD and how this serves as a selective advantage for D. magna within its natural habitat, especially for receptors often involved in neural and optic development (Miyawaki et al., 2004; Weber et al., 2012).
In general, there are few differences between the intron phases and intron-exon boundaries between D. magna and D. pulex NRs (Additional Files 5-8). However, there are some significant differences in exon number and intron phases between homologous D. pulex and D. magna NR2 members (Additional File 7). Some NR2E/F members, SVP, DSF, and to a lesser extent TLL, differ significantly with changes in exon number and different intron phases. Interestingly, these changes in exon number and intron phases have no effect on the conservation of the DBD and little effect on the conservation of the LBD in NR2 members as most NR2 members with the exception of NR2E6 and TLL are highly conserved (Table 1).
NR subfamilies 3-6
Phylogenetic analysis of subfamilies 3-6 produced few disagreements within the NR groups (Fig. 7). Disagreements were primarily caused by changes in which species of arthropods showed closer relationships to Daphnia NRs. Disagreements on the left hand side of the tree were primarily caused by the inability of maximum parsimony or NJ to resolve these phylogenetic relationships. BI and ML were able to resolve most of the relationships with probabilities greater than 0.99 with the exception of the 5 and 6 subfamilies where the proximal probability is only 0.85.
In D. magna, these four subfamilies only account for 5 NRs (Fig. 1). Subfamily 3 is represented only by estrogen-related receptor (ERR). The 4 subfamily is represented by HR38, which is regulated by ecdysteroids and involved in cuticle formation in insects (Kozlova et al., 1998). Subfamily 5 NRs are divided into two groups, A and B of which 5A (FTZ-F1) is crucial in development and regulated by ecdysteroids (Yamada et al., 2000; Palanker et al., 2006), and 5B (HR39) is involved in the development of female reproductive glands (Sun and Spradling, 2012). The only subfamily 6 receptor, HR4, is also regulated by ecdysteroids (Palanker et al., 2006) and involved in growth and maturation (King-Jones et al., 2005). In general, NR groups 3 (ERR), 4 (HR38), 5A (FTZ), and 5B (HR39) are well conserved; group 6 (HR4) is poorly conserved between D. pulex and D. magna (Table 1). This suggests the potential for differences in ecdysone regulation or HR4-regulated growth and maturation.
Conclusions
There are 26 nuclear receptors in both D. magna and D. pulex spanning all seven gene subfamilies of which half are in the NR1 subfamily often involved in energy metabolism and resource allocation. Most of the receptors are highly conserved between the two cladoceran species. However, there are several poorly conserved NRs such as THRL_11, RARL_10, EcRb, and HR4, many of which are subfamily 1. These differences within the DBD and/or LBD, suggest that D. magna and D. pulex may in some cases respond to different internal or external environmental cues or regulate different sets of genes.
Supplementary Material
RESEARCH HIGHLIGHTS.
The D. magna NRs were annotated and compared to D. pulex and other species.
D. magna and D. pulex contain 26 NRs spanning each of the seven gene subfamilies.
Thirteen of the 26 receptors phylogenetically fit within the NR1 subfamily
Several homologous D. magna and D. pulex NRs have nearly identical LBDs or DBDs
However, about 20% of the homologous NRs show high divergence between the species
Fig 8. Phylogenetic relationship of nuclear receptors in subfamilies 3-6.
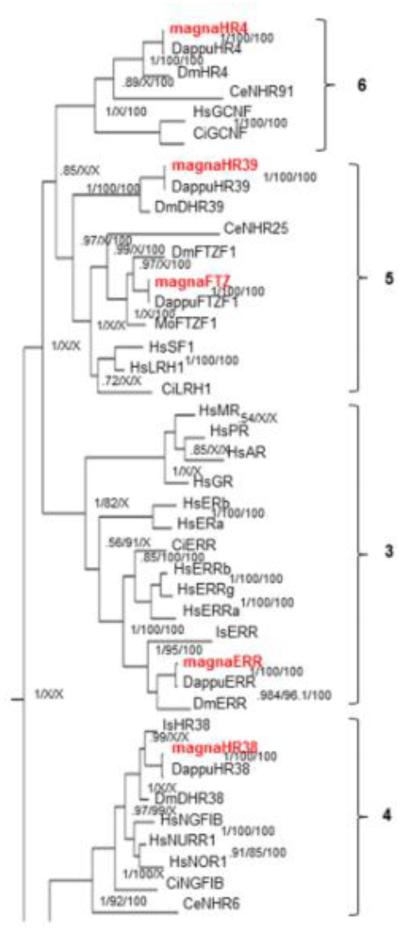
The subfamily 3,4,5, and 6 nuclear receptors from different species were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Neighbor-Joining and Maximum Parsimony trees provided in order from left to right, respectively. Probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Species included are D. pulex (Dappu), D. magna (magna), D. melanogaster (Dm), H. sapiens (Hs), I. scapularis (Is), C. elegans (Ce), M. occidentalis (Mo) and C. intestinalis (Ci). Numbers at nodes are posterior probabilities. All D. magna sequences are in red.
Acknowledgements
The authors would like to thank Dr. Patrick Gerard at Clemson University for his statistical advice. Funds for this project were provided through a Clemson University Creative Inquiry project, Clemson University start-up funds, National Institutes of Health grant R15-ES017321, and an NIH-ARRA supplement that provided summer support for TEG.
Abbreviations
- NR
Nuclear Receptor
- CDD
conserved domain database
- DBD
DNA binding Domain
- LBD
Ligand binding Domain
- Pfam
Protein family
- ML
Maximum Likelihood
- EcR
Ecdysone Receptor
- KNR
Knirp
- THRL
Thyroid hormone receptor-like
- RARL
Retinoid Acid Receptor-like
- PPAR
Peroxisome Proliferator Activated Receptor
- HR3
Hormone Receptor 3
- HR4
Hormone Receptor 4
- HR38
Hormone Receptor 38
- HR39
Hormone Receptor 39
- HR78
Hormone Receptor 78
- HR96
Hormone Receptor 96
- HR97
Hormone Receptor 97
- HNF4
Hepatocyte Nuclear Factor 4
- NR2E6
Nuclear Receptor 2E6
- RXR
Retinoid X Receptor
- DSF
Dissatisfaction
- PNR
Photoreceptor cell-specific nuclear receptor
- TLL
Tailless
- SVP
Seven-up
- ERR
Estrogen Related Receptor
- FTZF1
Fushi Tarazu Factor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have no conflicts of interest to declare.
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amoutzias GD, Pichler EE, Mian N, De Graaf D, Imsiridou A, Robinson-Rechavi M, Bornberg-Bauer E, Robertson DL, Oliver SG. A protein interaction atlas for the nuclear receptors: properties and quality of a hub-based dimerisation network. BMC Systems Biology. 2007;1:34. doi: 10.1186/1752-0509-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin WS, LeBlanc GA. Identification of multiple steroid hydroxylases in Daphnia magna and their modulation by xenobiotics. Environ Toxicol Chem. 1994;13:1013–1021. [Google Scholar]
- Bonneton F, Chaumot A, Laudet V. Annotation of Tribolium nuclear receptors reveals an increase in evolutionary rate of a network controlling the ecdysone cascade. Insect Biochem Mol Biol. 2008;38:416–429. doi: 10.1016/j.ibmb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Eick GN, Larroux C, Deshpande K, Harms MJ, Gauthier MEA, Ortlund EA, Degnan BM, Thornton JW. Protein Evolution by Molecular Tinkering: Diversification of the Nuclear Receptor Superfamily from a Ligand-Dependent Ancestor. PLoS Biology. 2010;8:e1000497. doi: 10.1371/journal.pbio.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chen CK, Kuhnlein RP, Eulenberg KG, Vincent S, Affolter M, Schuh R. The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development. 1998;125:4959–4968. doi: 10.1242/dev.125.24.4959. Development 125:4959–4968. [DOI] [PubMed] [Google Scholar]
- Cheung D, Xia Q, Duan J, Wei L, Huang C, Li Z, Wang G, Xiang Z. Nuclear receptors in Bombyx mori: Insights into genomic structure and developmental expression. Insect Biochem Mol Biol. 2008;38:1130–1137. doi: 10.1016/j.ibmb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Hebert PDN, Taylor DJ. Evolutionary origins of phenotypic diversity in Daphnia. In: Givnish TJ, Sytsma KJ, editors. Molecular Evolution and Adaptive Radiation. Cambridge University Press; London: 1997. pp. 163–188. [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi J-H, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak R, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee NRN. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Cordeiro A, Souza LL, Einicker-Lamas M, Pazos-Moura CC. Non-classic thyroid hormone signalling involved in hepatic lipid metabolism. J Endocrinol. 2013;216:R47–R57. doi: 10.1530/JOE-12-0542. [DOI] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am. 2012;96:269–281. doi: 10.1016/j.mcna.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Eick GN, Colucci JK, Harms MJ, Ortlund EA, Thornton JW. Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors. PLoS Genet. 2012;8:e1003072. doi: 10.1371/journal.pgen.1003072. doi:10.1371/journal.pgen.1003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM. The nuclear receptor superfamily: A rosetta stone for physiology. Mol Endocrinol. 2005;19:1429–1434. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Smagghe G, Velarde RA. Insect nuclear receptors. Annu Rev Entomol. 2012;57:83–106. doi: 10.1146/annurev-ento-120710-100607. [DOI] [PubMed] [Google Scholar]
- Fisk GJ, Thummel CS. Isolation, regulation, and DNA-binding properties of three Drosophila nuclear hormone receptor superfamily members. Proc Natl Acad Sci U S A. 1995;92:10604–10608. doi: 10.1073/pnas.92.23.10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss B, Meissner T, Bauer R, Lehmann C, Eckardt F, Hoch M. Control of endoreduplication domains in the Drosophila gut by the knirps and knirps-related genes. Mech Dev. 2001;100:15–23. doi: 10.1016/s0925-4773(00)00512-8. [DOI] [PubMed] [Google Scholar]
- Gao J, Xie W. Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab Dispos. 2010;38:2091–2095. doi: 10.1124/dmd.110.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M, Rothe M, Wimmer EA, Taubert H, Jackle H. Redundant functions of the genes knirps and knirps-related for the establishment of anterior Drosophila head structures. Proc Natl Acad Sci USA. 1994;91:8567–8571. doi: 10.1073/pnas.91.18.8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Chambon P. Chimeric receptors used to probe the DNA-binding domain of the estrogen and glucocorticoid receptors. Cancer Res. 1989;49:2282s–2285s. [PubMed] [Google Scholar]
- Hannas BR, Wang YH, Baldwin WS, Li Y, Wallace AD, LeBlanc GA. Interactions of the crustacean nuclear receptors HR3 and E75 in the regulation of gene transcription. Gen Comp Endocrinol. 2010;167:268–278. doi: 10.1016/j.ygcen.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann L-H, Sibly RM, Connon R, Hooper HL, Hutchinson TH, Maund SJ, Hill CJ, Bouetard A, Callaghan A. Systems biology meets stress ecology: linking molecular and organismal stress responses in Daphnia magna. Genome Biol. 2008;9:R40. doi: 10.1186/gb-2008-9-2-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacog Personal Med. 2009;7:81–105. doi: 10.2174/187569209788654005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg SM, Giguere V, Segui P, Evans RM. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987;49:39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Horner MA, Pardee K, Liu S, King-Jones K, Lajoie G, Edwards A, Krause HM, Thummel CS. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009;23:2711–2716. doi: 10.1101/gad.1833609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Wang W, Miner J, Fromm M. Cross regulation of sirtuin 1, AMPK, and PPARγ in conjugated linoleic acid treated adipocytes. PLoS One. 2012;7:e48874. doi: 10.1371/journal.pone.0048874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimullina E, Li Y, Gingupalli GK, Baldwin WS. HR96 is a promiscuous endo- and xeno-sensing nuclear receptor. Aquat Toxicol. 2012;116-117:69–78. doi: 10.1016/j.aquatox.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Charles JP, Lam G, Thummel CS. DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell. 2005;121:773–784. doi: 10.1016/j.cell.2005.03.030. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors: A perspective from Drosophila. Nature Rev Genetics. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid × receptor interacts with nuclear receptors in retinoic acid, thyroid hormone, and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova T, Pokholkova GV, Tzertzinis G, Sutherland JD, Shimulev IF, Kafatos FC. Drosophila hormone receptor 38 functions in metamorphosis: A role in adult cuticle formation. Genetics. 1998;149:1465–1475. doi: 10.1093/genetics/149.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 2005;155:111–28. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kumar S, Skjæveland A, Orr RJS, Enger P, Ruden T, Mevik B-H, Burki F, Botnen A, Shalchian-Tabrizi K. AIR: A batch-oriented web program package for construction of supermatrices ready for phylogenomic analyses. BMC Bioinformatics. 2009;10 doi: 10.1186/1471-2105-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc GA, Norris DO, Kloas W, Kullman SW, Baldwin WS, Greally JM. Detailed Review Paper on the State of the Science on Novel In Vitro and In Vivo Screening and Testing Methods and Endpoints for Evaluating Endocdrine Disruptors Series on Testing & Assessment: No. 178. Organisation for Economic Co-operation and Development; Paris: 2012. p. 213. [Google Scholar]
- Lehman N, Pfrender ME, Morin PA, Crease TJ, Lynch M. A hierarchical molecular phylogeny within the genus Daphnia. Mol Phylog Evol. 1995;4:395–407. doi: 10.1006/mpev.1995.1037. [DOI] [PubMed] [Google Scholar]
- Li Y, Ginjupalli GK, Baldwin WS. The HR97 (NR1L) Group of Nuclear Receptors: A New Group of Nuclear Receptors Discovered in Daphnia species. Gen Comp Endocrinol. 2014;206:30–42. doi: 10.1016/j.ygcen.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, Ke Z, Krylov D, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Thanki N, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:237–240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T. Tlx, an Orphan Nuclear Receptor, Regulates Cell Numbers and Astrocyte Development in the Developing Retina. J Neurosci. 2004;24:8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TM, O'Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2014;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- Palanker L, Naecakov AS, Sampson HM, Ni R, Hu C, Thummel CS, Krause HM. Dynamic regulation of Drosophila nuclear receptor activity in vivo. Development. 2006;133:3549–3562. doi: 10.1242/dev.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel AD, Chipman AD, Akam M. Arthropod segmentation: beyond the Drosophila paradigm. Nat Rev Genetics. 2005;6:905–916. doi: 10.1038/nrg1724. [DOI] [PubMed] [Google Scholar]
- Perl TN, Schmid BGM, Schwirz J, Chipman AD. The evolution of the knirps family of transcription factors in arthropods. Mol Biol Evol. 2013;30:1348–1357. doi: 10.1093/molbev/mst046. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rothe M, Pehl M, Taubert H, Jackle H. Loss of gene function through rapid mitotic cycles in the Drosophila embryo. Nature. 1992;359:156–159. doi: 10.1038/359156a0. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JR, Pfrender ME, Eads BD, Klaper R, Callaghan A, Sibly R, Colson I, Jansen B, Gilbert D, Colbourne JK. Daphnia as an emerging model for toxicological genomics. In: Hogstrad C, Kille P, editors. Comparative Toxicogenomics. Vol. 2. Elsevier; London: 2008. pp. 165–219. Recent Advances in Experimental Biology. [Google Scholar]
- Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Spradling AC. NR5A Nuclear Receptor Hr39 Controls Three-Cell Secretory Unit Formation in Drosophila Female Reproductive Glands. Current Biol. 2012;22:862–871. doi: 10.1016/j.cub.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustalx windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SA, Baldwin WS, Wang YH, Kwon G, LeBlanc GA. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genomics. 2009;10:500. doi: 10.1186/1471-2164-10-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P, Zhang XK, Salbert G, Hermann T, Lehmann JM, Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992;12:4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Kwon G, Li H, Leblanc GA. Tributyltin synergizes with 20-hydroxyecdysone to produce endocrine toxicity. Toxicol Sci. 2011;123:71–79. doi: 10.1093/toxsci/kfr154. [DOI] [PubMed] [Google Scholar]
- Weber KP, Alvaro CG, Baer GM, Reinert K, Cheng G, Clever S, Wightman B. Analysis of C. elegans NR2E nuclear receptors defines three conserved clades and ligand-independent functions. BMC Evol Biol. 2012;12:81. doi: 10.1186/1471-2148-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Xu J, Tan A, Palli SR. The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum. J Insect Physiol. 2010;56:1471–1480. doi: 10.1016/j.jinsphys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M.-a., Murata T, Hirose S, Lavorgna G, Suzuki E, Ueda H. Temporally restricted expression of transcription factor bFTZ-F1: significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127 doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, Ma H, Kasim N, Edwards PA, Novak CM. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–280. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Sladek FM, Darnell JEJ. The expression pattern of a Drosophila homolog to the mouse transcription factor HNF-4 suggests a determinative role in gut formation. EMBO J. 1993;12:537–544. doi: 10.1002/j.1460-2075.1993.tb05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.