Abstract
Background
Patients presenting to thoracic surgeons with pulmonary nodules suspicious for lung cancer have varied diagnostic options including navigation bronchoscopy (NB), computed tomography fine needle aspiration (CT-FNA), 18F-fluoro-deoxyglucose positron emission tomography (FDG-PET) and video assisted thoracic surgery (VATS). We studied the relative cost-effective initial diagnostic strategy for a 1.5-2 cm nodule suspicious for cancer.
Methods
A decision analysis model was developed to assess the costs and outcomes of four initial diagnostic strategies for diagnosis of a 1.5-2 cm nodule with either a 50% or 65% pretest probability of cancer. Medicare reimbursement rates were used for costs. Quality adjusted life years were estimated using patient survival based on pathologic staging and utilities derived from the literature.
Results
When cancer prevalence was 65%, tissue acquisition strategies of NB and CT-FNA had higher quality adjusted life years compared to either FDG-PET or VATS and VATS was the most costly strategy. In sensitivity analyses NB and CT-FNA were more cost-effective than FDG-PET when FDG-PET specificity was less than 72%. NB, CT-FNA, and FDG-PET had similar cost effectiveness when cancer prevalence was 50%.
Conclusions
NB and CT-FNA diagnostic strategies are more cost-effective than either VATS biopsy or FDG-PET scan to diagnose lung cancer in moderate to high-risk nodules and resulted in fewer nontherapeutic operations when FDG-PET specificity was less than 72%. FDG-PET scan for diagnosis of lung cancer may not be cost-effective in regions of the country where specificity is low.
Introduction
The management of pulmonary nodules suspicious for lung cancer is a combination of art and science as the clinician balances the advantages and disadvantages of a range of tests at each stage in the diagnostic process. The United Stated Preventive Services Task Force recently recommended low dose computed tomographic (CT) screening of healthy individuals at high risk for lung cancer.1 Based on current smoking estimates in the U.S. population and given the rates of nodule discovery found in the National Lung Screening Trial, as many as 1 to 2 million more suspicious nodules will be discovered annually given current clinical screening recommendations.2 Patients with suspicious nodules will require additional tests for diagnosis, and some will require evaluation by a surgeon.
Currently, 18F-fluoro-deoxyglucose positron emission tomography (FDG-PET) is suggested for non-invasive diagnosis of a nodule larger than 0.8 cm with a clinical probability for lung cancer between 5% and 65%. Computed tomography guided fine-needle aspiration (CT-FNA) is an alternative diagnostic technique with a diagnostic accuracy of 77% (59-96% ) in peripheral nodules accessible to needle biopsy; however, as many as 41% of CT-FNA's are non-diagnostic.3
Several image guided-bronchoscopy techniques have been developed to improve the yield of transthoracic and transbronchial biopsy for lung nodule diagnosis. Computer assisted navigation bronchoscopy (NB), virtual bronchoscopy and radial endobronchial ultrasound allow the clinician to navigate beyond the hilum to biopsy suspicious lesions in the peripheral lung fields with increased diagnostic yield.4,5 Using a decision analysis model, we examined the cost-effectiveness of NB compared to FDG-PET, CT-FNA and video-assisted thoracic surgery (VATS) biopsy.
Material and Methods
A decision analysis model was developed to estimate the costs and outcomes of four different diagnostic strategies for the workup of a patient with a 1.5 to 2 cm nodule detected by CT. Compared strategies included FDG-PET scan, NB, CT-FNA, and surgical biopsy (Figure 1). The model includes key outcomes after each treatment or diagnostic alternative with estimated probabilities of these events, quality adjusted life years (QALYs) and total costs associated with each strategy. Model construction and cost-effectiveness analysis were performed using TreeAge Pro 2013 (Williamstown, MA). This study was not deemed human research by Vanderbilt Institutional Review Board.
Figure 1. Decision analysis model for patient presenting with a 1.5-2 cm nodule and likelihood of lung cancer is 65%.
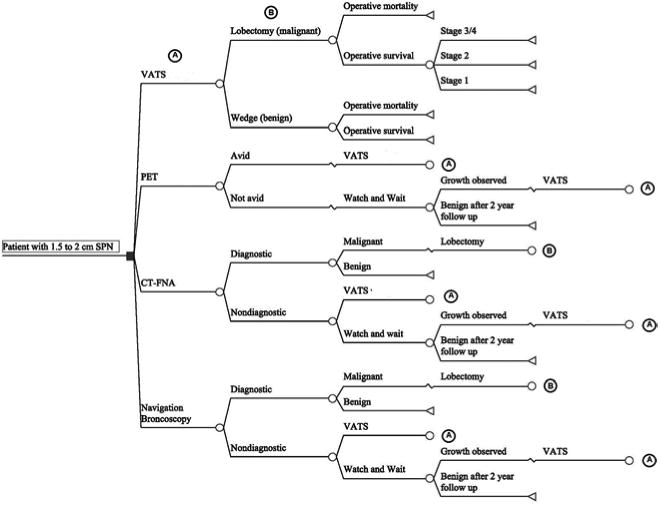
(A) Outcomes resulting from a VATS biopsy
(B) Outcomes resulting from a lobectomy given pathologically determined malignancy
The base case is a 60 year old male with a 15 pack year smoking history, no prior history of lung cancer, and a 1.5 to 2 cm nodule in an upper lobe incidentally observed on a CT scan. The nodule is either spiculated or has grown at least 15% in diameter on serial radiographs, but does not have both radiographic risk factors. The individual is a good operative candidate and would tolerate a lobectomy. With a clinical risk for lung cancer of approximately 65% based upon the Mayo Clinic model, this case reflects a patient presenting to thoracic surgeons for surgical evaluation for suspected lung cancer without a preoperative diagnosis.6 Several diagnostic choices are available to the surgeon for diagnostic work up of this patient who is at the margin between high and intermediate risk for lung cancer.7 A second base case with a clinical risk for lung cancer of 50% while holding all other model parameters constant was also considered.
Data sources
We examined published literature for estimates and ranges of diagnostic test characteristics (sensitivity and specificity), complication and outcome probabilities, 5-year survival rates, and costs included in the decision analysis model (Table 1). Recent meta-analyses examining values of a model component (such as 5-year survival) were the preferred source.8,9 Next, systematic reviews were considered with preference for evidence-based reviews to support practice guidelines. 7,10-12 When neither systematic reviews nor meta-analyses were available, a limited review of the relevant literature was conducted and applicable but broad ranges of values were used in sensitivity analysis of the characteristic or event's impact on cost and outcomes. We used Medicare reimbursable amounts for societal costs representing the mean Medicare hospital reimbursement for the indicated inpatients procedure using a base year of 2011 based upon combined diagnostic resource group reimbursement and Common Procedural Terminology (CPT). Outpatient procedures were calculated using their respective combined professional and facility CPT code reimbursement for 2011. Costs were abstracted from existing literature or Center for Medicare Services publications (Table 2).13 Cost ranges were assumed to be base reimbursable amount plus or minus 50.
Table 1. Variables used in the model.
| Test Characteristics assuming lesion ≤2 cm | |||
|---|---|---|---|
| Variable | Value | Range | Source |
| PET sensitivity | 0.87 | 0.80 - 0.96 | 9 |
| PET specificity | 0.76 | 0.40 - 1.00 | 9 |
| Nav Bronch. % diagnostic | 0.61 | 0.54 - 0.68 | 8,12 |
| Nav Bronch sensitivity | 0.98 | ||
| Nav Bronch specificity | 1.00 | 0.85 - 1.00 | |
| CT-FNA % Diagnostic | 0.77 | 0.40 - 0.90 | 12,23 |
| CT-FNA sensitivity | 0.92 | 0.85 - 1.00 | |
| CT-FNA specificity | 1.00 | ||
| Surgical Biopsy sensitivity | 1.00 | Reference | |
| Surgical Biopsy specificity | 1.00 | Reference | |
| QALYs and 5 Year Survival Rates | ||||
|---|---|---|---|---|
| QALYs | 5 year survival | Prevalence in base case | Reference | |
| Benign | 92%18 | 35% | Base Case | |
| Stage 1 | 13.14 | 68%11 | 49% | 24 |
| Stage 2 | 6.06 | 44%11 | 11% | 24 |
| Stage 3/4 | 3.2 | 21%11 | 5% | 24 |
Table 2. Cost of the diagnostic strategies.
| Costs | |||
|---|---|---|---|
| Base Estimate (2011 dollars) | Range (2011 dollars) | References | |
| Procedures | |||
| FDG-PET | 1,170 | 580 - 1750 | 26 |
| CT-FNA | 571 | 286 – 857 | 5 |
| Nav Bronch | 1,228 | 614 – 1,842 | 5 |
| Lobectomy | 11,493 | 5,747 – 17,293 | 26 |
| Wedge | 10,944 | 5,472 – 16,416 | 26 |
| Surgery with complications | 18,614 | 9,307 – 27,921 | |
| Computed Tomography | 311 | 156 – 467 | 26 |
| Complications | |||
| Pneumothorax needing observation | 557 | 279 – 836 | 5 |
| Pneumothorax needing chest tube | 5844 | 2,922 – 8,766 | 5 |
| Hemorrhage | 11751 | 5,876 – 17,627 | 5 |
Outcomes
CT-FNA and NB biopsies could be either diagnostic (malignant or definitively benign) or non-diagnostic. We refer to diagnostic yield as the percent of biopsies that result in a malignant or definitively benign diagnosis. Patients with malignant biopsies underwent a lobectomy (Figure 1). All diagnostic biopsies, FDG-PET avid lesions and underwent VATS. We assumed 85% of all non-diagnostic biopsies underwent VATS biopsy. The remaining 15% of non-diagnostic biopsies and all non-avid FDG-PET nodules entered the watchful waiting strategy. In the watchful waiting strategy, a nodule was followed with 3 CT scans over 2 years with the first scan occurring 3 months after the decision to initiate the watch and wait strategy. Patients with malignancy after a VATS biopsy were assumed to undergo lobectomy while patients with a pathologically determined benign nodule at VATS biopsy were assumed to undergo a wedge resection. Surgical biopsy was considered the gold standard for diagnosis.
In the watch and wait strategy, 10% of benign lesions were assumed to demonstrate malignant growth on the 3 month follow-up CT scan as used in previous cost effectiveness studies.14 Lesions demonstrating malignant growth underwent VATS biopsy. Malignant nodules were assumed to be identified at 3 months as used in previous effectiveness analyses.14 To incorporate the hazard of delayed diagnosis from the watchful waiting strategy, disease progression due to delayed diagnosis resulted in stage progression and reduced life expectancies as modeled in previous effectiveness analyses.15 Patients with benign lesions who underwent surgical biopsy were assumed to have a nontherapeutic wedge resection that would not affect life expectancy if the initial surgery did not result in mortality. The Society of Thoracic Surgeons National General Thoracic Surgery Database was the source for perioperative mortality and complications with lobectomy and National Lung Cancer Screening trial data was used for perioperative mortality from wedge resection. 16,17
The changes in survival due to a diagnosis of benign disease or cancer were converted to Quality Adjusted Life Years (QALYs) by multiplying the expected years of survival by the utility or quality of life of the patient for those remaining years. One QALY represents the quality and quantity of life equivalent to a healthy individual with no disease burden for a year. United States life expectancy tables were used to calculate life expectancy for patients with benign nodules.18 The utility or quality of life of this patient population would be 1.0. The only decrease in QALYs in this group was from morbidity and mortality from diagnostic procedures. Survival curves for lung cancer were used to determine stage-specific survival after resection.11 Expected 5-year survival was converted to overall life expectancy using the declining exponential approximation for life expectancy method to estimate excess mortality due to disease. This method uses a competing risks for mortality model where risks of mortality are a function of age.19 Since older patients have multiple competing risks for death, the absolute effect of a new diagnosis on life expectancy is often relatively small. The prevalence of each pathologic stage for suspected clinical stage 1 disease (T1N0M0) was based upon the American College of Surgeons, Oncology Group Z4031 trial.20 Prior publication by Gould and colleagues was the source for utilities associated with patient health states arising from procedures and the complications associated with those procedures.15
Baseline and Sensitivity Analysis
Baseline estimates were used to calculate outcomes for the model. One-way sensitivity analyses were performed by varying single parameters across clinically plausible ranges to assess changes in cost effectiveness of modeled strategies and to identify potential thresholds where the preferred treatment option would change. Two-way sensitivity analyses were performed by varying two parameters simultaneously to assess whether the optimal treatment strategy changed within clinically plausible values. Incremental cost-effectiveness ratio (ICER) is used to compare the cost-effectiveness of different treatments and indicates the additional cost required to gain one additional QALY. Differences in the incremental cost-effectiveness were expressed as an ICER and were calculated using the additional cost associated with a diagnostic decision and its associated net increase or decrease in quality adjusted life.
Results
The FDG-PET had the lowest expected cost for diagnosing patients ($10,410) with an expected QALY of 14.12 (Table 3). Compared with FDG-PET, patients diagnosed using NB incurred an expected incremental cost of $191 to obtain an additional 0.05 QALYs and resulted in an ICER of $4,602 per additional QALY. CT-FNA had a similar cost ($193) and efficacy with a QALY of 14.17 as compared to FDG-PET and marginally higher QALY (<0.01) when compared to navigation bronchoscopy. Diagnosis by VATS had both higher expected cost of $11,720 for lower effectiveness (14.15 QALYs) and the other two biopsy strategies provided higher QALYs at a lower cost than VATS biopsy.
Table 3. Results of the decision tree analysis.
| Strategy | Total Cost ($) | Incremental Cost ($) | QALYs | Incremental effectiveness | ICER*($ perQALY) |
|---|---|---|---|---|---|
| Base case scenario- 65% prevalence of cancer | |||||
| FDG-PET | 10,411 | 14.12 | |||
| CT-FNA | 10,603 | 193 | 14.17 | .05 | 3,998 |
| Nav Bronch | 10,601 | 191 | 14.17 | .04 | 4,602 |
| VATS | 11,720 | 1,294 | 14.15 | .03 | 43,578 |
| Base case scenario- 50% prevalence of cancer | |||||
| FDG-PET | 9,256 | 15.55 | |||
| CT-FNA | 9,542 | 286 | 15.58 | .03 | 9,533 |
| Nav Bronch | 9,476 | 220 | 15.58 | .03 | 7,333 |
| VATS | 11,623 | 2,367 | 15.54 | -.02 | -118,350 |
Incremental Cost effectiveness Ratio is the ratio of the differences in the cost of decisions divided by the increase in QALY, where FDG-PET is the reference decision (Costdecision–CostFDG-PET)/(QALYdecision – QALYFDG-PET)
In sensitivity analysis of individual model components, decreasing the diagnostic yield of NB below 54% causes FDG-PET to be preferred. When the diagnostic yield of NB is greater than 65%, then tissue acquisition by NB is the preferred decision for diagnosis. Similarly, for CT-FNA, increasing the diagnostic yield to 85% caused it to be preferred over FDG-PET or NB; however, FDG-PET was preferred when CT-FNA diagnostic yield dropped below 70%. When FDG-PET sensitivity was fixed at 87% and the specificity of a FDG-PET scans fell below 72%, then CT-FNA or NB were the preferred diagnostic strategies. When the sensitivity of FDG-PET was above 94% and specificity was fixed at 77%, then FDG-PET was less costly than tissue biopsy or surgery and similarly effective.
In two-way sensitivity analysis, FDG-PET remained the least costly diagnostic strategy across all combinations of sensitivity between 80% and 100% and specificity between 60% and 90% (Figure 2). Efficacy for FDG-PET ranged from 14.08 to 14.22 QALYs across these combinations of sensitivity and specificity. FDG-PET was the most effective and least costly strategy at the upper ranges of sensitivity and specificity when expected QALYs exceeded 14.17.
Figure 2. Sensitivity analysis–Cost –effectiveness threshold given various combinations of FDG-PET sensitivity and specificity.
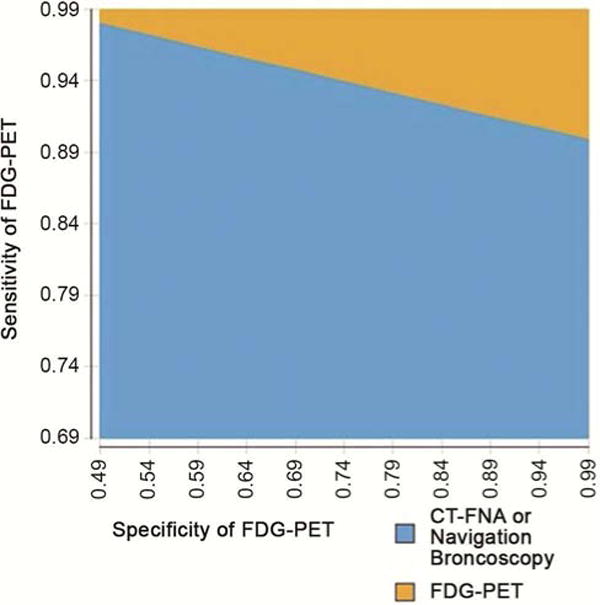
Graph displays the most cost-effective treatment across sensitivity and specificity combinations with all other model variables held constant at baseline values. For example, FDG-PET is the most cost effective choice if the sensitivity and specificity of FDG-PET are both 94%. If the sensitivity of FDG-PET is 94% and specificity is 60%, then CT-FNA or NB is the most cost-effective strategy.
When the risk of malignancy was decreased to 50% in the base case, then all estimated QALYs increased, making the non-VATS strategies similar in efficacy (Table 3). The cost of care for each diagnostic modality was lower compared to the model with higher prevalence of lung cancer (Table 3). FDG-PET and CT-FNA were the preferred strategies compared to NB and VATS biopsy, although the difference between CT-FNA and NB in overall cost-effectiveness was negligible. Sensitivity analysis on single diagnostic components when cancer prevalence was 50% was similar to that observed when the prevalence of malignancy was 65%.
Comment
In clinical practice, surgeons are frequently asked to see patients with suspicious lesions. Based on American College of Chest Physicians 2013 guidelines, and estimation of the likelihood of cancer should be made first and the subsequent workup follow this estimation.3 Predictive models such as the Mayo Clinic model exist to help clinicians but these cancer risk prediction models are poorly calibrated for the higher prevalence of cancer a surgeon encounters, so we are left with our best clinical judgment.21 In the diagnostic algorithm presented in the updated guidelines, patients with a probability of cancer in the 5-65% range needed a diagnostic workup such as FDG-PET or a non-invasive biopsy (CT-FNA or NB). Patients who have greater than a 65% likelihood of cancer were recommended to go to VATS biopsy. In this study we focused on the patient with a 65% likelihood of cancer where a difficult decision exists between VATS or further diagnostic workup. We also performed sensitivity analyses for cancer likelihoods between 50% to 85% range to represent the range cancer prevalence range frequently encountered in surgical practices. Within these cancer likelihood and practice prevalence patterns we varied the model parameters to determine the important drivers of the outcomes.
In populations where the prevalence of lung cancer is at least 65%, we found that pursuit of tissue diagnosis with biopsy (CT-FNA or NB) was more cost effective than FDG-PET or VATS. The method of biopsy, made little difference in effectiveness as measured by quality of life. Navigation bronchoscopy is slightly less expensive than CT-FNA in this analysis due to fewer complications, but given the range in costs of each method, they are equivalent in this scenario. The use of CT-FNA or NB is highly dependent upon both clinical expertise and physical accessibility of the nodule to the proposed modality. For 1.5 to 2.0 cm nodules, centralized location, lesions distant from an airway, or smaller lesions increase the likelihood of a non-diagnostic biopsy and decrease the efficacy of their respective diagnostic modalities.4,12 In our model we assumed that 85% of non-diagnostic biopsies would be followed by VATS biopsy. The aggressive pursuit of tissue diagnosis is logical in populations where the prevalence of malignancy is high, as we assumed here. The increased quality of life after tissue biopsy (0.05 QALYs) compared to imaging was modest; however, the under $200 cost for this increase of life was modest as well. Instituting a strategy of aggressive tissue diagnosis in a population with a moderate to high likelihood of cancer would result in a gain of about 1 QALY per 20 patients, assuming an increase of adjusted life year of 0.05, treated at a modest cost of an additional $4,000 to Medicare for those 20 patients.
In sensitivity analysis the preferred strategy of tissue biopsy did not change when the prevalence of morbidity and mortality from VATS ranged from half to 2.5 times the reported rates found in the literature.16,17,22 VATS biopsy for diagnosis was not preferred over the other diagnostic methods unless the prevalence of malignancy was over 85% (data not shown). In our base case scenario, VATS biopsy for diagnosis became competitive as a diagnostic strategy but remained inferior to CT-FNA or NB when the likelihood of mortality dropped to half that observed for wedge resection (0.6%) and lobectomy (1.1%). Parameters materially influencing diagnostic method choice were the rates of non-diagnostic biopsy for NB and CT-FNA and the sensitivity and specificity of FDG-PET. Increases in diagnostic yield by 6% for NB and 7% for CT-FNA, caused each modality to be preferred over FDG-PET. Comparable decreases in diagnostic biopsy yield made FDG-PET preferred. As the prevalence of lung cancer decreases, the non-invasive FDG-PET scan becomes more effective as a lower cost alternative to tissue diagnosis. However, surgeons should also consider tissue acquisition strategies even in instances when the likelihood of cancer is lower (50%) if there is reason to believe that the accuracy of FDG-PET is reduced due to smaller lesion diameter or high rates of false positive scans.
Following the recommendations of Gold and colleagues, we used Medicare reimbursable charges when possible to estimate the relative cost of performing a procedure or diagnostic test.13 The lack of uniformity of costs or charges across providers required reliance on published reimbursement from Medicare and a perspective that can be considered from the payors perspective. Societal costs arising from lost work days, transportation costs, and waiting-induced anxiety were not included in this analysis. One weakness of our study was the use of QALY data from previous studies in lung cancer and cancer treatment rather than direct estimation of utilities from surgery patients. We assumed that the stress from watchful waiting would be equivalent to that incurred from a benign pathological diagnosis after VATS wedge biopsy. Our analysis of the preferred diagnostic strategy may not be applicable to nodules <1.5 cm where the sensitivity of FDG-PET is lower, the diagnostic yield of FNA and NB decrease, and the risk of lung cancer is lower. Conversely, FDG-PET accuracy and diagnostic yields of biopsy likely increase for nodules greater than 2cm in diameter.
When evaluating a suspicious lung lesion, the surgeon should first provide their best estimate of the likelihood of cancer. Guidelines suggest using either a validated clinical risk model, like the Mayo Clinic model, or physician expertise to estimate a lesions risk for cancer. Based on our effectiveness model, if the likelihood of cancer approaches 85%, VATS would be the most cost effective choice. If the likelihood of cancer is 50-85% then a tissue acquisition is best. Navigational bronchoscopy should be used when expected diagnostic yield is over 65% and CT-FNA chosen if its expected yield is over 85%. If the lesion has an estimated likelihood of cancer less than 50%, then FDG-PET is a cost effective option provided that the sensitivity and specificity are above 87% and 76%, respectively.
Conclusions
Navigational bronchoscopy and CT-FNA diagnostic strategies were more cost-effective than VATS biopsy or FDG-PET strategies in the work up of a 1.5-2 cm nodule in populations with lung cancer prevalence greater than 50%. In circumstances where FDG-PET accuracy is reduced, the cost-effectiveness of FDG-PET scans for diagnosis of lung cancer may be reduced. Selection of a predefined diagnostic strategy using the surgeon's estimate of the likelihood of lung cancer and the accuracy of the diagnostic tests available to them will be cost effective and may result in fewer non-therapeutic operations.
Discussion
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Bryan F. Meyers, M.D., St. Louis, Missouri. E-mail: meyersb@wustl.edu
Dr. B. Meyers (St. Louis, Missouri): Nice job on your presentation. I have followed your work. I think Dr. Grogan does a great job with this type of analysis of what we do in clinical practice. I think this type of work is very important because it allows us to look simultaneously at all aspects of the whole process of taking care of a patient and then focus on the multiple decisions we make and try to figure out where our weakest area of decision-making lies.
With these cost-effectiveness studies, which I think are quite valuable, there are times where the differences in effectiveness are so small that it actually becomes a different type of a study. I did the quick math on your survival estimates and they describe days, less than a month, of survival difference for all the different pathways, so it really then becomes a cost-reduction study rather than a cost-effectiveness study because the effectiveness is essentially equivalent. How big of an effectiveness difference do you think there ought to be in order to consider the effectiveness as part of the answer?
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: That's a great question. Let me see if I can actually go back. This is sort of the way that my statisticians helped me understand this, and you have been doing this for even longer than I have, but the way that I understand this is, the incremental effectiveness, which is .5, what that would mean is that you would have to basically make an impact on 20 different people for one QALY at $4,000, so the incremental cost-effectiveness for $4,000 falls well beneath what we are used to as a country. It's well beneath the $50,000 to $100,000 limit. So the incremental cost-effectiveness is small.
Dr. Meyers: To elaborate a little on that slide, a QALY is a quality-adjusted life year, and so if you just assume that the quality of life scores are the same for all paths, you are really looking at years of survival from the point of the decision-making. If you need to go 2 digits beyond the decimal point to show a difference, one tenth of a QALY is 30 days and one one-hundredth is 3 days.
Dr. Grogan: Right.
Dr. Meyers: So you are looking at really 15 days of survival difference between arms. So it's a cost study more than anything else.
Dr. Grogan: It is, and that is a great point. So when you look at these two strategies, which it's decimal-point differences, it doesn't matter, but when you start comparing this and you start comparing the cost and the effectiveness, then the incremental cost-effectiveness ratio starts to matter. I think your point is excellent. That's one of the reasons why we did the sensitivity analysis with FNA and navigational bronchoscopy, because then it becomes what is the best strategy as clinicians. When I look at the lesion, am I going to have a better diagnostic yield with an FNA or a better diagnostic yield with the navigational bronchoscopy. That actually begins to start driving it a little bit.
Dr. Meyers: And that is my other question. When the survival is identical and the costs are pretty close, I mean the difference of $191, I think the sequential compression boots to prevent DVTs cost more than that, so you are finding that the differences are small. Then it falls to local expertise. If you have a radiologist who is really good or really lousy at your local hospital, that's going to affect your decision-making here: local strengths and weaknesses rather than some global answer.
Dr. Grogan: That is correct. The other thing that we can't forget is actually PET and the accuracy of PET. PET where we are is not as good. When you start varying the sensitivity and specificity of PET at your local institution, then it becomes less and less attractive as an option.
Your points are well taken and I have followed your work as well and I appreciate your comments.
Dr. Meyers: Thank you.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Kamal G. Khalil, M.D., Houston, Texas, E-mail: kamal.khalil@uth.tmc.edu
Dr. K. Khalil (Houston, Texas): Congratulations on considering cost as part of the decision tree. The problem I have is a patient with a central solitary lesion who had negative tissue sampling by limited means. You take him to VATS. You can't do VATS on a central lesion. Would you then do a lobectomy for diagnosis or would you back off and observe?
My second question is the medicolegal implications of depending on limited sampling that is negative and then a year later you come back with metastases and so forth.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: That's a great question. It's hard to take a case-by-case basis because our decision-making is all driven by the likelihood and the probability that we as clinicians feel that this lesion is cancer, and that can only drive how willing we are to wait and watch this, and if there is a high probability of cancer, there are other options, such as navigational bronchoscopy, that are a possibility. A lobectomy is still well within the realms of our diagnostic capabilities to perform for a highly-suspicious lesion. However, I think all of us are starting to see, even from a medicolegal perspective, cases of physicians being sued for benign diagnoses and doing lobectomies. So all of these things must be balanced and I'm not sure I have the perfect solution for that.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Laurie B. Reeder, M.D., Warwick, Rhode Island. E-mail: lbreeder@yahoo.com
Dr. L. Reeder (Warwick, Rhode Island): Did size play any role in the differences? I mean it's a wide range from .8 to 3.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: Correct. Size is actually very problematic from a decision analysis modeling standpoint, which is why we focused on that size, less than 2 cm, basically between 1.5 and 2 cm. As you start to vary the size, the probabilities change. So I can't comment for the smaller lesions or the larger lesions. I can really only comment for the ones that we modeled here. But that is a very good question.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Walter J. Scott, M.D., Philadelphia, Pennsylvania. E-mail: walter.scott@fccc.edu
Dr. W. Scott (Philadelphia, Pennsylvania): Very nice presentation.
We had some experience with this back in the dark ages of 1998 when we looked at PET and ways to diagnose mediastinal lymph nodes. Decision analysis is a very sensitive tool and the assumptions are very important. In the watch-and-wait arm of the analysis, the number of tests can add up and lead to increasing expenses for multiple CT scans and other tests. Did you assume that there would be just one CT scan, 3 CT scans?
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: We assumed following for up to 2 years, and when we put our model together, we made some assumptions about serial CT scans at 3-month and 6-month intervals and tried to put that in from a cost perspective. When you model that, though, as you can see, the watch-and-wait strategy is really in all of the trees, so when you change that number, if you change it in all of them, it kind of washes out in the model, if that makes sense.
Dr. Scott: I see. Again, nice work. Thank you.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Larry L. Stept, M.D., Pittsburgh, Pennsylvania. E-mail: lstept@verizon.net
Dr. L. Stept (Pittsburgh, Pennsylvania): On the CT-fine-needle aspiration and on the navigational bronchoscopy, you said that 85% of the people eventually went to VATS or some sort of invasive diagnostic procedure. Is that because 85% of the people had a positive tissue diagnosis or is that because in 85% you eventually had to do something in order to get to the bottom of this lesion?
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: That's a great question. Let me see if I can go back here real quick. With regard to the model itself, we modeled the non-diagnostic and diagnostic probabilities of CT-FNA. So you could say 60% of the time you had a non- diagnostic biopsy rate. If you are at our institution, our radiologists for CT-FNA, it seems like it's even higher than that. In this probability for the non-diagnostic, then you can model the VATS probability, and we chose a very conservative estimate that 85% of the time, because this is a suspicious lesion, I'm not going to trust that negative or the non-diagnostic FNA. I'm going to go to VATS because I'm worried about this lesion. So this is a conservative approach, and when we did sensitivity analyses, it didn't seem to change our results when you varied that number.
Dr. Stept: And of those patients who went to VATS because they were in the watch-and-wait, what percentage of those patients actually turned up with a malignant diagnosis?
Dr. Grogan: In the model, then, and this is based on the probability of a 65% chance of being cancer, you can go back to that A category, which is the VATS model, and model the probabilities that it's going to be 35% cancer and 35% benign disease. So it's a model and there are limitations, but we did our best.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Paper presented by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Discussion by Thomas K. Waddell, M.D., Toronto, Ontario, Canada. E-mail: tom.waddell@uhn.ca
Dr. T. Waddell (Toronto, Ontario, Canada): I enjoyed it as well. It was very well done.
My question is about the sensitivity analysis about the basal prevalence estimate. You set up the question very nicely from the CT screening, but I think it's worth remembering that the prevalence of cancer in the screened group is about 1%, the prevalence in the group that has a nodule is only 4%, and you presented that algorithm where right at the beginning there was a certain strategy, but then you did the modeling on 65%.
100. COST-EFFECTIVENESS OF INITIAL DIAGNOSTIC STRATEGIES FOR SOLITARY PULMONARY NODULES PRESENTING TO THORACIC SURGEONS. Response by Eric L. Grogan, M.D., Nashville, Tennessee. E-mail: eric.grogan@vanderbilt.edu
Dr. Grogan: That's a great question. I didn't have time to present this, but if you change the base scenario to 50% prevalence of cancer --
Dr. Waddell: I would like to know if you modeled it down to 5%.
Dr. Grogan: But those aren't the people that I'm seeing as a surgeon. That's a good point, but I had to pick something, so I chose the suspicious lesions, and as you get down to 50%, PET becomes more and more a better option for diagnosis. It's more and more cost-effective. Once you get up to above 80% to 85%, VATS actually becomes the best strategy. So we have got a little bit more work to do to figure out sort of what these breakpoints are and where the best options are for us as surgeons. But I can tell you that clinically, this will drive my practice a little bit. I will be doing a lot more tissue-acquisition strategies because it does appear to be more cost-effective for those intermediate lesions.
Dr. Waddell: I am really surprised if you say that you are seeing patients at the 50% from the screening program, because that means somebody else, radiologists or pulmonary people, are removing 90% of the false-positives, in which case I would like to know what strategies they are using. It sounds like they are doing a very good job.
Acknowledgments
Dr. Grogan is a recipient of the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service Career Development Award (10-024). This work was also supported by an AHRQ (1 R03 HS021554-01), Vanderbilt Institute for Clinical and Translational Research grant support (UL1TR000011, NCATS/NIH) for the REDCap database. Dr. Aldrich is supported by a K07 NCI grant (1K07CA172294-01A1). The authors had full control of the study design, methods, outcome parameters and results, data analysis, and manuscript production. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations and Acronyms
- CT-FNA
computed tomography fine needle aspiration
- FDG-PET
18F-fluoro-deoxyglucose positron emission tomography
- ICER
incremental cost-effectiveness ratio
- NB
navigation bronchoscopy
- QALYs
quality adjusted life years
- VATS
video assisted thoracic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Humphrey LL, Deffebach M, Pappas M, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine. 2013;159(6):411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 2.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? Journal of Medical Screening. 2012 Sep 1;19(3):154–156. doi: 10.1258/jms.2012.012010. 2012. [DOI] [PubMed] [Google Scholar]
- 3.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2013;143(5_suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamprecht B, Porsch P, Wegleitner B, Strasser G, Kaiser B, Studnicka M. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respiratory Medicine. 2012;106(5):710–715. doi: 10.1016/j.rmed.2012.02.002. 5// [DOI] [PubMed] [Google Scholar]
- 5.Dale CR, Madtes DK, Fan VS, Gorden JA, Veenstra DL. Navigational bronchoscopy with biopsy versus computed tomography-guided biopsy for the diagnosis of a solitary pulmonary nodule: a cost-consequences analysis. Journal of bronchology & interventional pulmonology. 2012 Oct;19(4):294–303. doi: 10.1097/LBR.0b013e318272157d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008 Apr;63(4):335–341. doi: 10.1136/thx.2007.084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AF, Hastie ND. Complex genetic diseases: controversy over the Croesus code. Genome Biol. 2001;2(8) doi: 10.1186/gb-2001-2-8-comment2007. COMMENT2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-Analysis of Guided Bronchoscopy for the Evaluation of the Pulmonary Nodule. Chest. 2011 Oct 6; doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deppen SA. Development of a lung cancer prediction model for surgeons and factors affecting its national application. Thesis/Dissertation. 2013 http://etd.library.vanderbilt.edu/available/etd-06062013-113320/
- 10.Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007 Sep;132(3 Suppl):94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 11.Goldstraw PF, Crowley JP, Chansky KMS, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 12.Logan RFA, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2011 Dec 7; doi: 10.1136/gutjnl-2011-300843. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold MR, editor. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 14.Gambhir S, Shepherd J, Shah B, et al. Analytical decision model for the cost-effective management of solitary pulmonary nodules. J Clin Oncol. 1998;16(6):2113–2125. doi: 10.1200/JCO.1998.16.6.2113. [DOI] [PubMed] [Google Scholar]
- 15.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003 May 6;138(9):724–735. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 16.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozower BD, Sheng S, O'Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010 Sep;90(3):875–881. doi: 10.1016/j.athoracsur.2010.03.115. discussion 881-873. [DOI] [PubMed] [Google Scholar]
- 18.Miniño A, Heron M, Murphy S, Kochanek K. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004. Natl Vital Stat rep. 2007;55(19):1–120. 2007. [PubMed] [Google Scholar]
- 19.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating Treatment Benefits for the Elderly: The Effect of Competing Risks. Annals of Internal Medicine. 1996;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Grogan EL, Deppen SA, Ballman KV, et al. Accuracy of fluorodeoxyglucose-positron emission tomography within the clinical practice of the american college of surgeons oncology group z4031 trial to diagnose clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2014 Apr;97(4):1142–1148. doi: 10.1016/j.athoracsur.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isbell JM, Deppen S, Putnam JB, Jr, et al. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann Thorac Surg. 2011 Jan;91(1):227–233. doi: 10.1016/j.athoracsur.2010.08.054. discussion 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan EL, Weinstein JJ, Deppen SA, et al. Thoracic Operations for Pulmonary Nodules Are Frequently Not Futile in Patients with Benign Disease. J Thorac Oncol. 2011 Oct;6(10):1720–1725. doi: 10.1097/JTO.0b013e318226b48a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno Y, Hatabu H, Takenaka D, et al. CT-Guided Transthoracic Needle Aspiration Biopsy of Small (<= 20 mm) Solitary Pulmonary Nodules. Am J Roentgenol. 2003 Jun 1;180(6):1665–1669. doi: 10.2214/ajr.180.6.1801665. 2003. [DOI] [PubMed] [Google Scholar]
- 24.Grogan E, Deppen SA, Ballman K, et al. Accuracy of FDG-PET within the clinical practice of the ACOSOG Z4031 trial to diagnose clinical stage 1 NSCLC. Annals of Thoracic Surgery. 2014 doi: 10.1016/j.athoracsur.2013.12.043. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-Based Risk for Complications After Transthoracic Needle Lung Biopsy of a Pulmonary Nodule: An Analysis of Discharge Records. Ann Intern Med. 2011 Aug 2;155(3):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Services HaH., editor. Services CfMM. Details for Regulation CMS-1503 and 1524. Washington DC: 2011. [Google Scholar]


